Back to Journals » Journal of Asthma and Allergy » Volume 13
Exhaled Nitric Oxide in Wheezy Infants Predicts Persistent Atopic Asthma and Exacerbations at School Age
Authors White MP, Kolstad TK , Elliott M, Cochrane ES , Stamey DC , Debley JS
Received 18 August 2019
Accepted for publication 21 November 2019
Published 7 January 2020 Volume 2020:13 Pages 11—22
DOI https://doi.org/10.2147/JAA.S227732
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amrita Dosanjh
Maria P White,1 Tessa K Kolstad,1 Molly Elliott,2 Elizabeth S Cochrane,2 David C Stamey,2 Jason S Debley1,2
1Center for Immunity and Immunotherapies, Seattle Children’s Research Institute, Seattle, WA, USA; 2Department of Pediatrics, Division of Pulmonary and Sleep Medicine, Seattle Children’s Hospital, University of Washington, Seattle, WA, USA
Correspondence: Jason S Debley
Department of Pediatrics, Division of Pulmonary and Sleep Medicine, Seattle Children’s Hospital, University of Washington, 4800 Sand Point Way NE, Seattle, WA 98105, USA
Tel +1 206-987-2174
Fax +1 206-987-2639
Email [email protected]
Background: There are limited data assessing the predictive value of fraction of exhaled nitric oxide (FENO) in infants/toddlers with recurrent wheezing for asthma at school age.
Objectives: In a cohort of infants/toddlers with recurrent wheezing determine the predictive values of sedated single-breath FENO (SB-FENO) and awake tidal-breathing mixed-expired FENO (tidal-FENO) for active asthma, severe exacerbations, and lung function at age 6 years.
Methods: In 44 infants/toddlers, SB-FENO was measured under sedation at 50 mL/sec in conjunction with forced expiratory flow and volume measurements, and tidal-FENO was measured during awake tidal breathing. Clinical outcomes and lung function were assessed at age 6 years in 36 subjects.
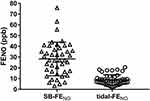
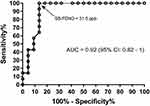
Results: Enrollment SB-FENO was significantly higher among subjects with active asthma at age 6 years than among subjects without asthma (36.4 vs. 16.9 ppb, p < 0.0001), and the odds of asthma was 7.6 times greater (OR 7.6; 95% CI 1.8–31.6) for every 10 ppb increase in enrollment SB-FENO. A ROC analysis demonstrated that an enrollment SB-FENO > 31.5 ppb predicted active asthma at age 6 years with an area under the curve (AUC) of 0.92 (95% CI: 0.82–1). SB-FENO was also higher among subjects who experienced severe asthma exacerbations during the year preceding age of 6 years. SB-FENO at enrollment and lung function measures at age 6 years were modestly correlated (FEV1: r = −0.4; FEF25-75: r = −0.41; FEV1/FVC ratio: r=−0.46), and SB-FENO was significantly higher among subjects with bronchodilator responsiveness (BDR) at age 6 years. Tidal-FENO was not predictive of active asthma, exacerbations, or lung function at age 6 years.
Conclusion: In wheezy infants/toddlers, SB-FENO was predictive of school-age asthma and associated with lung function measures at age 6 years.
Keywords: exhaled nitric oxide, FENO, recurrent wheezing, infants, asthma, pulmonary function, exacerbations
Plain Language Summary
The fraction of exhaled nitric oxide (FENO) is an adjunctive test for atopic asthma in school-age children, and its use to monitor eosinophilic airway inflammation and tailor therapy may decrease the frequency of asthma exacerbations in children. The aim of this study was to determine the predictive value of FENO among infants and toddlers with recurrent wheezing, using both a flow-controlled method under sedation and an awake tidal-breathing method, for the development of school-age asthma and exacerbations and lung function at age 6 years. We found that among wheezy infants and toddlers FENO measured using a flow-controlled technique was predictive of school-age asthma and associated with lung function measures at age 6 years. These results will inform future efforts to develop feasible point-of-care FENO testing methods in wheezy infants and toddlers at risk for asthma.
Introduction
Approximately 50% of school-age children with persistent asthma developed their first symptoms, typically recurrent episodes of wheezing, between birth and age 3 years.1 Recurrent wheezing affects up to a third of infants and toddlers, yet resolves in at least half of these children by school age. Clinicians generally cannot confidently differentiate preschool-age children with transient wheezing from those children with persistent asthma, and an accurate clinical test to predict asthma among infants and toddlers does not currently exist. Current national and international clinical guidelines recommend initiating daily use of inhaled corticosteroids (ICS) for children under age 5 years with >3 prior episodes of wheezing and epidemiologic risk factors for asthma.2,3 These guidelines are based primarily on the Asthma Predictive Index (API) proposed by Castro-Rodriquez et al4 which is limited by the fact that when validated in a large independent population-based cohort5 the API was found to have a high negative predictive value yet a sensitivity ranging from 28% to 37% and positive predictive value of only 40–48% for asthma at school age.
