Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Exercise Ameliorates Emphysema Of Cigarette Smoke-Induced COPD In Mice Through The Exercise-Irisin-Nrf2 Axis
Authors Kubo H , Asai K , Kojima K, Sugitani A , Kyomoto Y , Okamoto A, Yamada K, Ijiri N, Watanabe T, Hirata K, Kawaguchi T
Received 9 August 2019
Accepted for publication 31 October 2019
Published 14 November 2019 Volume 2019:14 Pages 2507—2516
DOI https://doi.org/10.2147/COPD.S226623
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Hiroaki Kubo, Kazuhisa Asai, Kazuya Kojima, Arata Sugitani, Yohkoh Kyomoto, Atsuko Okamoto, Kazuhiro Yamada, Naoki Ijiri, Tetsuya Watanabe, Kazuto Hirata, Tomoya Kawaguchi
Department of Respiratory Medicine, Graduate School of Medicine, Osaka City University, Osaka, Japan
Correspondence: Kazuhisa Asai
Department of Respiratory Medicine, Graduate School of Medicine, Osaka City University, 1-4-3 Asahimachi, Abeno-Ku, Osaka 545-8585, Japan
Tel +81 6 6645 3916
Fax +81 6 6646 6160
Email [email protected]
Background: Oxidative stress is one of the important mechanisms underlying the pathogenesis of chronic obstructive pulmonary disease (COPD). Irisin is a type of myokine secreted from the muscle during exercise and acts against oxidative stress via nuclear factor erythroid 2-related factor 2 (Nrf2), a transcription factor with antioxidant properties. Here, we examined the emphysema suppressive effects of the exercise-irisin-Nrf2 axis in mice.
Methods: Mice were divided into three groups, namely, the control, smoking, and exercise + smoking groups. All mice from the smoking and exercise + smoking groups were exposed to cigarette smoke once a day. The mice from the exercise + smoking group were adapted to a treadmill once a day. To investigate the Nrf2 cascade, after 12 weeks, serum irisin concentration and Nrf2 and heme oxygenase-1 (HO-1) expression in the lung homogenate were determined. To evaluate cigarette smoke-induced COPD, the number of inflammatory cells in bronchoalveolar lavage fluid (BALF), mean linear intercept (MLI), and destructive index in the lung tissue were examined.
Results: Serum irisin concentration and the expression levels of Nrf2 and HO-1 in the lung homogenate were significantly higher in mice from the exercise + smoking group than in those from the control and smoking groups. The proportion of neutrophils in the BALF was significantly lower in the exercise + smoking group than in the smoking group. The MLI and destructive index were also significantly smaller in mice from the exercise + smoking group than mice from the smoking group.
Conclusion: Irisin secreted from the muscle during exercise may exert protective effects against oxidative stress via Nrf2 and HO-1, and ameliorate emphysema of cigarette smoke-induced COPD. The exercise-irisin-Nrf2 axis may serve as a novel target for COPD treatment.
Keywords: chronic obstructive pulmonary disease, oxidative stress, exercise, irisin, nuclear factor erythroid 2-related factor 2, heme oxygenase-1
Background
Chronic obstructive pulmonary disease (COPD) is a pulmonary disease caused by prolonged inhalation of noxious gas, mainly cigarette smoke, and results in poorly reversible airflow limitation.1 There are 98 hazardous components found in smoke.2 Moreover, cigarette smoke contains a large amount of oxidants.3 Oxidative stress is considered one of the important mechanisms of COPD pathogenesis.4 Airflow limitation in COPD follows a progressive course characterized by chronic cough, sputum production, shortness of breath,and a reduction in the ability to perform physical activity, such as walking and climbing stairs.5 The decline in physical activity was recently shown to be correlated with COPD prognosis, and physical activity level is most associated with COPD mortality.6 Therefore, there is a growing interest in maintaining and improving the physical activity of patients with COPD. Muscle mass is reduced due to cachexia in COPD,7 and a previous study reported a significant association between quadricep strength and physical activity level in patients with COPD.8 However, the biological mechanism underlying the improvement in the prognosis of patients with COPD following maintenance of physical activity has not been elucidated.
