Back to Journals » International Journal of Nanomedicine » Volume 17
Exosomal lncRNA HOTAIR Promotes the Progression and Angiogenesis of Endometriosis via the miR-761/HDAC1 Axis and Activation of STAT3-Mediated Inflammation
Authors Zhang L , Yu Z, Qu Q, Li X, Lu X, Zhang H
Received 22 December 2021
Accepted for publication 4 March 2022
Published 16 March 2022 Volume 2022:17 Pages 1155—1170
DOI https://doi.org/10.2147/IJN.S354314
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Israel (Rudi) Rubinstein
Lu Zhang,1 Zitong Yu,2 Qingxi Qu,1 Xiao Li,1 Xue Lu,1 Hui Zhang1
1Department of Obstetrics and Gynecology, Qilu Hospital of Shandong University, Jinan, 250012, People’s Republic of China; 2Department of Obstetrics, Shouguang People’s Hospital, Shouguang, 262700, People’s Republic of China
Correspondence: Hui Zhang, Department of Obstetrics and Gynecology, Qilu Hospital of Shandong University, No. 107 Wenhua West Road, Jinan, 250012, People’s Republic of China, Email [email protected]
Background: Long non-coding RNA (lncRNA) and exosomes are implicated in endometriosis development. We measured the expression of an exosomal lncRNA, homeobox transcript antisense RNA (HOTAIR), and explored its molecular mechanism in endometriosis progression.
Methods: Expression of HOTAIR and microRNA (miR)-761 in different endometrial tissues was measured. Exosomes were isolated from a culture medium of endometrial stromal cells (ESCs). RT-qPCR was used to measure HOTAIR expression in different exosome types. CCK-8, Edu, wound healing, transwell assays, flow cytometry and tube formation were used to detect the role of exosomal HOTAIR on ESCs and human umbilical vein endothelial cells (HUVECs). The relationship among miR-761, HOTAIR, and histone deacetylase 1 (HDAC1) was verified by dual-luciferase reporter assay. ESCs were transfected with miR-761 mimics or HDAC1 small interfering RNA (si-RNA) to ascertain if alterations in expression of miR-761 or HDAC1 could reverse the effect of exosomal HOTAIR. Then, we detected the effect of the HOTAIR/miR-761/HDAC1 axis on signal transducer and activator of transcription 3 (STAT3)-mediated inflammation. In vivo experiments were conducted to verify in vitro results.
Results: HOTAIR expression was upregulated and miR-761 expression was downregulated in ectopic endometrium tissues. HOTAIR was packaged into exosomes and transported from ESCs to surrounding cells. Exosomal HOTAIR promoted the proliferation, migration, and invasion, and inhibited the apoptosis of ESCs. Angiogenesis of HUVECs was enhanced after cultured with exosomal HOTAIR. HOTAIR acted as a competing endogenous RNA to downregulate miR-761 and increase HDAC1 expression. miR-761 overexpression or HDAC1 knockdown reversed the role of exosomal HOTAIR on ESCs and HUVECs. The HOTAIR/miR-761/HDAC1 axis could activate STAT3-related proinflammatory cytokines and stattic (inhibitor of phosphorylated-STAT3) could reverse the effect of HOTAIR on ESCs and HUVECs. In vivo experiments suggested that exosomal HOTAIR promoted the growth of endometrial lesions in vivo.
Conclusion: Exosomal HOTAIR promoted the progression and angiogenesis of endometriosis by regulating the miR-761/HDAC1 axis and activating STAT3-mediated inflammation in vitro and in vivo, which may provide promising treatment for endometriosis.
Keywords: endometriosis, HOTAIR, exosomes, miR-761, HDAC1, STAT3, inflammation
Introduction
Endometriosis is a chronic inflammatory disease. It is characterized by endometrial tissue being present outside of the uterus.1 Endometriosis is a leading cause of pelvic pain and infertility that affects the health and quality of life of 6–10% of women of childbearing age.2 The etiology of endometriosis is complicated and multifactorial: retrograde menstruation, hormone secretion, local immunity, environment, and susceptible genes.3 The exact pathogenesis of endometriosis is largely unclear. Hence, more efforts are needed to explore the underlying mechanisms of endometriosis to create new therapeutic regimens.
Long non-coding RNAs (lncRNAs) have a length >200 nucleotides and have no protein-coding function.4 Recently, lncRNAs have been shown to have crucial roles in regulation of gene expression through modulation of transcription and post-transcription regulation.5 Accumulating evidence has demonstrated lncRNAs to be involved in numerous physiological and pathological processes, such as the proliferation, migration, invasion, and differentiation of cells.6–8
Several lncRNAs have been reported to be dysregulated upon endometriosis development. For instance, lncRNA H19 promotes the proliferation and invasion of cells of ectopic endometrium via the miR-124-3p/ITGB3 pathway.9 LncRNA LINC00261 inhibits the growth and migration of cells in endometriosis.10 Homeobox transcript antisense RNA (HOTAIR) can serve as a functional lncRNA in several disease types by sponging specific miRNAs and regulating downstream gene transcription. HOTAIR promotes hepatocellular carcinoma by sponging miR-23b-3p and targeting ZEB1.11 HOTAIR can also serve as a sponge of miR-331-3p to regulate HER2 expression in gastric cancer.12 However, the role of HOTAIR in endometriosis is not clear.
