Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Examining Skin Recovery After a 3% Aqueous Hydrogen Peroxide (H2O2) Treatment Using ATP Biofluorescence
Received 26 February 2022
Accepted for publication 18 May 2022
Published 24 May 2022 Volume 2022:15 Pages 929—937
DOI https://doi.org/10.2147/CCID.S363723
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
James V Gruber,1 Jed Riemer2
1Research, BotanicalsPlus, Fairfield, NJ, USA; 2Research, Jeen International, Fairfield, NJ, USA
Correspondence: James V Gruber, Email [email protected]
Introduction: Since its complete mapping, the human skin microbiome has become an important area of research related to skin health. The human skin is populated by an environment of microorganisms, fungi, insects, and viruses that is collectively known as the microbiota, and the complete genomic contribution to the skin is called the microbiome. The terms are different but frequently used interchangeably. Measuring the skin’s microbial diversity can be done, but it is a sophisticated technique that is performed using expensive instruments that can sequence the 16S ribosomal RNA of the microorganisms. Finding more rapid and less costly methods to analyze the changes in the skin’s microbial biome is desirable.
Methods: A study was conducted on thirty (30) inner volar forearms to see if ATP biofluorescence could be employed to examine skin microbial dysbiosis caused by the application of 3% hydrogen peroxide. Fifteen individuals were examined on both arms for a total of thirty inner volar forearms using a Charm Science® NovaLum® ATP analyzer to examine in a broad sense the skin’s total microbial population and how it is affected after surface treatment with 3% hydrogen peroxide over a 24-hour period.
Results: It was found that surface treatment of the skin with three cotton swab applications of 3% hydrogen peroxide five minutes apart was able to statistically significantly suppress the expression of ATP biofluorescence compared against un-swabbed sites and the effects remained significant for six hours following the H2O2 treatment. After 8 hours, and into the 24th hour, the ATP biofluorescence difference returns to non-statistical significance indicating potential return of the stable microbiota.
Discussion: Using ATP biofluorescence to detect possible sanitizer-induced microbial dysbiosis may be a rapid way to examine how skin treatments may impact the return of microbially disrupted skin to its normal state and how surface treatments may impact the rate of return to normal after a disruptive event.
Keywords: skin microbiome, skin microbiota, adenosine triphosphate, ATP, biofluorescence, dysbiosis
Introduction
Since the groundbreaking studies by Elizabeth Grice at the University of Pennsylvania to map the total human skin microbiota, interest in the relationship between the microbiome and the skin’s health have exploded.1–3 Grice’s studies demonstrated clearly that different areas of the body carry different populations of microorganisms depending on several factors such as body moisture, oil content and exposure to ultraviolet radiation, among others. Interest in the role of the skin’s microbiome and microbiota and how they relate to the skin’s health are important and continue to expand. For instance, it is now well established that skin suffering from atopic dermatitis will typically have an abnormally high population of Staphylococcus aureus.4 Likewise, there appears to be a link between acne and the occurrence of Cutibacterium acnes (formerly Propionibacterium acnes).5,6 Dandruff has been linked to the presence of Malassezia fungi which are known to be present in higher numbers in populations of people with the condition.7,8 However, whether these opportunistic microorganisms lead to the skin dysfunction, or the occurrence of the abnormal microorganism populations is due to problems with the skin’s innate health remains elusive.
The skin microbiota is populated by over 19 phyla of microorganisms with the four dominant ones Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria typically comprising over 95% of the phyla present on healthy skin.2,6 Measuring the diversity of microorganisms on the skin is sophisticated and requires careful swabbing, tape stripping or biopsy techniques followed by analysis of the various microorganism populations using techniques that rely on sequencing of the bacterial and fungal RNA. Early work relied on the use of 16S ribosomal RNA sequencing, real-time PCR for bacteria and ITS1 sequencing for fungal RNA.9,10 However, newer metagenomic sequencing methods have evolved to shotgun metagenomic sequencing methods that are proving faster and more precise. Byrd et al have published a recent review of the methods used to analyze skin microbial and fungal populations, as well as the emerging science around viral analysis that summarizes these advanced technologies in excellent detail.11 These unique instruments are expensive and require time and considerable skill to use and interpret data. Other methods to examine more global populations of skin microbes can include agar plating or even the application of newer microbial testing strips, such as the 3M Petrifilm® that allow for more rapid examination of microbial contamination in a global fashion.12 Even these unique testing techniques require that the skin be carefully swabbed, and the swab applied to the strips, which must then be cultured over a 24-hour period to determine microbial content. And, importantly, some skin commensal microorganisms do not grow readily outside of the milieu of the skin. Thus, using plating techniques presents some of its own problems. Also, while these strips are useful for determining if something is contaminated, to quantitatively determine the level of microbial contamination requires counting the colony forming units (CFU) that grow on the strips.