Irisin, a circulating hormone-like myokine secreted from the muscle during exercise, has gained much attention owing to its mechanism of action.9 We reported that serum irisin level was significantly lower in patients with COPD than in healthy subjects and that it correlated with physical activity level.10 We also showed that serum irisin level significantly correlated with lung diffusing capacity for carbon monoxide divided by alveolar volume and the percentage of low-attenuation area in patients with COPD.11
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor exhibiting antioxidant capacity. Following oxidative stress, Nrf2 translocates to the nucleus of a cell and binds to antioxidant response element (ARE) to initiate the transcription of antioxidant genes and the subsequent expression of the corresponding proteins. The activation of the Nrf2-ARE signaling pathway is a major mechanism involved in cellular defense against oxidative stress.12 A previous report showed that Nrf2-deficient mice were highly susceptible to cigarette smoke-induced lung injury.13,14 However, overexpression of Nrf2 is known to protect against cigarette smoke-induced cell apoptosis.15 We found that irisin significantly enhanced the expression of Nrf2 and reduced the cigarette smoke extract (CSE)-induced apoptosis of A549 cells.11 Based on these reports, we hypothesize that irisin secreted from the muscle during exercise may enhance Nrf2 expression in the lung and ameliorate emphysema of cigarette smoke-induced COPD. To address this hypothesis, we examined emphysema suppression and Nrf2-ARE signaling pathway activation by irisin secreted from the muscle in response to exercise in a COPD mouse model.
Materials And Methods
Animals
C57BL/6 male mice (4 weeks old, weighing 18–20 g) were obtained from Japan SLC (Shizuoka, Japan) and maintained under pathogen-free conditions at a controlled temperature of 23 °C ± 2 °C and a 12 h light/dark cycle. The mice were fed a standard diet and had free access to water. The mice were divided into three groups as follows: 1) control (n = 8), 2) smoking (n = 8), 3) exercise + smoking (n = 8) group. The animals were acclimatized to the environment for a week before experiments began. Animal experimentation was approved by the Ethics Committee of the Institutional Animal Care and Use of Osaka City University Graduate School of Medicine (17,023, 6/11/2017). All animal experiments were conducted in accordance with the Regulations on Animal Experiments in Osaka City University following the Guidelines for Proper Conduct of Animal Experiments in Japan.
Experimental Model Of Smoke-Induced COPD
All mice from the smoking and exercise + smoking groups were exposed to cigarette smoking (18 cigarettes/day) once a day, for 60 min per session, five times per week. Commercially marketed Peace non-filter cigarette (2.3 mg nicotine and 28 mg tar/cigarette; Japan Tobacco, Tokyo, Japan) and a cigarette smoke inhalation experimental system for small animals (SG-300; Shibata Scientific Technology, Tokyo, Japan) were used for the exposure to cigarette smoke. Cigarette smoke exposure was performed for 12 weeks.
Treadmill Aerobic Training
All mice from the exercise + smoking group were forced to run on a motor-driven treadmill (TMS-8B; Melquest, Toyama, Japan). After gradually increasing the speed during a 5 min preparation time, the mice were subjected to the same treadmill protocol (0° incline, 18 m/min) once a day, for 30 min per session, five times per week. Treadmill aerobic training was performed before the exposure to cigarette smoke for 12 weeks.
Treatments And Preparation For Evaluation
At the end of the 12-week experimental period, the mice were sacrificed under isoflurane, 3 h after the last exercise session or 24 h after the last exposure to smoke. Laparotomy was performed and 0.5–1 mL of blood was collected from the cava vein. Following blood collection, the mice were tracheotomized and cannulated, and the lung was lavaged thrice with 0.5 mL phosphate-buffered saline (PBS) for collection of bronchoalveolar lavage fluid (BALF). After bronchoalveolar lavage, the right lung was carefully excised. The right lower lobe was soaked in RNAlater (Invitrogen by Thermo Fisher Scientific, Waltham, MA, USA) for gene expression analysis, while the other right lobe was instantly frozen in liquid nitrogen for protein expression analysis. The left lung was carefully excised and soaked in 10% formalin for histological analysis.
Evaluation Of Serum Irisin Level
The collected blood was centrifuged at 8,200 × g at 4 °C for 10 min, and the serum was stored at −80 °C. Serum irisin concentration was evaluated with a commercially available enzyme-linked immunosorbent assay kit (EK-067-29, Phoenix Pharmaceuticals, Inc., Burlingame, USA) according to the manufacturer’s instructions.