Exosomes can mediate cell-to-cell communication by transmitting active molecules, including lipids, proteins, and non-coding RNAs.13 We have demonstrated that exosomes are deeply involved in endometriosis development.14–16 but the role of lncRNAs packaged in exosomes in endometriosis needs further investigation. Exosomal lncRNAs have certain roles in mediating cell proliferation, migration, apoptosis, and autophagy.17–19 Exosomal HOTAIR has been shown to reduce the radiosensitivity of laryngeal cancer cells by regulating the miR-454-3p/E2F2 pathway.20 Exosomal HOTAIR promotes the progression of lung cancer by regulating miR-203 expression.21 However, the role of exosomal HOTAIR in endometriosis has not been elucidated.
In the present study, expression of HOTAIR and miR-761 in the normal endometrium, eutopic endometrium, and ectopic endometrium was measured. Then, we explored the biological functions of exosomal HOTAIR in the activity of endometrial stromal cells (ESCs) and human umbilical vein endothelial cells (HUVECs). Furthermore, we investigated the downstream mechanisms by which exosomal HOTAIR has crucial roles in endometriosis as a competing endogenous RNA (ceRNA).
Materials and Methods
Ethical Approval
The study protocol was approved by the Medical Ethics Committee of Qilu Hospital of Shandong University. Written informed consent was obtained from all women to use their samples for analyses. The study protocol complied with the Declaration of Helsinki was obtained from all participants.
Patients
Patients with ovarian endometrial cysts served as the endometriosis group (n = 50). The control group (n = 50) was defined as patients who had undergone laparoscopic surgery for obstruction of fallopian tubes. The exclusion criteria were ovarian cancer, other benign ovarian cysts, pelvic inflammation, and polycystic ovarian syndrome. Normal endometria from the control group, and samples of eutopic endometrium and ectopic endometrium from the endometriosis group were collected. All women had regular menstrual cycles and had not taken any hormonal medications within 6 months before tissue collection.
Isolation and Culture of ESCs
ESCs were isolated from endometrial tissues. Briefly, the endometrial tissues were minced into pieces and digested with type-IV collagenase (0.1%; MilliporeSigma, USA) for 45 min at 37°C. To remove undigested epithelial cells and debris, the tissue pieces were filtered through sterile sieves of size 150 μm and 37.4 μm. Then, the suspension was centrifuged at 1000 × g for 5 min at room temperature. This action was followed by culture in red blood cell lysis buffer for 10 min to remove erythrocytes. After centrifugation at 1000 × g for 5 min at room temperature, the ESCs were plated in flasks. Subsequently, the ESCs were cultured in Dulbecco’s modified Eagle’s medium (DMEM)/F12 supplemented with 10% fetal bovine serum (Gibco, USA), penicillin (100 µ/mL), and streptomycin (100 mg/mL) in a humidified atmosphere of 5% CO2 at 37°C.
RNA Extraction and Real-Time Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA from tissues, cells, or exosomes was extracted with TRIzol Reagent (Invitrogen, USA) according to manufacturer instructions. Real-time RT-qPCR was undertaken using a SYBR Green-based PCR kit (Takara, Japan). GAPDH or U6 was used as an endogenous reference gene. The 2−ΔΔCt method was employed to calculate relative gene expression. The results were normalized to the expression of GAPDH (for lncRNA and mRNA) or U6 (for miRNA).
Isolation and Identification of Exosomes
The culture supernatants of ESCs were harvested for exosome isolation by sequential centrifugation (Optima L-100 ultracentrifuge; Beckman Coulter, USA). Briefly, supernatants were centrifuged at 300 × g for 10 min followed by 2000 × g for 10 min at room temperature. Then, the supernatants were centrifuged at 10,000 × g for 30 min at room temperature to remove cell debris, followed by ultracentrifugation twice at 100,000 × g for 70 min at room temperature. The final pellets were exosomes. Transmission electron microscopy (TEM) using a JEOL (Tokyo, Japan) system was employed to identify exosome morphology. The size and concentration of exosomes were analyzed by Nanoparticle-tracking analysis (NTA) using an analyzer (NanoSight NS300; Malvern Instruments, Malvern, UK). Specific markers for exosomal proteins, CD9 and CD63, were identified by Western blotting.
Labeling and Internalization of Exosomes
Exosomes were labeled with Dil dye (1 mM) for 30 min. ESCs were co-cultured with DiI-labeled exosomes for 24 h. ESCs were incubated with 4’,6-diamidino-2-phenylindole (DAPI; Invitrogen, USA) for 10 min at 37°C. Fluorescence was detected under a fluorescence microscope (Olympus, Japan).
Cell Transfection
HOTAIR small interfering RNA (si-HOTAIR), HOTAIR siRNA control (si-HOTAIR-control), HOTAIR-overexpression plasmid (Oe-HOTAIR), HOTAIR-overexpression plasmid negative control (Oe-control), miR-761 mimics, miR-761 mimics negative control (mimics NC), HDAC1 siRNA (si-HDAC1), and HDAC1 siRNA control (si-HDAC1-control) were designed, synthesized, and transfected into ESCs by Lipofectamine 2000 (Invitrogen, USA).