Clinicians and parents are often reluctant to initiate infants/toddlers on inhaled steroid therapy unless confident about the diagnosis of asthma, a concern heightened by some trials suggesting that treatment of viral-induced wheezing with oral or inhaled steroids in preschool children is ineffective6–8 and that treatment with inhaled steroids may be associated with reduced growth velocity in children.9–11 Therefore, a biomarker able to predict atopic asthma among preschool-age children with recurrent wheezing or other chronic respiratory symptoms would be useful to clinicians as well as investigators testing potential asthma early intervention therapies in infants and toddlers. The fractional concentration of exhaled nitric oxide (FENO) is a biomarker for which an extensive body of clinical data support its use in adults and school-age children to identify eosinophilic airway inflammation, support the diagnosis of allergic asthma, and to determine the likelihood of steroid responsiveness in patients with chronic respiratory symptoms.12,13 However, there is limited prospective data assessing FENO prospectively among wheezy infants/toddlers who were followed into their school-age years to determine associations with persistent asthma,14–18 and to our knowledge, there are no prospective data assessing associations between FENO measured in wheezy infants and toddlers with asthma exacerbations or lung function at school age among those children. Because the majority of infants and preschool children with recurrent wheezing who go on to develop persistent childhood asthma can be characterized as having an atopic phenotype of asthma,19 FENO may be well suited as a biomarker to separate infants/toddlers with evolving atopic asthma from those with wheezing due to other etiologies.
In a prospective study Singer et al reported that offline FENO collected during tidal breathing among 166 preschool-age children (median age 36 months, interquartile range 24–42 months) was associated with an increased risk for asthma at school age; however, there was considerable overlap between enrollment FENO levels among children with and without asthma at school age, and the association was no stronger than the association between the API at enrollment and asthma at school age in this cohort.20 Unsedated tidal-breathing FENO measurements can be technically challenging to measure in infants/toddlers.21 Additional limitations of tidal breathing-FENO in infants include: mixing of nasal and lower airway NO sources, and variable expiratory flow which is problematic due to the high flow-dependent nature of FENO.22,23 Despite these potential limitations, it is easier to measure FENO in infants/toddlers during tidal breathing than to measure FENO using a flow-regulated exhalation method.
We have previously reported that single-breath flow-regulated FENO (SB-FENO) in a cohort of infants and toddlers (mean enrollment age 15.6 months) with a history of recurrent episodes of physician-diagnosed and treated wheezing measured at enrollment was associated with the persistence of wheezing at age 3 years.24 We now report follow-up of this cohort through age 6 years. In a cohort of infants and toddlers enrolled at < age 2 years with recurrent wheezing, our aims were to assess associations between FENO (both SB-FENO measured during sedated infant pulmonary function testing and mixed expired exhaled nitric oxide concentration measured during awake tidal breathing) measured at enrollment and: 1) active persistent asthma at age 6 years, 2) severe asthma exacerbations during the year prior to age 6 years, and 3) lung function measured at age 6 years. We hypothesized that enrollment FENO would be associated with persistent asthma, asthma exacerbations, and/or lung function age at 6 years. Some of these results have been reported in abstract form.25
Methods
Subjects
Children 6–24 months of age with ≥3 episodes of physician-diagnosed wheezing treated with bronchodilators or corticosteroids were recruited for a single-center longitudinal study. Subjects with birth <36 weeks gestation, congenital heart disease, dysphagia, severe gastroesophageal reflux, or upper airway obstruction were excluded. Systemic or inhaled corticosteroid treatment was not permitted for at least 3 weeks prior to baseline lung function and FENO measurement. Thereafter, corticosteroid treatment was at the discretion of the subject’s primary care or emergency department provider who did not have access to FENO data. Written informed consent was obtained from parents of subjects. The study was conducted in accordance with the Declaration of Helsinki and approved by the Seattle Children’s Hospital Institutional Review Board.
Study Visits
Study visits occurred a minimum of 3 weeks following resolution of an upper or lower respiratory infection, or acute exacerbation of wheezing. At enrollment medical history was reviewed, length and weight were measured with a calibrated stadiometer and digital scale, SB-FENO and tidal-FENO were measured, and infant lung function testing performed. We have previously reported study outcomes for this cohort between enrollment and age 3 years.24 Between age 3 and 6 years of age, parents completed online interval medical history and medication use surveys electronically every 6 months. A follow-up study visit that included review of interval medical history, medication use and completion of lung function tests was completed at age 6 years.
Lung Function Measurements
As previously reported, at enrollment, 6 months post-enrollment, and age 3 years forced expiratory volumes and flows were measured using the RVRTC technique according to ATS/ERS guidelines for raised volume forced expirations in infants26 via the InSpire® Infant Pulmonary Lab. Lung function tests were performed at least 3 weeks after the resolution of acute exacerbations of wheezing or respiratory illnesses. At age 6 years forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), and forced expiratory flow between 25% and 75% of FVC (FEF25-75) were measured according to ATS guidelines using a VMAX® series 2130 spirometer, and percent predicted parameters were assessed using reference equations by Hankinson.27 Among children with asthma, spirometry measurements were obtained when subjects were at their clinical baseline, and were repeated 15 mins following administration of 4 puffs of albuterol from a metered-dose inhaler via a spacer to determine if a bronchodilator response was present.