Gene Expression Analysis
The right lower lobe was disrupted and homogenized in RLT lysis buffer (Qiagen NV, Venlo, Netherlands). Total RNA was extracted with the RNeasy mini kit (Qiagen NV, Venlo, Netherlands). Complementary DNA (cDNA) was obtained by reverse transcription of the mRNA with the Ready-to-Go T-primed first-strand kit (GE Healthcare, Little Chalfont, UK). The cDNA was subjected to real-time quantitative polymerase chain reaction (PCR) on an Applied Biosystems 7500 real-time PCR system (Thermo Fisher Scientific) with TaqMan gene expression assays for Nrf2 (Mm00477784_m1). The relative level of Nrf2 mRNA was normalized to that of the housekeeping gene 36B4 (Mm00725448_s1), as previously described.16
Western Blot Analysis
The right lung other than the lower lobe was subjected to Western blot analysis. About 30 mg of lung sample was treated with 300 µL of radioimmunoprecipitation assay buffer (Beyotime Biotechnology, Shanghai, China) supplemented with the protease inhibitor phenylmethanesulfonyl fluoride (Beyotime Biotechnology) and Protease Inhibitor Cocktail (Cell Signaling Technology, USA) for homogenization. After homogenization, the samples were placed on ice for 5 min and centrifuged at 11,800 × g and 4 °C for 4 min. The supernatant was collected and the protein concentration was determined using the colorimetric bicinchoninic acid protein assay kit (Pierce, Waltham, MA, USA) according to the manufacturer’s recommendations. The proteins were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis with Mini-PROTEAN TGX Precast Protein Gels (4561023, Bio-Rad, Hercules, California, USA) and the separated bands were transferred onto polyvinylidene fluoride membranes. The membranes were incubated with primary anti-Nrf2 antibody (1:500; ab137550, Abcam, Cambridge, UK), anti-HO-1 antibody (1:250; ab13248, Abcam), or anti-β-actin antibody (1:1,000; ab8227, Abcam) at 4 °C overnight. The membranes were then incubated with the corresponding secondary antibodies for 2 h at room temperature. After washing the membranes, SuperSignal West Dura Extended Duration Substrate (Thermo Fisher Scientific, Rockford, IL, USA) was used for detection. Western blot signals were acquired using a Fuji LAS-4000 fluorescence imager (Fujifilm Corporation, Tokyo, Japan). β-actin was used to normalize the level of target protein.
Bronchoalveolar Lavage Fluid Analysis
The recovered BALF was centrifuged at 1,200 × g and 4 °C for 10 min, and the supernatant collected. The cell pellet was resuspended in 1 mL of PBS and subjected to a cytospin procedure using a Shandon Cytospin 3 centrifuge (Shandon Scientific Co., London, England). The slides were stained with Diff-Quick, and enumeration of cells and differential cell counts (neutrophils, lymphocytes, macrophages, and eosinophils) were performed according to the hematological criteria.
Quantitative Evaluation Of Lung Emphysema
The left lung was perfused and fixed with 10% formalin for 24–48 h at positive pressure (25 cm H2O), and submitted to histological routine. Three-micrometer thick slices were stained with hematoxylin and eosin for analysis of the level of emphysema. Airspace size was evaluated by determining the mean linear intercept (MLI), as previously described.17 Moreover, the destructive index was also determined to evaluate destruction, as previously described.18
Statistical Analysis
Data are expressed as mean ± standard deviation. Differences were evaluated with one-way analysis of variance, followed by a multiple comparison procedure. Differences were considered significant for p < 0.05. All statistical analyses were performed with GraphPad Prism 7.04 (GraphPad Software, San Diego, CA, USA).
Results
Mice From The Smoking Group Gained Less Weight Than The Control And Exercise + Smoking Groups
One mouse from the control group was excluded from the analysis due to missing. Three mice from the exercise + smoking group were excluded from the analysis, owing to their inability to run or death due to treadmill accident. In addition, one mouse from the smoking group was excluded from the analysis due to blood collection failure.
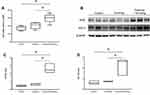
After the 12-week experimental period, the mice from the smoking group did not gain as much weight as those from the control group. Although the mice from the exercise + smoking group gained more weight than those from the smoking group, these mice did not gain as much weight as the mice from the control group (Figure 1A).
Serum Irisin Concentration Was Elevated In Mice From The Exercise + Smoking Group
Serum irisin concentration was significantly elevated in mice from the exercise + smoking group compared to concentrations from the control and smoking groups (p < 0.05; Figure 1B). In contrast, no significant differences in serum irisin concentration were observed between the mice from the control and smoking groups.