HOTAIR-Interfering Exosomes
ESCs were transfected transiently with si-HOTAIR, si-HOTAIR-control, Oe-HOTAIR, or Oe-control by Lipofectamine 2000 (Invitrogen, USA) for 48 h. exosi-HOTAIR, exosi-HOTAIR-control, exoOe-HOTAIR, and exoOe-control were isolated from the culture supernatants of HOTAIR-interfering ESCs.
Cell Proliferation Assay
ESCs proliferation was measured using Cell Counting Kit-8 (CCK-8; Dojindo, China) according to manufacturer protocols. Briefly, ESCs were added to 96-well culture plates. CCK-8 reagent (10 μL) was added to each well at 24, 48, 72, 96, and 120 h respectively, and the mixture allowed to incubate for an additional 4 h at 37°C. The absorbance at 450 nm was measured using a microplate spectrometer (Thermo Fisher Scientific, USA).
5-Ethynyl-2′-Deoxyuridine (EdU) Assay
Treated ESCs were plated on 96-well plates (5×103 cells per well) followed by incubation with 100 μL of EdU solution (50 μm; RiboBio, China) for 2 h, fixed with 4% paraformaldehyde (50 μL) for 30 min and finally, incubated with 50 μL of glycine (2 mg/mL) for 5 min at room temperature. Then, cells were incubated with 100 μL of an osmotic agent (0.5% Triton X-100) for 10 min, incubated in the dark with 100 μL of Apollo solution for 30 min, and decolorized with methanol, at room temperature. Finally, cells were stained with DAPI and observed under a fluorescence microscope (Olympus, Japan).
Cell Migration and Invasion Assay
Transwell chambers were precoated with Matrigel (BD Biosciences, USA). ESCs in DMEM/F12 containing 0.1% bovine serum albumin were added to the upper chamber. DMEM/F12 medium supplemented with 20% fetal bovine serum was added to the bottom chamber. After incubation for 24 h at 37°C, non-invading cells were removed. Cells invading into the bottom chamber were fixed with methanol and stained with 0.1% crystal violet. Cell invasion was photographed under a microscope and quantified by counting five random fields. Cell migration assays were also carried out using the same method but without Matrigel.
Flow Cytometry
ESCs apoptosis was detected by flow cytometry using annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) staining assay kits (Solarbio, China) according to manufacturer instructions. The fluorescence of FITC and PI was detected via a FACSCalibur flow cytometer (BD Biosciences, USA) and apoptosis was analyzed.
Tube Formation Assay
Different exosomes were added to HUVECs. Matrigel was thawed at 4°C overnight and placed in 96-well plate at 37°C for 30 min. HUVECs (1 × 105 cells per well) were seeded in 96-well plates for 6 h at 37°C in a 5% CO2 humidified incubator. Tube formation was observed and imaged with a light microscopy. The branch points per well were scanned and quantified by Image J.
Luciferase Activity Assay
Potential miRNA binding sites in HOTAIR sequences were predicted by StarBase V2.0 (http://starbase.sysu.edu.cn/starbase2/). Wild-type (wt) or mutant (mut) HOTAIR binding sites was subcloned into the pGL3-control vector (Promega, USA). miR-761 mimics or mimics NC were co-transfected with wt-HOTAIR or mt-HOTAIR into ESCs. Similarly, putative miR-761 binding sites in the 3′-untranslated region (UTR) of HDAC1 mRNA were synthesized and inserted into the pGL3-control vector. ESCs were transfected with wt or mut HDAC1 and miR-761 mimics or mimics NC. A dual-luciferase reporter assay system (Promega, USA) was employed to measure fluorescence intensity according to manufacturer instructions.
RNA Immunoprecipitation (RIP) Assay
The binding of HOTAIR to Ago2 was detected by a RIP kit (Millipore, USA). Cells were collected and resuspended in an equal volume of RNA RIP lysis buffer plus protease and RNase inhibitors. Then, they were centrifuged at 12,000 × g for 30 min at room temperature, and incubated with anti-Ago2 magnetic beads for 4 h at 4°C with anti-immunoglobulin G magnetic beads as negative control. The magnetic beads were washed thrice with lysis buffer. Expression of HOTAIR in the RNA–protein complex was detected by RT-qPCR.
RNA Pull-Down Assay
ESCs were treated with biotin-labeled Bio-miR-761-wt, Bio-miR-761-mut, or the corresponding Bio-NC for 48 h. Cells were incubated in specific lysis buffer (Ambion, USA). Lysates were centrifuged at 14,000 × g at room temperature to extract the supernatant, and then incubated with M-280 streptavidin beads (MilliporeSigma, USA) that had been precoated with bovine serum albumin without RNase and yeast tRNA (MilliporeSigma, USA). After cleansing with washing buffer, the bound RNA was purified by TRIzol Reagent, and enrichment of HOTAIR expression was detected by RT-qPCR.
Western Blotting
The protein extracted from cells was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis on 10% gels, and then transferred onto polyvinylidene-fluoride membranes (Millipore, USA). We used antibodies against CD9 (ab2215, 1:1000), CD63 (ab59479, 1:1000), HDAC1 (ab19845, 1:1000), STAT3 (ab68153, 1:1000), p-STAT3 (ab76315, 1:1000) and GAPDH (ab181602; 1:10,000). Bands were visualized using a chemiluminescent substrate (Thermo Fisher Scientific, USA).