Exhaled Nitric Oxide Measurements
As we have previously described, at study enrollment while under sedation with chloral hydrate at least 3 flow-regulated SB-FENO measurements were performed in each subject immediately prior to infant lung function testing.28 Mixed expired FENO measurements during tidal breathing (tidal-FENO) were obtained prior to administration of chloral hydrate while subjects were awake and seated in parent’s lap watching a video for distraction. Mixed expired collections were obtained over 30 s of quiet regular breathing with a full facemask over the mouth and nose following 5 breaths to washout circuit dead space. Nitric oxide-free air (<2 ppb) was provided during inspiration via a filter attached to the collection circuit with a one-way valve. Nitric oxide was measured using a Sievers® NOA 280 chemiluminescence analyzer (GE Analytical Instruments; Boulder, CO, USA).
At age 6 years, FENO was measured according to American Thoracic Society (ATS) guidelines using a NIOX MINO nitric oxide analyzer (Aerocrine®, Sweden).12
RAST Allergy Testing
At age 6 years, a blood sample was drawn from each subject and used to measure total serum IgE and allergen-specific IgE to dust mites (Dermatophagoides farinae and Dermatophagoides pteronyssinus), cat epithelium, dog epithelium, Alternaria alternata, Aspergillus fumigatus, and timothy grass.
Statistical Analysis
Forty subjects were estimated to provide 80% power to identify SB-FENO as a significant predictor of FEV0.5 if SB-FENO accounted for ≥15% of the total variance in FEV0.5 (i.e. r2=0.15). SB-FENO and lung function data were determined to be normally distributed by the D’Agostino and Pearson as well as Kolmogorov–Smirnov tests. As appropriate based on the normality of data distributions, summary statistics, Pearson’s or Spearman correlation coefficients and unpaired t-tests or the Mann Whitney test were used in data analyses.
Multivariate logistic regression was used to assess associations between enrollment FENO measures (SB-FENO and tidal-FENO) and: 1) acute severe asthma exacerbations (defined as ≥1 episode of respiratory distress lasting ≥3 days treated with both albuterol and systemic corticosteroids) during the year prior to age 6-year follow-up visit, and 2) active persistent asthma at age 6 years (meeting NHLBI criteria for asthma diagnosis and ≥ mild persistent asthma severity, as well as a history of ≥1 episode of an acute respiratory illness treated with albuterol for ≥3 days during the year prior to age 6 years). Enrollment age, gender, family history of asthma, eczema, sustained inhaled corticosteroid treatment, and environmental tobacco smoke exposure (parental report of an active smoker in the home both at the time of subject enrollment and follow-up at age 6 years) were included as covariates in the logistic regression models. Receiver operating characteristics (ROC) analyses were performed to determine the enrollment SB-FENO concentration that best predicted active asthma at age 6 years. Using the best cutoff point for SB-FENO, the sensitivity, specificity, positive predictive value, negative predictive value, and relative risk of enrollment SB-FENO as a predictor of active asthma were calculated.
Multivariate linear regression models were used to assess associations between enrollment SB-FENO and tidal FENO, baseline subject characteristics, and lung function at age 6 years. All reported p-values are two-sided. Data analyses were conducted using GraphPad® Prism 7.0 (La Jolla, CA, USA) and Intercooled Stata for Windows® version 10.1 (College Station, Texas, USA).
Results
Forty-eight infants and toddlers between ages 6 and 24 months of age with recurrent wheezing were recruited and enrolled in the study. At enrollment, technically acceptable SB-FENO measurements during sedated RVRTC lung function testing and tidal-FENO measurements while awake were obtained in 44 subjects, with a mean age at enrollment of 15.5 (SD ± 5.4) months. Baseline characteristics of the cohort are presented in Table 1. At age 6 years, 36 subjects completed clinical follow-up (82% of subjects who completed enrollment SB-FENO measurements), including measurement of lung function by spirometry.