Nrf2 And HO-1 Expression Levels Were Elevated In Mice From The Exercise + Smoking Group
We evaluated Nrf2 mRNA expression in the lung homogenate by real-time PCR as an indicator of antioxidant capacity. Nrf2 mRNA expression was significantly higher in mice from the exercise + smoking group than in mice from the control and smoking groups (p < 0.05; Figure 2A). No significant difference was observed in Nrf2 mRNA expression between the mice from the control and smoking groups. Furthermore, we evaluated protein expression of Nrf2 and HO-1, which was regulated by Nrf2, with Western blot analysis (Figure 2B). Nrf2 protein expression was upregulated in mice from the exercise + smoking group compared to the expression in mice from the control and smoking groups (p < 0.05; Figure 2C). HO-1 protein expression was also upregulated in mice from the exercise + smoking group compared to the expression in mice from the control and smoking groups (p < 0.05; Figure 2D). There was no significant difference in Nrf2 and HO-1 protein expression between the mice from the control and smoking groups.
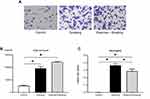
Proportion Of Neutrophils In The BALF Was Suppressed Following Exercise
To examine the influence of cigarette smoke exposure on BALF and the changes induced by exercise, we enumerated cell population in the BALF and evaluated the proportion of different cells. Representative images of BALF from each group are shown in Figure 3A. Total cell count and proportion of neutrophils in the BALF were significantly higher in mice from the smoking group than in those from the control group (p < 0.05; Figure 3B and C). No significant difference was noted in the total cell count in the BALF of mice from the exercise + smoking and smoking groups (Figure 3B). However, the proportion of neutrophils in BALF was significantly lower in mice from the exercise + smoking group than in mice from the smoking group (p < 0.05; Figure 3C).
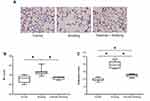
Exercise Ameliorated Emphysema Of Cigarette Smoke-Induced COPD
Representative histologic images of the lung from each group are shown in Figure 4A. Exposure to cigarette smoke for 12 weeks resulted in the development of pulmonary emphysema in mice from the smoking group. Interestingly, lung tissues from the exercise + smoking group showed lower alveolar destruction than that observed in the lung tissues of the mice from the smoking group. The MLI was significantly larger in mice from the smoking group than in mice from the control group, and the MLI for mice from the exercise + smoking group was significantly lower than that of mice from the smoking group (p < 0.05; Figure 4B). No significant difference was reported in the MLI between the mice from the control and exercise + smoking groups. Moreover, the destructive index was significantly larger in mice from the smoking group than in those from the control group, and the destructive index was significantly lower in mice from the exercise + smoking group than in those from the smoking group (p < 0.05; Figure 4C). However, unlike the MLI, the destructive index in mice from the exercise + smoking group was not as small as that in mice from the control group.
Discussion
The present study demonstrated that treadmill exercise increased the serum concentration of irisin, enhanced Nrf2 and HO-1 expression in the lung, and suppressed emphysema of cigarette smoke-induced COPD in mice. We have previously reported the irisin-mediated significant increase in the expression of Nrf2 and the reduction in the CSE-induced alveolar epithelial cell apoptosis, which are deemed important in emphysema progression.11 Our findings indicate that the exercise-induced irisin expression activates the Nrf2-ARE signaling pathway and attenuates lung damage induced by cigarette smoke. In addition, we showed that treadmill exercise inhibited cigarette smoke-induced neutrophil infiltration in BALF.
COPD is the third leading cause of death worldwide following ischemic heart disease and stroke.19 Hence, the development of a novel prevention and treatment strategy for COPD is desirable. Oxidant-antioxidant imbalance and protease-antiprotease imbalance hypotheses are associated with COPD etiology.20,21 Cigarette smoke is a major cause of COPD and contains many oxidants, which are known to induce apoptosis of alveolar epithelial cells.3 The intervention against oxidative stress could serve as a novel strategy for COPD prevention and treatment. Nrf2 is expressed in various human organs, including the lung. The activation of the Nrf2-ARE signaling pathway is a major cellular defense mechanism against oxidative stress.22,23 Nrf2 and other downstream antioxidants, such as HO-1, play a protective role against oxidative stress caused by harmful substances, such as cigarette smoke.24 Previous studies have reported that Nrf2-deficient mice were very sensitive to cigarette smoke-induced lung injury.13,14 However, the overexpression of Nrf2 is thought to mediate protective effects against cigarette smoke-induced cell apoptosis.15 We have previously shown that Nrf2 expression was significantly decreased in the bronchial and alveolar epithelial cells of patients with COPD compared to those from control subjects, and that Nrf2 exhibited a protective role against CSE-induced apoptosis.25 These reports suggest that the Nrf2-ARE pathway protects against the oxidant-antioxidant imbalance in cigarette smoke-induced emphysema. In our study, Nrf2 expression was slightly higher in mice from the smoking group than in those from the control group, but no significant difference was observed. Nrf2 expression tended to increase as an endogenous antioxidant mechanism against cigarette smoke. However, these inductions by cigarette smoke were considered potentially weaker than the oxidants contained in cigarette smoke, and thus the mice developed emphysema. In contrast, Nrf2 expression significantly increased in mice from the exercise + smoking group compared to the expression in those from the smoking group. Antioxidants could exert stronger effects than cigarette smoke-induced oxidants and suppress the development of emphysema.