Enzyme-Linked Immunosorbent Assay (ELISA)
The culture supernatants of ESCs were collected and analyzed using ELISA kits (Thermo Fisher Scientific) according to manufacturer protocols. The endometrial lesions was weighed and homogenized with RIPA buffer on ice followed by centrifugation at 12,000 × g for 15 min at 4°C. Expression of proinflammatory cytokines in supernatants was also measured by ELISA kits.
Endometriosis Mouse Model
A model of endometriosis mouse was established according to the method described in our previous study.22 Six-week-old female nude mice were purchased from Beijing HFK Bioscience (Beijing, China). Mice suffering from endometriosis were divided into five groups: blank group (no treatment); exosi-HOTAIR-control group (exosi-HOTAIR-control injection); exosi-HOTAIR group (exosi-HOTAIR injection); exoOe-control group (exoOe-control injection); exoOe-HOTAIR group (exoOe-HOTAIR injection). Equal volumes of various solutions were injected into the tail vein of mice. The injections were repeated every 2 days until day-14. Mice were killed 24 h after the last injection, and endometrial lesions were collected to record their weight and calculate the volume (volume = 0.5 × (length × width2)) after measuring their length and width. Animal experiments were approved by the Medical Ethics Committee of Qilu Hospital of Shandong University and conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals (US National Institutes of Health).
Statistical Analysis
Statistical analyses were undertaken using Prism 7.0 (GraphPad, USA). Comparisons of the data from two groups were done using the Student’s t-test. Multiple comparisons were carried out with one-way ANOVA. Data are the mean ± SD. Experiments were repeated at least three times. Differences were considered significant if P < 0.05.
Results
HOTAIR Expression is Upregulated and miR-761 Expression is Downregulated in Ectopic Endometrial Tissues
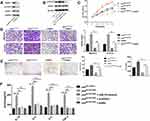
Expression of HOTAIR and miR-761 in different endometrial tissues was measured by RT-qPCR. The ectopic endometrium exhibited higher expression of HOTAIR (Figure 1A) and lower expression of miR-761 (Figure 1B) compared with that in paired eutopic endometrium and normal endometrium. Correlation analysis indicated that HOTAIR expression and miR-761 expression in ectopic endometrium was negatively correlated (r = −0.855, P < 0.001) (Figure 1C). Then, we analyzed the relationship between HOTAIR expression and clinicopathological features in endometriosis. HOTAIR expression was independent of dysmenorrhea, abortion times, and cyst size >3 cm. Endometriosis patients at stages III + IV (according to the revised American Fertility Society classification, r-AFS) or with infertility and deeply infiltrating endometriosis had higher expression of HOTAIR (Table 1).
 |
Table 1 Relationship Between the Expression of HOTAIR and Clinicopathological Features in Endometriosis |
ESCs Transport HOTAIR to Surrounding Cells Through Exosomes
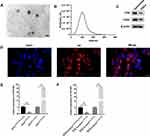
Exosomes were isolated from the culture medium of ESCs by differential centrifugation and identified by TEM, NTA, and Western blotting. TEM showed that exosomes were round or oval with a bilayer membrane (Figure 2A). According to NTA, the diameter of exosomes was 50–200 nm (Figure 2B). We measured expression of the exosomal markers D63 and CD9 via Western blotting (Figure 2C). Fluorescence microscopy revealed that Dil-labeled exosomes were distributed around the DAPI-labeled nuclei of ESCs (Figure 2D). Differential expression of HOTAIR was compared between exosi-HOTAIR-control, exosi-HOTAIR, exoOe-control, and exoOe-HOTAIR groups. We documented a decrease in HOTAIR expression in the exosi-HOTAIR group and an increase in HOTAIR expression in the exoOe-HOTAIR group (Figure 2E). ESCs were co-cultured with different exosomes: treatment with exosi-HOTAIR induced a decrease in HOTAIR expression in ESCs, whereas treatment with exoOe-HOTAIR revealed the reverse phenomenon (Figure 2F).
Exosomal HOTAIR Promotes the Proliferation, Migration, and Invasion of ESCs, and Inhibits ESCs Apoptosis
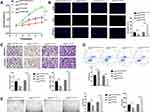
To explore the biological effect of exosomal HOTAIR on ESCs, ESCs were transfected with exosi-HOTAIR-control, exosi-HOTAIR, exoOe-control, or exoOe-HOTAIR. CCK-8 and Edu assays revealed that exosi-HOTAIR inhibited ESCs proliferation significantly, but exoOe-HOTAIR showed the opposite effect (Figure 3A and B). Transwell assay demonstrated that exosi-HOTAIR induced a decrease in the invasion and migration of ESCs (Figure 3C). Flow cytometry revealed apoptosis promotion in ESCs treated with exosi-HOTAIR (Figure 3D). Additionally, exoOe-HOTAIR induced more tube formation of HUVECs than that observed in exoOe-control group (Figure 3E).