 |
Table 1 Baseline Characteristics of Subjects Completing Enrollment FENO Measurement |
The mean FEV0.5 and FEF25–75 z-scores for the cohort at enrollment were significantly less than zero (a z-score equal to zero represents the mean value of published normative data).28,29 Enrollment of subjects was evenly distributed across seasons.28 As previously reported, 8 subjects used inhaled corticosteroids on a sustained basis between the enrollment and 6-month follow-up visits, and 9 of the 34 subjects who completed lung function testing at age 3 years used inhaled steroids on a sustained basis during the 6 months prior to the visit at age 3 years. At 6 years of age, 6 subjects (14%) reported using inhaled steroids on a daily basis, and 3 subjects (7%) reported using inhaled steroids intermittently during the preceding year. We previously reported that SB-FENO measured at enrollment was associated with both age and a history of eczema, but was not associated with gender, family history of asthma, environmental tobacco smoke exposure, wheezing apart from viral respiratory infections, enrollment lung function measures, hospitalization for wheezing, or oral steroid treatment prior to enrollment.28 At enrollment, the median SB-FENO for the cohort was 27.1 ppb (Figure 1; IQR 16.4–38.1) and median awake tidal-FENO was 7.9 ppb (IQR 5.7–11.3), and the two measurements of FENO were weakly correlated.28
At age 6 years, 14 subjects met our definition for active asthma. Enrollment SB-FENO concentrations were significantly higher among subjects with active asthma at age 6 years than among those subjects without asthma (median 36.4 ppb, IQR 32.4–42.2 vs. 16.9 ppb, IQR 11.3–26.8, p < 0.0001; Figure 2A). The odds of active asthma at age 6 years were 7.6 times greater (OR 7.6; 95% CI 1.8–31.6) for every 10 ppb increase in enrollment SB-FENO (Table 2). Ten subjects (28%) experienced an acute severe asthma exacerbation in the year preceding their age 6-year follow-up visit. Enrollment SB-FENO concentrations were also significantly higher among subjects who experienced severe asthma exacerbations during the year preceding age 6 years than among those subjects who did not (median 34.7 ppb, IQR 32.1–46.4 vs. 21.0 ppb, IQR 12.2–35.1; p = 0.003; Figure 2B). The odds of an asthma exacerbation were 2.5 times higher (OR 2.5; 95% CI 1.2–5) for every 10 ppb increase in enrollment SB-FENO (Table 2). Enrollment tidal-FENO concentrations were not significantly different between children with and without active asthma at age 6 years (mean 10.5 ppb, SD 4.8 vs. 7.9 ppb, SD 4.9, p = 0.1; Figure 2C), or between those who experienced severe asthma exacerbations during the year preceding age 6 years and those that did not (mean 9 ppb, SD 3.8 vs. 8.3 ppb, SD 5.3, p = 0.7; Figure 2D).
 |
Table 2 Associations Between Enrollment SB-FENO and Active Asthma and Severe Exacerbations at Age 6 Years |
An ROC analysis demonstrated that an enrollment SB-FENO concentration ≥31.5 ppb in this cohort of wheezy infants and toddlers predicted active asthma at age 6 years with an area under the curve (AUC) of 0.92 (95% CI: 0.82–1; Figure 3). An enrollment SB-FENO concentration ≥31.5 ppb predicted active asthma at age 6 years (n=14 subjects) with a sensitivity of 93%, a specificity of 86%, a positive predictive value of 81%, negative predictive value of 95% and a relative risk of 16.3 (95% CI: 3.3–92.4). (Table 3).There was a modest correlation between SB-FENO concentration at subject enrollment and lung function measures at age 6 years (FEV1: r = −0.4, p = 0.02; FEF25–75: r = −0.41, p = 0.02; FEV1/FVC ratio: r=−0.46, p = 0.003; Figure 4). Multivariable linear regression models to assess associations between enrollment SB-FENO and lung function at age 6 years were constructed following univariate analysis of associations between SB-FENO and gender, eczema at enrollment, environmental tobacco smoke exposure, sustained use of inhaled corticosteroids between ages 3–6 years, and family history of asthma. Covariates associated with SB-FENO with a p<0.25 were included in the final multivariable linear regression models (enrollment SB-FENO, gender, eczema, and tobacco smoke exposure). Each 10 ppb increase in enrollment SB-FENO was associated with a 2.9% reduction in FEV1% predicted, 5.6% reduction in FEF25–75, and 0.02 decrease in FEV1/FVC ratio (Table 4). Tidal-FENO measured at study enrollment was not correlated with lung function measured at age 6 years (FEV0.5: r = 0.07, p = 0.5; FEF25–75: r = −0.05, p = 0.8; FEV1/FVC ratio: r=−0.01, p=0.9).
 |
Table 3 Sensitivity, Specificity, PPV, NPV, and RR of Enrollment SB-FENO, for Active Asthma at Age 6 Years |
 |
Table 4 Associations Between Enrollment SB-FENO and Lung Function at Age 6 Years |
During spirometry performed at age 6 years, bronchodilator responsiveness (BDR) was present in 18 of 36 subjects. The SB-FENO concentration measured in subjects at study enrollment was significantly higher among subjects found to have BDR at age 6 years than among those without BDR (median 35.1 ppb, IQR 22.3–42.2 vs. 24.5 ppb, IQR 12.2–32.1; p = 0.02; Figure 5A). In contrast, enrollment tidal-FENO did not differ between subjects with and without BDR at age 6 years (median 8.5, IQR 6.2–10.8 ppb vs. 6.7 ppb, IQR 4.2 −12.6; p = 0.6; Figure 5B).
Neither SB-FENO or tidal-FENO concentrations at study enrollment were significantly different between subjects with one or more positive RAST IgE tests to a common aeroallergen at age 6 years and subjects with no positive RAST IgE tests (SB-FENO: median 32.5 vs. 24.5 ppb, p = 0.08, Figure 6A; tidal FENO: median 8.8 vs. 6.6 ppb, p = 0.3, Figure 6B), or between subjects with or without physician-diagnosed eczema at age 6 years (SB-FENO: median 31.9 vs. 25.3 ppb, p = 0.2, Figure 6C; tidal FENO: median 8.6 vs. 6.9 ppb, p = 0.2, Figure 6D). There was also no correlation between SB-FENO or tidal-FENO concentrations at study enrollment and total serum IgE levels measured at age 6 years (SB-FENO: r = 0.27, p = 0.1, Figure 6E; tidal FENO: r = 0.18, p = 0.3, Figure 6F).