Irisin is a novel myokine mainly released by skeletal muscles in response to exercise. Exercise induces the expression of peroxisome proliferator-activated receptor- γ co-activator 1α (PGC-1α), a transcriptional cofactor expressed in the skeletal muscle. PGC-1α stimulates the synthesis and secretion of fibronectin type III domain-containing protein 5 (FNDC5). Irisin is secreted as a product of FNDC5.26–28 In the present study, the serum concentration of irisin was elevated 3 h after treadmill running. Previous studies have also reported an increase in the serum concentration of irisin 3 to 6 h after treadmill running in mice, using a similar protocol.29–32 We previously reported that serum irisin levels in patients with COPD were significantly lower than those in healthy subjects.10 Serum irisin concentration has been reported to correlate with muscle mass.33 Cachexia is a common and serious comorbidity of COPD associated with increased mortality and loss of peripheral muscle mass.7 Therefore, serum irisin concentration in patients with COPD may be lower than that in healthy subjects because of the reduction in muscle mass due to cachexia in COPD. Moreover, shortness of breath and reduction of muscle mass due to cachexia in COPD may result in lower levels of physical activity and serum irisin. In our results, the mice in the exercise + smoking group had a larger weight gain than the mice in the smoking group. Therefore, daily exercise has the potential to prevent reduction of muscle mass due to cachexia in COPD. Since the discovery of irisin in 2012, several studies have reported its physiological role. Irisin circulates in the blood and induces browning of the white adipose tissue through an increase in the expression of uncoupling protein-1. As a result, there is an increase in heat production in adipocytes and a subsequent amelioration in metabolic diseases such as obesity, dyslipidemia, and diabetes. A recent report revealed the important role of irisin in bone and neurogenesis.34,35 Considering the metabolic properties of irisin, several studies have focused on its therapeutic potential for the treatment of metabolic disorders such as type 2 diabetes and obesity.9 Moreover, the anti-inflammatory and anti-oxidative effects of irisin have also gained attention of the scientific community,36 and several reports on the antioxidant effects of irisin have been published. Irisin was shown to alleviate the endothelial dysfunction in type 2 diabetes, at least in part, by reducing oxidative/nitrative stress.37 Furthermore, irisin exerts a beneficial role in the prevention of hepatic steatosis through the attenuation of oxidative stress in an arginine methyltransferase-3-dependent manner.38 Other studies showed that irisin alleviated hepatic ischemia/reperfusion injury by relieving oxidative stress,39 and protected the heart against ischemia/reperfusion-induced oxidative stress.40 Zhang et al have recently reported the irisin-mediated attenuation of vascular injury through the activation of the protein kinase B/mechanistic target of rapamycin/Nrf2 pathway.41 These reports support our hypothesis that irisin enhances the expression of Nrf2 and suppresses emphysema of cigarette smoke-induced COPD.
Whether exercise improves emphysema of cigarette smoke-induced COPD in humans is not yet completely understood. However, several studies have shown that exercise suppressed pulmonary emphysema and inflammatory cell infiltration in the BALF of COPD mice, consistent with the results of the present study.42–44 However, these reports mainly focused on different mechanisms other than irisin and Nrf2,such as modulation of signal transducer and activator of transcription 3, anti-inflammatory mediators, and antioxidant enzymes. A report described the elevation in Nrf2 expression in the diaphragm in response to exercise and showed that treadmill aerobic training protected diaphragm muscle wasting induced by cigarette smoke exposure through the upregulation in the expression of antioxidant genes such as Nrf2 and HO-1.45 We focused on the serum level of irisin and expression of Nrf2 in the lung tissue and found that the MLI and destructive index were significantly decreased in mice from the exercise + smoking group compared to those from the smoking group, suggesting that these mice had decreased emphysema. This result may be related to the antioxidant and antiprotease effects observed along with the increase in Nrf2 expression. A previous study reported that smoking elevated neutrophil elastase activity in Nrf2-deficient mice compared to activity in wild-type mice.13 The high elastase activity observed in Nrf2-deficient mice may be attributed to the reduced anti-elastase activity. Thus, Nrf2 may be protective against the development of emphysema through the regulation of not only the oxidant-antioxidant balance but also the protease-antiprotease balance.