HOTAIR Acts as a ceRNA to React with miR-761 to Regulate HDAC1
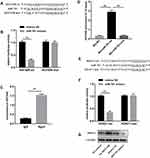
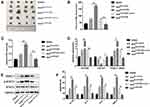
An online database (http://starbase.sysu.edu.cn/starbase2/) predicted potential binding sites between HOTAIR and miR-761 (Figure 4A). The luciferase reporter assay showed that co-transfection with miR-761 mimics and the HOTAIR-wt vector lowered the luciferase activity significantly, whereas co-transfection with miR-761 mimics and the HOTAIR-mut vector did not affect the luciferase activity (Figure 4B). The RIP assay revealed higher specific adsorption of HOTAIR on Ago2 (Figure 4C). The RNA pull-down assay indicated that HOTAIR enrichment was increased in the Bio-miR-761-wt group than that in Bio-miR-761-mut group (Figure 4D).
Then TargetScanHuman database (www.targetscan.org/vert_71/) was used to predict the target genes of miR-761. HDAC1 was targeted by miR-761 because HDAC1 had potential complementary binding sites for miR-761 within its 3′-UTR (Figure 4E). To validate HDAC1 as a direct target of miR-761, we used luciferase reporter plasmids of HDAC1-wt and HDAC1-mut. We discovered that miR-761 overexpression decreased the luciferase activity of the HDAC1-wt 3ʹUTR significantly, but not that of the HDAC1-mut in ESCs (Figure 4F). We also revealed that HOTAIR overexpression led to higher protein expression of HDAC1, and that miR-610 mimics led to a reduction in the protein expression of HDAC1 (Figure 4G).
Exosomal HOTAIR Promotes the Proliferation, Migration, and Invasion of ESCs and Angiogenesis of HUVECs, and Inhibits ESCs Apoptosis Through Sponging miR-761
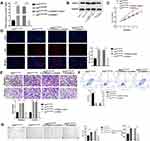
We wished to further investigate the role of the exosomal HOTAIR/miR-761/HDAC1 axis in endometriosis. Hence, ESCs were transfected with exoOe-control or exoOe-HOTAIR, followed by co-culture with miR-761 mimics or mimics NC. miR-761 mimics significantly reversed the exosomal HOTAIR-mediated decrease in miR-761 expression (Figure 5A). miR-761 mimics significantly reversed the exosomal HOTAIR-mediated proliferation, migration, and invasion induction of ESCs (Figure 5B–D), and inhibition of ESCs apoptosis (Figure 5E). Additionally, the exosomal HOTAIR-mediated tubes formation by HUVECs was also reversed by miR-761 mimics (Figure 5F).Collectively, these results indicated that miR-761 overexpression reversed the role of exosomal HOTAIR on ESCs and HUVECs.
Exosomal HOTAIR Promotes the Proliferation, Migration and Invasion of ESCs and Angiogenesis of HUVECs, and Inhibits ESCs Apoptosis Through Upregulating HDAC1
Next, ESCs were co-cultured with exoOe-control or exoOe-HOTAIR followed by the transfection with si-HDAC1 or si-HDAC1-control. Knockdown of HDAC1 expression significantly reversed the HOTAIR-mediated increase in mRNA and protein expression of HDAC1 (Figure 6A and B). si-HDAC1 significantly reversed the exosomal HOTAIR-mediated proliferation, migration, and invasion induction of ESCs (Figure 6C–E), and inhibition of ESCs apoptosis (Figure 6F). Additionally, the exosomal HOTAIR-mediated tubes formation by HUVECs was also reversed by si-HDAC1 (Figure 6G).Taken together, these results indicated that knockdown of HDAC1 expression reversed the role of exosomal HOTAIR.
The HOTAIR/miR-761/HDAC1 Axis Activates STAT3-Mediated Inflammation
Considering the interaction between HDAC1 and STAT3,23 several experiments were undertaken to explore the underlying signaling pathway which mediated the effects of the HOTAIR/miR-761/HDAC1 axis. Western blotting showed that the protein expression of p-STAT3 was decreased in the group with knockdown of HDAC1 expression (Figure 7A). Exosomal HOTAIR could enhance the protein expression of p-STAT3, whereas overexpression of miR-761 could reverse these effects (Figure 7B). The viability, migration, and invasion of ESCs were decreased after treatment with stattic (inhibitor of p-STAT3), and stattic could reverse the promoting effects of exosomal HOTAIR on the viability, migration, and invasion induction of ESCs (Figure 7C and D). Moreover, the tube formation of HUVECs was obviously restrained by stattic (Figure 7E).
ELISA was used to measure expression of STAT3-related proinflammatory cytokines, including IL-1β, IL-6, IL-8, and TNF-α. The levels of IL-1β, IL-6, IL-8, and TNF-α were enhanced markedly by upregulation of exosomal HOTAIR expression, but were rescued by miR-761 mimics, si-HDAC1, or stattic (Figure 7F).