Discussion
In a prospective longitudinal study of infants and toddlers (mean age 15.5 months) with a history of recurrent episodes of physician-diagnosed wheezing, we found that flow-controlled SB-FENO measured at enrollment in conjunction with sedated infant pulmonary function testing was associated with active asthma at 6 years of age as well as a history of severe asthma exacerbations between ages 5 and 6 years. Furthermore, higher concentrations of SB-FENO measured in these infants and toddlers was associated with modestly lower lung function at age 6 years. Finally, subjects with BDR during spirometry at age 6 years had higher enrollment SB-FENO than subjects without BDR at age 6 years. In contrast to flow-controlled sedated SB-FENO measurements, awake tidal-FENO measurements at enrollment were not associated with active asthma, a history of severe exacerbations, lower lung function, or BDR at age 6 years.
Our SB-FENO results are generally consistent with the only other published longitudinal study that we are aware of that attempted to study the predictive power of flow-controlled FENO measurements in infants and toddlers for subsequent asthma during the school-age years. Chang et al reported that in their prospective cohort of infants with atopic dermatitis but without prior wheezing, SB-FENO measured at enrollment (mean age 10.7 months) was predictive of asthma as well as airway reactivity at age 5 years.30 However, we believe that our study is the first to perform SB-FENO measurements in a cohort of infants and toddlers with recurrent physician-diagnosed wheezing that were then prospectively followed into the school-age years to determine the predictive value of SB-FENO for school-age asthma, exacerbations, and lung function. Furthermore, we found that an elevated SB-FENO (concentration ≥31.5 ppb) in wheezy infants and toddlers predicted active asthma at age 6 years with a high degree of accuracy (AUC of 0.92, sensitivity of 93%, specificity of 86%, positive predictive value of 81%, negative predictive value of 95% and a relative risk of 16).
Although the prevalence of recurrent wheezing among infants/toddlers is high, this group of children is heterogeneous. Approximately two thirds of wheezy infants/toddlers will not go on to develop persistent school-age asthma, but rather they wheeze for a variety of anatomic and/or pathophysiologic reasons, including but not limited to small airway caliber, post-viral airway inflammation and injury, dysphagia, or tracheobronchomalacia.31 For most of these young children, episodic wheezing resolves between ages 3–5 years. However, discriminating between infants/toddlers with transient wheezing and young children with evolving asthma is a major challenge for clinicians.31 Prospectively identifying atopic asthma in infants and toddlers with recurrent wheeze using an objective test such as FENO could greatly facilitate more targeted use of asthma therapies, prevent under- and over-treatment with corticosteroids, and be potentially very useful in selecting young children that would be appropriate candidates for future early intervention therapeutic trials targeting children at high risk for atopic endotypes of asthma.
Although not directly related to our assessment of FENO in this cohort, we observed that parental report of environmental tobacco smoke exposure was not associated with active asthma at age 6 years; however, was associated with an increased risk of severe asthma exacerbation between ages 5 and 6 years (Table 2). Our observations are consistent with recent studies in the literature reporting a lack of association between early life tobacco smoke exposure and the development of asthma by school age,32 yet multiple studies over the past decade have found that environmental tobacco smoke exposure is associated with an increased risk of asthma exacerbations in children with existing asthma.33–35 A weakness of our study is that we did not collect objective markers of environmental tobacco smoke exposure (e.g. cotinine levels) at study enrollment or during follow-up. However, the subjects in whom parents reported tobacco smoke exposure at enrollment also reported tobacco smoke exposure at age 6 years. Our findings, in the context of published literature, support the concept that although early life environmental tobacco smoke does not appear to be a causal factor in the development of asthma, it is a risk factor for asthma exacerbations in children with asthma.