We showed that treadmill exercise inhibited cigarette smoke-induced neutrophil infiltration in the BALF. Although Nrf2 suppresses inflammation as a secondary consequence of its antioxidant effect, Nrf2 also directly suppresses the inflammatory response. Nrf2 was bound to the proximity of the proinflammatory cytokine genes, including IL-6 and IL-1b, and inhibited the lipopolysaccharide-induced expression of these genes.46 The Nrf2-mediated transcriptional interference was reported to be independent of reactive oxygen species level and ARE.
This study has some limitations. First, we could not definitively show a causal relationship between irisin and Nrf2, while our previous study showed the direct irisin-Nrf2 pathway in vitro.11 The suppressive effect on emphysema could also be related to other mechanisms such as other hormones or myokines induced by exercise. Second, we failed to analyze the mice from the exercise group without smoking. Therefore, we do not know what the levels of markers might be with exercise only. Third, the gastrocnemius muscle and adipose tissue were not measured or analyzed. Thus, we did not evaluate the effects of exercise and cachexia. Fourth, male mice were used in this study because COPD is more common in male. Therefore, we did not discuss any gender differences in response to exercise. To clarify the irisin-Nrf2 axis and the effect on emphysema suppression, additional studies with Nrf2 knockout mice or mice injected with irisin are warranted. However, the overexpression of irisin in mice was shown to cause mitochondrial overdrive with the production of reactive oxygen species, eventually resulting in an increase in the apoptosis of cardiomyocytes under a hypoxic environment.47 Therefore, the dose of irisin injection should be carefully chosen. Furthermore, irisin was reported to bind to the αV class of integrins as an agonist.48 This report enabled us to perform a detailed investigation of the irisin-Nrf2 axis.
Conclusion
Irisin secreted from the muscles in response to exercise circulates in the blood, increases the expression of Nrf2 in the lung, and suppresses cigarette smoke-induced alveolar apoptosis, all of which may potentially ameliorate emphysema development. Our results provide a model to explain why COPD patients with high physical activity have better prognosis and may highlight a promising approach for the development of a strategy for the treatment of patients with COPD.
Acknowledgments
This work was supported by research grants from the Japan Society for the Promotion of Science KAKENHI, Grant Number 19K08660 to K. Asai.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol. 2009;4:435–459. doi:10.1146/annurev.pathol.4.110807.092145
2. Talhout R, Schulz T, Florek E, van Benthem J, Wester P, Opperhuizen A. Hazardous compounds in tobacco smoke. Int J Environ Res Public Health. 2011;8(2):613–628. doi:10.3390/ijerph8020613
3. Pryor WA, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann N Y Acad Sci. 1993;686:
4. Sundar IK, Yao H, Rahman I. Oxidative stress and chromatin remodeling in chronic obstructive pulmonary disease and smoking-related diseases. Antioxid Redox Signal. 2013;18(15):1956–1971. doi:10.1089/ars.2012.4863
5. Wheaton AG, Cunningham TJ, Ford ES, Croft JB. Employment and activity limitations among adults with chronic obstructive pulmonary disease–United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(11):289–295.
6. Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140(2):331–342. doi:10.1378/chest.10-2521
7. Remels AH, Gosker HR, Langen RC, Schols AM. The mechanisms of cachexia underlying muscle dysfunction in COPD. J Appl Physiol (1985). 2013;114(9):1253–1262. doi:10.1152/japplphysiol.00790.2012
8. Waschki B, Spruit MA, Watz H, et al. Physical activity monitoring in COPD: compliance and associations with clinical characteristics in a multicenter study. Respir Med. 2012;106(4):522–530. doi:10.1016/j.rmed.2011.10.022
9. Mahgoub MO, D’Souza C, Al Darmaki R, Baniyas M, Adeghate E. An update on the role of irisin in the regulation of endocrine and metabolic functions. Peptides. 2018;104:15–23. doi:10.1016/j.peptides.2018.03.018
10. Ijiri N, Kanazawa H, Asai K, Watanabe T, Hirata K. Irisin, a newly discovered myokine, is a novel biomarker associated with physical activity in patients with chronic obstructive pulmonary disease. Respirology. 2015;20(4):612–617. doi:10.1111/resp.12513
11. Sugiyama Y, Asai K, Yamada K, et al. Decreased levels of irisin, a skeletal muscle cell-derived myokine, are related to emphysema associated with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2017;12:765–772. doi:10.2147/COPD
12. Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi:10.1146/annurev.pharmtox.46.120604.141046
13. Iizuka T, Ishii Y, Itoh K, et al. Nrf2-deficient mice are highly susceptible to cigarette smoke-induced emphysema. Genes Cells. 2005;10(12):1113–1125. doi:10.1111/j.1365-2443.2005.00905.x
14. Rangasamy T, Cho CY, Thimmulappa RK, et al. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114(9):1248–1259. doi:10.1172/JCI200421146
15. Huang C, Wang JJ, Ma JH, Jin C, Yu Q, Zhang SX. Activation of the UPR protects against cigarette smoke-induced RPE apoptosis through up-regulation of Nrf2. J Biol Chem. 2015;290(9):5367–5380. doi:10.1074/jbc.M114.603738
16. Okamoto A, Nojiri T, Konishi K, et al. Atrial natriuretic peptide protects against bleomycin-induced pulmonary fibrosis via vascular endothelial cells in mice: ANP for pulmonary fibrosis. Respir Res. 2017;18(1):1. doi:10.1186/s12931-016-0492-7
17. Thurlbeck WM. The internal surface area of nonemphysematous lungs. Am Rev Respir Dis. 1967;95(5):765–773. doi:10.1164/arrd.1967.95.5.765
18. Saetta M, Shiner RJ, Angus GE, et al. Destructive index: a measurement of lung parenchymal destruction in smokers. Am Rev Respir Dis. 1985;131(5):764–769. doi:10.1164/arrd.1985.131.5.764
19. Quaderi SA, Hurst JR. The unmet global burden of COPD. Glob Health Epidemiol Genom. 2018;3:e4. doi:10.1017/gheg.2018.1
20. McGuinness AJ, Sapey E. Oxidative stress in COPD: sources, markers, and potential mechanisms. J Clin Med. 2017;6:2. doi:10.3390/jcm6020021
21. Houghton AM. Matrix metalloproteinases in destructive lung disease. Matrix Biol. 2015;44–46:167–174. doi:10.1016/j.matbio.2015.02.002
22. Bocci V, Valacchi G. Nrf2 activation as target to implement therapeutic treatments. Front Chem. 2015;3:4. doi:10.3389/fchem.2015.00004
23. Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284(20):13291–13295. doi:10.1074/jbc.R900010200
24. Cho HY, Kleeberger SR. Association of Nrf2 with airway pathogenesis: lessons learned from genetic mouse models. Arch Toxicol. 2015;89(11):1931–1957. doi:10.1007/s00204-015-1557-y
25. Yamada K, Asai K, Nagayasu F, et al. Impaired nuclear factor erythroid 2-related factor 2 expression increases apoptosis of airway epithelial cells in patients with chronic obstructive pulmonary disease due to cigarette smoking. BMC Pulm Med. 2016;16:27. doi:10.1186/s12890-016-0189-1
26. Bostrom P, Wu J, Jedrychowski MP, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–468. doi:10.1038/nature10777
27. Castillo-Quan JI. From white to brown fat through the PGC-1alpha-dependent myokine irisin: implications for diabetes and obesity. Dis Model Mech. 2012;5(3):293–295. doi:10.1242/dmm.009894
28. Schumacher MA, Chinnam N, Ohashi T, Shah RS, Erickson HP. The structure of irisin reveals a novel intersubunit beta-sheet fibronectin type III (FNIII) dimer: implications for receptor activation. J Biol Chem. 2013;288(47):33738–33744. doi:10.1074/jbc.M113.516641
29. Liu J, Cui XY, Yang YQ, et al. Effects of high-intensity treadmill training on timeliness and plasticity expression of irisin in mice. Eur Rev Med Pharmacol Sci. 2015;19(12):2168–2173.