Exosomal HOTAIR Promotes the Growth of Endometrial Lesions in vivo
To investigate if exosomal HOTAIR was crucial for endometriosis progression in vivo, we established an endometriosis model in mice. Once mice had been killed after the injection of different types of exosomes, we observed a significant reduction of the ectopic endometrial cysts in the exosi-HOTAIR group and a significant increment of the ectopic endometrial cysts in the exoOe-HOTAIR group (Figure 8A). The volume and weight of ectopic endometrial cysts was increased significantly in the exoOe-HOTAIR group compared with that in the exoOe-control group and blank group (Figure 8B and C). To ascertain if these effects were due to the abnormal expression of HOTAIR, miR-761, and HDAC1, we undertook RT-qPCR and Western blotting. Expression of HOTAIR and HDAC1 mRNA was enhanced in the exoOe-HOTAIR group and decreased in the exosi-HOTAIR group, whereas the change in miR-761 expression revealed the reverse phenomenon (Figure 8D). Protein expression of HDAC1 and p-STAT3 was enhanced in the exoOe-HOTAIR group and decreased in the exosi-HOTAIR group (Figure 8E). Levels of IL-1β, IL-6, IL-8, and TNF-α in endometrial lesions were increased in the exoOe-HOTAIR group and decreased in the exosi-HOTAIR group (Figure 8F). Therefore, exosomal HOTAIR exerted a stimulating effect in a mouse model of endometriosis via the miR-761/HDAC1/STAT3 axis.
Discussion
Recently, numerous studies have demonstrated that lncRNA dysregulation has crucial functions in various diseases.24 lncRNAs have been shown to have important roles in the development and progression of endometriosis.25 We found that HOTAIR was overexpressed in the ectopic endometrium and promoted the proliferation, migration, and invasion of ESCs, and the angiogenesis of HUVECs by targeting the miR-761/HDAC1 axis and activating STAT3-mediated inflammation via an exosomal pathway. We revealed that an exosomal HOTAIR/miR-761/HDAC1/STAT3 regulatory network participated in endometriosis development. Our data provide a new molecular target for endometriosis treatment.
HOTAIR is a lncRNA that has been reported to show abnormal expression in several diseases, and to be implicated in the development of those diseases.26,27 Wang et al reported that HOTAIR can promote osteosarcoma development by sponging miR-217 and regulating ZEB1 expression.28 HOTAIR targets miR-217 to promote progression of gastric cancer by upregulating expression of GPC5.29 Chang et al found that HOTAIR had a prominent role in endometriosis.30 Similar to those findings, we also found that HOTAIR expression was increased significantly in the ectopic endometrium, which may promote endometriosis progression.
Exosomes can protect lncRNAs from degradation,31 and serve as a bridge of bioinformation transmission between different cells.32 Yuan et al demonstrated that laryngeal cancer cells can transport HOTAIR to surrounding cells, thereby negatively regulating the radiosensitivity of target cells.33 Similarly, we found that ESCs transmitted HOTAIR to surrounding cells through exosomes, and that exosomal HOTAIR could promote the growth, migration, and invasion and the angiogenesis of HUVECs, and inhibit the apoptosis of ESCs.
Several studies have reported a lncRNA-miRNA-mRNA regulatory network.34 lncRNAs can act as a ceRNA to sponge miRNAs in this network. To further investigate the underlying molecular mechanism by which exosomal HOTAIR regulates endometriosis, we used a bioinformatics tool to predict the potential target of HOTAIR and found miR-761 to be a potential candidate. Dual-luciferase reporter assays identified miR-761 as a downstream target of HOTAIR. Shi and collaborators demonstrated that miR-761 inhibited the proliferation and invasion of the ovarian cancer cells by targeting MSI1.35 Cho et al reported that miR-761 can inhibit the proliferation and migration of angiotensin II–induced vascular smooth muscle cells by targeting mTOR.36 In this study, we found that miR-761 overexpression reversed the promoting effect of exoOe-HOTAIR on the proliferation, migration, and invasion of ESCs and the angiogenesis of HUVECs, thereby indicating miR-761 overexpression could inhibit endometriosis progression.
We also explored the downstream mechanism of action of miR-761. Bioinformatics analysis showed that miR-761 interacted with the 3’-UTR of HDAC1, which was verified via the dual-luciferase reporter assay. HDAC1 regulates the acetylation status of histones.37 miR-34a inhibits the proliferation and chemoresistance of ovarian cancer cells by downregulating HDAC1 expression.38 The proliferation and invasion of colorectal cancer cells can be restrained by miR-761 through targeting HDAC1.39 In a previous report, it has been shown that HDAC1 expression was increased significantly in endometriosis.40 We demonstrated that knockdown of HDAC1 expression significantly reversed the exoOe-HOTAIR-mediated proliferation, migration, and invasion induction, as well as apoptosis inhibition of ESCs. Hence, HDAC1 serves as a promoting factor in endometriosis.
STAT3 has been found to mediate the biological action of HDAC1.41 STAT3 signaling is activated in endometriosis, which may be a viable method for endometriosis treatment.42 Okamoto et al demonstrated that miR-210 promoted endometriosis pathogenesis by activating STAT3.43 We found that HOTAIR and HDAC1 enhanced the protein expression of p-STAT3, and that inhibition of p-STAT3 expression could reverse the effects of HOTAIR on ESCs. The HOTAIR/miR-761/HDAC1 axis also enhanced the production of IL-1β, IL-6, IL-8, and TNF-α in ESCs by activating STAT3, which also verified the role of STAT3-mediated inflammation in endometriosis. In vivo experiments also showed that exoOe-HOTAIR promoted the growth of endometrial lesions via the miR-761/HDAC1 axis and activation of STAT3-mediated inflammation, which provides a new avenue for endometriosis treatment.