Our observed lack of an association between enrollment tidal-FENO measured while subjects were awake and active asthma or a history of severe exacerbations at age 6 years is consistent with several other studies of FENO measured during tidal breathing in young children with recurrent wheezing.36,37 There are several potential explanations for the lack of associations between tidal-FENO and asthma and lung function measures at age 6 years. First, given that during tidal breathing the soft palate is not closed and nasal NO concentrations are frequently at least 100-fold greater than lower airway NO concentrations there is significant contamination of sampled lower airway NO by nasal NO when measurements are performed during tidal breathing.38 Contamination of measurements by nasal NO therefore would therefore tend to obscure inter-individual differences in lower airway FENO and bias towards a lack of association between tidal-FENO and our clinical outcomes. Second, expiratory flow is often quite variable during tidal-breathing measurements conducted in awake young children, which is problematic due to the highly flow dependent nature of FENO measurements.22,23 In contrast, Singer et al demonstrated in a cohort of 391 pre-school age children that FENO measured during tidal breathing was modestly predictive of asthma at age 5 years (AUC of 0.62).20 Despite the limitations of the tidal breathing FENO method, they did demonstrate an association between preschool tidal-FENO and school-age asthma, likely due in part due to their larger sample size. However, the relatively weak predictive value of tidal-FENO in the Singer study limits the potential utility of tidal-FENO as a practical point-of-care clinical test in preschool-age children. Unfortunately, the lack of an association between tidal-FENO and asthma at school age dampens enthusiasm for FENO as practical biomarker that could be used clinically on a large scale in wheezy infants and toddlers given that SB-FENO measurements in this age range require that children undergo sedation using a drug that is increasingly difficult to obtain (chloral hydrate) and require highly specialized equipment as well as technical expertise available in a small number of centers worldwide. Although prior studies have demonstrated general associations between FENO and atopy,39 and some studies have reported that FENO is associated with atopy but not with asthma,40 a recent comprehensive comparative effectiveness review of the utility of FENO in asthma management that included 43 studies in adults and children found that although FENO is associated with atopy, FENO concentrations increased the odds of correctly diagnosing asthma between 5.9 and 17-fold, demonstrating that elevated FENO levels are also associated with asthma.13 We observed that enrollment FENO was not correlated with total IgE level or positive RAST specific IgE tests in subjects at age 6 years, or with a history of eczema at age 6 years; however, it is possible that with a sufficiently large sample size there might have been an association between FENO and other atopic features that our study was underpowered to detect. Although an association between FENO and atopy is well accepted,13,39 our data support the concept that FENO is not simply a marker of generalized atopy, but rather a marker of lower airway allergic inflammation that is associated with, and predictive of, atopic asthma in infants and toddlers with recurrent wheezing.
There are several additional limitations to this study. First, seven subjects were lost to follow-up between enrollment and age 6 years, thus we obtained clinical follow-up in 36 of the 44 subjects (82%) who completed enrollment FENO measurements. Second, although total IgE levels and RAST specific IgE to selected aeroallergens were measured at age 6 years, we did not perform skin or RAST testing of subjects to food or inhalant allergens at study enrollment. Kulig et al demonstrated in a birth cohort that the development of allergic sensitization to inhalant allergens occurs mostly beyond age 2 years,41 however, ideally we would have characterized the sensitization status of our subjects at both enrollment and age 6 years. Finally, although by study design none of the subjects were using inhaled corticosteroids at enrollment, nine subjects in our study were using inhaled steroids either on a sustained basis or intermittently at age 6 years. All nine subjects who reported sustained or intermittent ICS use at age 6 years were classified as having active asthma, however, ICS use could have decreased the incidence of asthma exacerbations between ages 5 and 6 years. This effect could have biased our results toward weaker associations between enrollment FENO and asthma exacerbations between ages 5 and 6 years or lung function at age 6 years.
Conclusions
We have demonstrated that higher concentrations of SB-FENO measured in infants and toddlers with a history of recurrent episodes of physician-diagnosed wheezing accurately predicted active asthma at age 6 years, a history of severe asthma exacerbations between ages 5 and 6 years, and lower lung function at age 6 years. Furthermore, enrollment SB-FENO was associated with the presence of BDR during lung function testing at age 6 years. In contrast, tidal-FENO in wheezy infants/toddlers were not associated with asthma, asthma exacerbations, or lung function measures at age 6 years. Unfortunately, SB-FENO measurements require sedation and complex equipment only available in a small number of centers worldwide. Therefore, SB-FENO is not a feasible point-of-care test to predict asthma in wheezy infants and toddlers. However, observations from this cohort strengthen our understanding of NO biology in early childhood asthma and the association of this biomarker with a common endotype of early childhood asthma. Hopefully, our findings will inspire current and future respiratory physiologists and bioengineers to develop new technologies that might allow for point-of-care testing methodologies feasible in awake young children that leverage the associations between airway NO biology and asthma risk to predict atopic asthma in wheezy infants and toddlers.
 |
Figure 6 Enrollment single-breath exhaled nitric oxide (SB-FENO) concentrations (A) and enrollment tidal-FENO concentrations (B) were not significantly different between subjects with ≥1 positive RAST IgE tests to a common aeroallergen (closed triangles) at age 6 years and subjects with no positive RAST IgE tests (open circles) (SB-FENO: median 32.5 vs. 24.5 ppb, p = 0.08; tidal FENO: median 8.8 vs. 6.6 ppb, p = 0.3). Analyses conducted using the Mann–Whitney test. Enrollment SB-FENO (C) and tidal-FENO (D) concentrations were not significantly different between subjects with (closed triangles) or without (open circles) physician-diagnosed eczema at age 6 years (SB-FENO: median 31.9 vs. 25.3 ppb, p = 0.2; tidal-FENO: median 8.6 vs. 6.9 ppb, p = 0.2). Analyses conducted using the Mann–Whitney test. There was no significant correlation between SB-FENO (E) or tidal-FENO (F) concentrations at study enrollment and total serum IgE levels measured at age 6 years (SB-FENO: r = 0.27, p = 0.1, Figure 6E; tidal FENO: r = 0.18, p = 0.3). Analyses conducted using the Spearman rank-order correlation coefficient. |
Abbreviations
FENO, fractional concentration of exhaled nitric oxide; SB-FENO, single-breath exhaled nitric oxide; Tidal-FENO, tidal-breathing mixed-expired; BDR, bronchodilator responsive; FVC, forced vital capacity; FEV0.5, forced expiratory volume in 0.5 s; FEF25–75, forced expiratory flow 25–75% of expiration; RVRTC, raised volume rapid thoracic compression; ROC, receiver operating characteristics; AUC, area under the curve; NHLBI, National Heart Lung Blood Institute; ATS, American Thoracic Society; ERS, European Respiratory Society.