30. Pang M, Yang J, Rao J, et al. Time-dependent changes in increased levels of plasma irisin and muscle PGC-1alpha and FNDC5 after exercise in mice. Tohoku J Exp Med. 2018;244(2):93–103. doi:10.1620/tjem.244.93
31. Brenmoehl J, Albrecht E, Komolka K, et al. Irisin is elevated in skeletal muscle and serum of mice immediately after acute exercise. Int J Biol Sci. 2014;10(3):338–349. doi:10.7150/ijbs.7972
32. Nygaard H, Slettalokken G, Vegge G, et al. Irisin in blood increases transiently after single sessions of intense endurance exercise and heavy strength training. PLoS One. 2015;10(3):e0121367. doi:10.1371/journal.pone.0121367
33. Huh JY, Panagiotou G, Mougios V, et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61(12):1725–1738. doi:10.1016/j.metabol.2012.09.002
34. Grygiel-Gorniak B, Puszczewicz M. A review on irisin, a new protagonist that mediates muscle-adipose-bone-neuron connectivity. Eur Rev Med Pharmacol Sci. 2017;21(20):4687–4693.
35. Rodrigues K, Pereira RM, de Campos TDP, et al. The role of physical exercise to improve the browning of white adipose tissue via POMC neurons. Front Cell Neurosci. 2018;12:88. doi:10.3389/fncel.2018.00088
36. Askari H, Rajani SF, Poorebrahim M, Haghi-Aminjan H, Raeis-Abdollahi E, Abdollahi M. A glance at the therapeutic potential of irisin against diseases involving inflammation, oxidative stress, and apoptosis: an introductory review. Pharmacol Res. 2018;129:44–55. doi:10.1016/j.phrs.2018.01.012
37. Zhu D, Wang H, Zhang J, et al. Irisin improves endothelial function in type 2 diabetes through reducing oxidative/nitrative stresses. J Mol Cell Cardiol. 2015;87:138–147. doi:10.1016/j.yjmcc.2015.07.015
38. Park MJ, Kim DI, Choi JH, Heo YR, Park SH. New role of irisin in hepatocytes: the protective effect of hepatic steatosis in vitro. Cell Signal. 2015;27(9):1831–1839. doi:10.1016/j.cellsig.2015.04.010
39. Bi J, Zhang J, Ren Y, et al. Irisin alleviates liver ischemia-reperfusion injury by inhibiting excessive mitochondrial fission, promoting mitochondrial biogenesis and decreasing oxidative stress. Redox Biol. 2019;20:296–306. doi:10.1016/j.redox.2018.10.019
40. Wang Z, Chen K, Han Y, et al. Irisin protects heart against ischemia-reperfusion injury through a SOD2-dependent mitochondria mechanism. J Cardiovasc Pharmacol. 2018;72(6):259–269. doi:10.1097/FJC.0000000000000608
41. Zhang M, Xu Y, Jiang L. Irisin attenuates oxidized low-density lipoprotein impaired angiogenesis through AKT/mTOR/S6K1/Nrf2 pathway. J Cell Physiol. 2019;234(10):18951–18962.
42. Toledo-Arruda AC, Vieira RP, Guarnier FA, et al. Time-course effects of aerobic physical training in the prevention of cigarette smoke-induced COPD. J Appl Physiol (1985). 2017;123(3):674–683. doi:10.1152/japplphysiol.00819.2016
43. Rodrigues Brandao-Rangel MA, Bachi ALL, Oliveira-Junior MC, et al. Exercise inhibits the effects of smoke-induced COPD involving modulation of STAT3. Oxid Med Cell Longev. 2017;2017:6572714. doi:10.1155/2017/6572714
44. Toledo AC, Magalhaes RM, Hizume DC, et al. Aerobic exercise attenuates pulmonary injury induced by exposure to cigarette smoke. Eur Respir J. 2012;39(2):254–264. doi:10.1183/09031936.00003411
45. Vieira Ramos G, Choqueta de Toledo-arruda A, Maria Pinheiro-Dardis C, et al. Exercise prevents diaphragm wasting induced by cigarette smoke through modulation of antioxidant genes and metalloproteinases. Biomed Res Int. 2018;2018:5909053. doi:10.1155/2018/5909053
46. Kobayashi EH, Suzuki T, Funayama R, et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun. 2016;7:11624. doi:10.1038/ncomms11624
47. Ho MY, Wen MS, Yeh JK, et al. Excessive irisin increases oxidative stress and apoptosis in murine heart. Biochem Biophys Res Commun. 2018;503(4):2493–2498. doi:10.1016/j.bbrc.2018.07.005
48. Kim H, Wrann CD, Jedrychowski M, et al. Irisin mediates effects on bone and fat via alphav integrin receptors. Cell. 2018;175(7):1756–1768.e1717. doi:10.1016/j.cell.2018.10.025
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.