Conclusion
In summary, our studies showed that exosomal HOTAIR served as a molecular sponge of miR-761 to promote the progression and angiogenesis of HUVECs endometriosis by regulating HDAC1 and activating STAT3-mediated inflammation. These findings provide a novel mechanism for endometriosis development and a new therapeutic target for this disease.
Acknowledgments
We thank the patients for their participation. This research was supported by the National Nature Science Foundation of China (82101724), and the Natural Science Foundation of Shandong Province, China (ZR2020MH243 and ZR2020QH043).
Disclosure
The authors report no conflicts of interest for this work.
References
1. Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789–1799. doi:10.1016/S0140-6736(04)17403-5
2. Giudice LC. Clinical practice endometriosis. N Engl J Med. 2010;362(25):2389–2398. doi:10.1056/NEJMcp1000274
3. Vercellini P, Vigano P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10(5):261–275. doi:10.1038/nrendo.2013.255
4. Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10(3):155–159. doi:10.1038/nrg2521
5. Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43(6):904–914. doi:10.1016/j.molcel.2011.08.018
6. Liu SJ, Dang HX, Lim DA, Feng FY, Maher CA. Long noncoding RNAs in cancer metastasis. Nat Rev Cancer. 2021;21(7):446–460. doi:10.1038/s41568-021-00353-1
7. Mirzadeh Azad F, Polignano IL, Proserpio V, Oliviero S. Long noncoding RNAs in human stemness and differentiation. Trends Cell Biol. 2021;31(7):542–555. doi:10.1016/j.tcb.2021.02.002
8. Shang P, Chen G, Zu G, et al. Long noncoding RNA expression analysis reveals the regulatory effects of nitinol-based nanotubular coatings on human coronary artery endothelial cells. Int J Nanomedicine. 2019;14:3297–3309. doi:10.2147/IJN.S204067
9. Liu S, Qiu J, Tang X, Cui H, Zhang Q, Yang Q. LncRNA-H19 regulates cell proliferation and invasion of ectopic endometrium by targeting ITGB3 via modulating miR-124-3p. Exp Cell Res. 2019;381(2):215–222. doi:10.1016/j.yexcr.2019.05.010
10. Sha L, Huang L, Luo X, et al. Long non-coding RNA LINC00261 inhibits cell growth and migration in endometriosis. J Obstet Gynaecol Res. 2017;43(10):1563–1569. doi:10.1111/jog.13427
11. Yang T, He X, Chen A, Tan K, Du X. LncRNA HOTAIR contributes to the malignancy of hepatocellular carcinoma by enhancing epithelial-mesenchymal transition via sponging miR-23b-3p from ZEB1. Gene. 2018;670:114–122. doi:10.1016/j.gene.2018.05.061
12. Liu XH, Sun M, Nie FQ, et al. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer. 2014;13:92. doi:10.1186/1476-4598-13-92
13. Ranjbaran A, Latifi Z, Nejabati HR, et al. Exosome-based intercellular communication in female reproductive microenvironments. J Cell Physiol. 2019;234(11):19212–19222. doi:10.1002/jcp.28668
14. Zhang L, Li HH, Yuan M, Li D, Wang GY. Exosomal miR-22-3p derived from peritoneal macrophages enhances proliferation, migration, and invasion of ectopic endometrial stromal cells through regulation of the SIRT1/NF-kappaB signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24(2):571–580. doi:10.26355/eurrev_202001_20033
15. Zhang L, Li H, Yuan M, Li D, Sun C, Wang G. Serum exosomal microRNAs as potential circulating biomarkers for endometriosis. Dis Markers. 2020;2020:2456340. doi:10.1155/2020/2456340
16. Liu T, Liu M, Zheng C, Zhang D, Li M, Zhang L. Exosomal lncRNA CHL1-AS1 derived from peritoneal macrophages promotes the progression of endometriosis via the miR-610/MDM2 axis. Int J Nanomedicine. 2021;16:5451–5464. doi:10.2147/IJN.S323671
17. Zhao W, Liu Y, Zhang C, Duan C. Multiple roles of exosomal long noncoding RNAs in cancers. Biomed Res Int. 2019;2019:1460572. doi:10.1155/2019/1460572
18. Wang Y, Zhang M, Zhou F. Biological functions and clinical applications of exosomal long non-coding RNAs in cancer. J Cell Mol Med. 2020;24(20):11656–11666. doi:10.1111/jcmm.15873
19. Chai Y, Wu HT, Liang CD, You CY, Xie MX, Xiao SW. Exosomal lncRNA ROR1-AS1 derived from tumor cells promotes glioma progression via regulating miR-4686. Int J Nanomedicine. 2020;15:8863–8872. doi:10.2147/IJN.S271795
20. Cui X, Xiao D, Cui Y, Wang X. Exosomes-derived long non-coding RNA HOTAIR reduces laryngeal cancer radiosensitivity by regulating microRNA-454-3p/E2F2 axis. Onco Targets Ther. 2019;12:10827–10839. doi:10.2147/OTT.S224881