Disclosure
All authors of this manuscript confirm that they have no known conflicts of interest associated with this manuscript and that they have not received financial support for this work that could have influenced its outcome.
References
1. Martinez FD. Development of wheezing disorders and asthma in preschool children. Pediatrics. 2002;109(2 Suppl):362–367.
2. NIH/NHLBI. National asthma education and prevention program Expert Panel Report 3 (EPR3): guidelines for the diagnosis and management of asthma. Available from: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm.
3. Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary (vol 31, pg 143, 2008). Eur Respir J. 2018;51(2):143.
4. Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1403–1406. doi:10.1164/ajrccm.162.4.9912111
5. Leonardi NA, Spycher BD, Strippoli MP, Frey U, Silverman M, Kuehni CE. Validation of the asthma predictive index and comparison with simpler clinical prediction rules. J Allergy Clin Immunol. 2011;127(6):1466–1472 e1466. doi:10.1016/j.jaci.2011.03.001
6. Panickar J, Lakhanpaul M, Lambert PC, et al. Oral prednisolone for preschool children with acute virus-induced wheezing. N Engl J Med. 2009;360(4):329–338. doi:10.1056/NEJMoa0804897
7. Clavenna A, Sequi M, Cartabia M, et al. Effectiveness of nebulized beclomethasone in preventing viral wheezing: an RCT. Pediatrics. 2014;133(3):e505–e512. doi:10.1542/peds.2013-2404
8. Jartti T, Nieminen R, Vuorinen T, et al. Short- and long-term efficacy of prednisolone for first acute rhinovirus-induced wheezing episode. J Allergy Clin Immunol. 2015;135(3):691–698 e699. doi:10.1016/j.jaci.2014.07.001
9. Ducharme FM, Lemire C, Noya FJ, et al. Preemptive use of high-dose fluticasone for virus-induced wheezing in young children. N Engl J Med. 2009;360(4):339–353. doi:10.1056/NEJMoa0808907
10. Kelly HW, Sternberg AL, Lescher R, et al. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367(10):904–912. doi:10.1056/NEJMoa1203229
11. Loke YK, Blanco P, Thavarajah M, Wilson AM, Zhang L. Impact of inhaled corticosteroids on growth in children with asthma: systematic review and meta-analysis. PLoS One. 2015;10(7):e0133428. doi:10.1371/journal.pone.0133428
12. Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–615. doi:10.1164/rccm.9120-11ST
13. Wang Z, Pianosi P, Keogh K, et al. The clinical utility of Fractional Exhaled Nitric Oxide (FeNO) in asthma management [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); AHRQ Comparative Effectiveness Reviews. 2017 Dec. Report No.: 17(18)-EHC030-EF.
14. Wildhaber JH, Hall GL, Stick SM. Measurements of exhaled nitric oxide with the single-breath technique and positive expiratory pressure in infants. Am J Respir Crit Care Med. 1999;159(1):74–78. doi:10.1164/ajrccm.159.1.9805021
15. Baraldi E, Dario C, Ongaro R, et al. Exhaled nitric oxide concentrations during treatment of wheezing exacerbation in infants and young children. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1284–1288. doi:10.1164/ajrccm.159.4.9807084
16. Gabriele C, Nieuwhof EM, Van Der Wiel EC, et al. Exhaled nitric oxide differentiates airway diseases in the first two years of life. Pediatr Res. 2006;60(4):461–465. doi:10.1203/01.pdr.0000238242.39881.64
17. Moeller A, Diefenbacher C, Lehmann A, et al. Exhaled nitric oxide distinguishes between subgroups of preschool children with respiratory symptoms. J Allergy Clin Immunol. 2008;121(3):705–709. doi:10.1016/j.jaci.2007.11.008
18. Meyts I, Proesmans M, Van Gerven V, Hoppenbrouwers K, De Boeck K. Tidal off-line exhaled nitric oxide measurements in a pre-school population. Eur J Pediatr. 2003;162(7–8):506–510. doi:10.1007/s00431-003-1215-x
19. Guilbert TW, Morgan WJ, Zeiger RS, et al. Atopic characteristics of children with recurrent wheezing at high risk for the development of childhood asthma. J Allergy Clin Immunol. 2004;114(6):1282–1287. doi:10.1016/j.jaci.2004.09.020
20. Singer F, Luchsinger I, Inci D, et al. Exhaled nitric oxide in symptomatic children at preschool age predicts later asthma. Allergy. 2013;68(4):531–538. doi:10.1111/all.2013.68.issue-4
21. Beigelman A, Mauger DT, Phillips BR, et al. Effect of elevated exhaled nitric oxide levels on the risk of respiratory tract illness in preschool-aged children with moderate-to-severe intermittent wheezing. Ann Allergy Asthma Immunol. 2009;103(2):108–113. doi:10.1016/S1081-1206(10)60162-7
22. Franklin PJ, Turner SW, Mutch RC, Stick SM. Measuring exhaled nitric oxide in infants during tidal breathing: methodological issues. Pediatr Pulmonol. 2004;37(1):24–30. doi:10.1002/ppul.v37:1
23. Franklin PJ, Turner SW, Mutch RC, Stick SM. Comparison of single-breath and tidal breathing exhaled nitric oxide levels in infants. Eur Respir J. 2004;23(3):369–372. doi:10.1183/09031936.04.00084604
24. Elliott M, Heltshe SL, Stamey DC, Cochrane ES, Redding GJ, Debley JS. Exhaled nitric oxide predicts persistence of wheezing, exacerbations, and decline in lung function in wheezy infants and toddlers. Clin Exp Allergy. 2013;43(12):1351–1361. doi:10.1111/cea.12171
25. Debley JS, Herrington-Shaner S, Stamey D, Redding GJ. Exhaled nitric oxide measured in wheezy infants and toddlers is associated with asthma, exacerbations, and lung function at age 6 years. Am J Respir Crit Care Med. 2014;189:A5117. doi:10.1164/rccm.201306-1150OC
26. Lum S, Stocks J, Castile R, Davis S. ATS/ERS statement: raised volume forced expirations in infants: guidelines for current practice. Am J Respir Crit Care Med. 2005;172(11):1463–1471. doi:10.1164/rccm.200408-1141ST
27. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi:10.1164/ajrccm.159.1.9712108
28. Debley JS, Stamey DC, Cochrane ES, Gama KL, Redding GJ. Exhaled nitric oxide, lung function, and exacerbations in wheezy infants and toddlers. J Allergy Clin Immunol. 2010;125(6):1228–1234 e1213. doi:10.1016/j.jaci.2010.03.023
29. Jones M, Castile R, Davis S, et al. Forced expiratory flows and volumes in infants. Normative data and lung growth. Am J Respir Crit Care Med. 2000;161(2 Pt 1):353–359. doi:10.1164/ajrccm.161.2.9903026
30. Chang DV, Sarria EE, Mattiello R, et al. Relationship of atopy, airway function, exhaled nitric oxide and cytokine production early in life to airway function and current asthma at 5-years of age. Am J Respir Crit Care Med. 2012;185:A2339.
31. Ren CL, Esther CR
32. O’Connor GT, Lynch SV, Bloomberg GR, et al. Early-life home environment and risk of asthma among inner-city children. J Allergy Clin Immunol. 2018;141(4):1468–1475. doi:10.1016/j.jaci.2017.06.040
33. Comhair SA, Gaston BM, Ricci KS, et al. Detrimental effects of environmental tobacco smoke in relation to asthma severity. PLoS One. 2011;6(5):e18574. doi:10.1371/journal.pone.0018574
34. Dick S, Doust E, Cowie H, Ayres JG, Turner S. Associations between environmental exposures and asthma control and exacerbations in young children: a systematic review. BMJ Open. 2014;4(2):e003827. doi:10.1136/bmjopen-2013-003827
35. Neophytou AM, Oh SS, White MJ, et al. Secondhand smoke exposure and asthma outcomes among African-American and Latino children with asthma. Thorax. 2018;73(11):1041–1048. doi:10.1136/thoraxjnl-2017-211383
36. Gabriele C, Jaddoe VW, van Mastrigt E, et al. Exhaled nitric oxide and the risk of wheezing in infancy, the generation R study. Eur Respir J. 2011.
37. van de Kant KD, Koers K, Rijkers GT, et al. Can exhaled inflammatory markers predict a steroid response in wheezing preschool children?Clin Exp Allergy. 2011;41(8):1076–1083. doi:10.1111/cea.2011.41.issue-8
38. American Thoracic S, European Respiratory S. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171(8):912–930. doi:10.1164/rccm.200406-710ST
39. Romero KM, Robinson CL, Baumann LM, et al. Role of exhaled nitric oxide as a predictor of atopy. Respir Res. 2013;14:48. doi:10.1186/1465-9921-14-48
40. Mikalsen IB, Halvorsen T, Oymar K. Exhaled nitric oxide is related to atopy, but not asthma in adolescents with bronchiolitis in infancy. BMC Pulm Med. 2013;13:66. doi:10.1186/1471-2466-13-66
41. Kulig M, Bergmann R, Klettke U, Wahn V, Tacke U, Wahn U. Natural course of sensitization to food and inhalant allergens during the first 6 years of life. J Allergy Clin Immunol. 1999;103(6):1173–1179. doi:10.1016/S0091-6749(99)70195-8
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.