21. Zhang C, Xu L, Deng G, et al. Exosomal HOTAIR promotes proliferation, migration and invasion of lung cancer by sponging miR-203. Sci China Life Sci. 2020;63(8):1265–1268. doi:10.1007/s11427-019-1579-x
22. Yuan M, Li D, An M, Li Q, Zhang L, Wang G. Rediscovering peritoneal macrophages in a murine endometriosis model. Hum Reprod. 2017;32(1):94–102. doi:10.1093/humrep/dew274
23. Du W, Wang N, Li F, et al. STAT3 phosphorylation mediates high glucose-impaired cell autophagy in an HDAC1-dependent and -independent manner in Schwann cells of diabetic peripheral neuropathy. FASEB J. 2019;33(7):8008–8021. doi:10.1096/fj.201900127R
24. Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152(6):1298–1307. doi:10.1016/j.cell.2013.02.012
25. Zhang C, Wu W, Zhu H, et al. Knockdown of long noncoding RNA CCDC144NL-AS1 attenuates migration and invasion phenotypes in endometrial stromal cells from endometriosisdagger. Biol Reprod. 2019;100(4):939–949. doi:10.1093/biolre/ioy252
26. Mozdarani H, Ezzatizadeh V, Rahbar Parvaneh R. The emerging role of the long non-coding RNA HOTAIR in breast cancer development and treatment. J Transl Med. 2020;18(1):152. doi:10.1186/s12967-020-02320-0
27. Yoon JH, Abdelmohsen K, Kim J, et al. Scaffold function of long non-coding RNA HOTAIR in protein ubiquitination. Nat Commun. 2013;4:2939. doi:10.1038/ncomms3939
28. Wang B, Qu XL, Liu J, Lu J, Zhou ZY. HOTAIR promotes osteosarcoma development by sponging miR-217 and targeting ZEB1. J Cell Physiol. 2019;234(5):6173–6181. doi:10.1002/jcp.27394
29. Dong X, He X, Guan A, et al. Long non-coding RNA Hotair promotes gastric cancer progression via miR-217-GPC5 axis. Life Sci. 2019;217:271–282. doi:10.1016/j.lfs.2018.12.024
30. Chang CY, Tseng CC, Lai MT, et al. Genetic impacts on thermostability of onco-lncRNA HOTAIR during the development and progression of endometriosis. PLoS One. 2021;16(3):e0248168. doi:10.1371/journal.pone.0248168
31. Wang M, Zhou L, Yu F, Zhang Y, Li P, Wang K. The functional roles of exosomal long non-coding RNAs in cancer. Cell Mol Life Sci. 2019;76(11):2059–2076. doi:10.1007/s00018-019-03018-3
32. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478). doi:10.1126/science.aau6977
33. Yuan Z, Yang Z, Li W, Wu A, Su Z, Jiang B. Exosome-mediated transfer of long noncoding RNA HOTAIR regulates temozolomide resistance by miR-519a-3p/RRM1 axis in glioblastoma. Cancer Biother Radiopharm. 2020. doi:10.1089/cbr.2019.3499
34. Huang Y. The novel regulatory role of lncRNA-miRNA-mRNA axis in cardiovascular diseases. J Cell Mol Med. 2018;22(12):5768–5775. doi:10.1111/jcmm.13866
35. Shi C, Zhang Z. miR-761 inhibits tumor progression by targeting MSI1 in ovarian carcinoma. Tumour Biol. 2016;37(4):5437–5443. doi:10.1007/s13277-015-4377-z
36. Cho JR, Lee CY, Lee J, et al. MicroRNA-761 inhibits Angiotensin II-induced vascular smooth muscle cell proliferation and migration by targeting mammalian target of rapamycin. Clin Hemorheol Microcirc. 2015;63(1):45–56. doi:10.3233/CH-151981
37. de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370(Pt 3):737–749. doi:10.1042/bj20021321
38. Lv T, Song K, Zhang L, et al. miRNA-34a decreases ovarian cancer cell proliferation and chemoresistance by targeting HDAC1. Biochem Cell Biol. 2018;96(5):663–671. doi:10.1139/bcb-2018-0031
39. Xiong W, Yang S, Zhang W, Chen Y, Wang F. MiR-761 inhibits colorectal cancer cell proliferation and invasion through targeting HDAC1. Pharmazie. 2019;74(2):111–114. doi:10.1691/ph.2019.8756
40. Samartzis EP, Noske A, Samartzis N, Fink D, Imesch P. The expression of histone deacetylase 1, but not other class I histone deacetylases, is significantly increased in endometriosis. Reprod Sci. 2013;20(12):1416–1422. doi:10.1177/1933719113488450
41. Pang M, Ma L, Liu N, et al. Histone deacetylase 1/2 mediates proliferation of renal interstitial fibroblasts and expression of cell cycle proteins. J Cell Biochem. 2011;112(8):2138–2148. doi:10.1002/jcb.23135
42. Kotlyar AM, Mamillapalli R, Flores VA, Taylor HS. Tofacitinib alters STAT3 signaling and leads to endometriosis lesion regression. Mol Hum Reprod. 2021;27(4). doi:10.1093/molehr/gaab016
43. Okamoto M, Nasu K, Abe W, et al. Enhanced miR-210 expression promotes the pathogenesis of endometriosis through activation of signal transducer and activator of transcription 3. Hum Reprod. 2015;30(3):632–641. doi:10.1093/humrep/deu332
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.