Development of methods to detect microbial ATP has expanded, and there are now several instruments that are offered commercially that use ATP biofluorescence as a means of detecting microbial contamination on surfaces. For example, 3M offers their Clean-Trace® Surface ATP Test and Charm Science® offers the PocketSwab® Plus and NovaLum® ATP analyzer for use principally in production plants requiring standardized and monitored sanitizing operations. The units are commonly used by many cosmetic and food manufacturing companies to monitor equipment and laboratory surface sanitizing operations. These units offer a very rapid way of swabbing surfaces and then analyzing the overall microbial content of the surface based on the presence of surface bacterial ATP. Studies that have examined the relationship between the Relative Fluorescence Units (RFU) expressed by these ATP instruments and the overall Colony Forming Units (CFU) have shown that the instruments have good sensitivity and linearity between the two measurements.13,14 Use of these instruments to examine oral cavity microbial burden after mouth rinsing, cow’s teats for microbial cleanliness and to monitor the cleanliness of poultry carcasses in poultry processing suggested that they might have potential for examination of the human skin microbiota.15–17 This paper will discuss a pilot study intended to look at the possibility of examining skin microbial dysbiosis by disruption of the skin’s microbiota with a well-known sanitizing agent, 3% hydrogen peroxide, and monitoring how quickly the disrupted skin is able to return to normal after the disruption.
Materials and Methods
Participant Selection
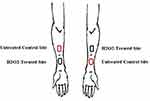
This study employed the use of fifteen individuals (9-Male, 6-Female, Ages 21–61, Fitzpatrick Skin Types II–III) who all signed Informed Consent to participate in the study. All the participants had healthy, undisrupted inner volar forearm skin and had not taken antibiotics or used any type of potential sanitizing or exfoliating products two weeks prior to participation in the study. Both arms of each participant were used that allowed inclusion of both a test site and a control, untreated, site on each arm for a total of n = 30 inner volar forearms to be examined as shown in Figure 1. Prior to participating in the study, everyone was instructed to rinse their arms with warm water in the morning of the study and not to apply any types of products to their arms for the duration of the study.
Charm Science PocketSwabs and NovaLum ATP Analyzer
For these studies, a NovaLum VX-II ATP Analyzer from Charm Sciences, Inc [Andover, MA] was employed to measure ATP biofluorescence. The company provides PocketSwab® Plus swabs that are designed to swab surfaces and then fit into the Charm RFU analyzer. A picture of a PocketSwab is shown below in Figure 2. The PocketSwab Plus is a self-contained unit that has the swab stored until use in a sterile chamber. When ready for use, the swab is withdrawn from the unit and is applied to the surface being tested using specific directions offered by the company, which includes a swabbing pattern that requires about sixteen square centimeters of surface while rotating the swab and applying pressure to the swab. The swab is then inserted back into the unit and with a twisting screw motion is thrust through a thin foil membrane that houses an oxidizing fluid. The tip of the swab and the oxidizing fluid drop into the clear chamber at the bottom of the device. The device houses a small tablet that contains a luciferin dye that is activated by the oxidizing fluid and a luciferase enzyme pellet that has been designed to detect adenosine triphosphate (ATP). The device then fluoresces in accordance with the amount of ATP present on the swab. The resulting fluorescing swabbing unit is placed into the NovaLum ATP analyzer shown in Figure 3. Prior to insertion of a treated swab, the NovaLum ATP analyzer is zeroed using an untreated swab that is thrust into the oxidizing fluid without prior microbial exposure to the swabbing unit. The NovalLum device measures the amount of luciferin biofluorescence and reports the values of Relative Fluorescent Units (RFUs) which can be recorded for testing purposes.
 |
Figure 2 Charm Sciences Pocketswab Plus unit. |
 |
Figure 3 Charm Novalum II–X ATP biofluorescence measuring unit. |
All measurements were made at a room temperature of 22–24°C and a RH of approximately 45–50% and each participant equilibrated to the room for 15 minutes prior to measurements being taken. The participants were tested initially at all four inner volar forearm locations before any treatments to gain a baseline level of ATP based on the RFUs noted from the unit for each site. Each participant was then swabbed with a cotton ball saturated with 3% hydrogen peroxide in the two test sites noted in Figure 1. The adjacent site was not swabbed and remained as an untreated control site on each arm. The participants then waited five minutes and the two sites were swabbed with hydrogen peroxide again. This sanitizing treatment was repeated a third time, and then the participants could continue their normal activities for one hour with the stipulation that they should take care not to rub their arms against any solid surfaces or any other skin surfaces for the duration of the first day of the study. The participants offered both arms, which doubled the number of treatment sites so a total of 30 arms were tested. The two arm test sites were also alternated as shown in Figure 1. Each participant was then measured using the Novalum ATP Analyzer with a single swab at each site at the following timepoints: T(1): 1 hr, T(2): 3 hrs, T(3): 6 hrs, T(4): 8 hrs and T(5): 24 hrs.
Statistical Analysis
The RFUs for each timepoint were collected and averaged. A total of twelve total measurements (T(0)-T(5)) were made through the course of each individual study using both arms, and the results at each test site for each time point were averaged. The results were then compared between the test sites (H2O2-treated) versus the control sites. In addition, the results at Timepoints T(1), T(2), T(3), T(4) and T(5) were normalized by dividing the average RFUs for each timepoint measurement by the averaged baseline measurement to provide normalized hourly comparisons accounting for initial variations in site ATP levels. This provided a unitless measure of normalized data for each timepoint. Using the statistical function in Microsoft Excel, the Student’s T-test (Paired, two-tailed) was employed to determine 95% confidence (p ≤ 0.05) for each datapoint.
Results and Discussion
Figure 4 shows the raw values for the averages of all measurements without normalization of the various timepoints against the initial baseline measurements for each site. The data indicate that only the baseline versus test 1-Hour and baseline versus test 3-Hour measurements (asterisked) are statistically different from one another. This indicates that the 3% hydrogen peroxide treatment reduces the level of ATP biofluorescence compared to the control sites, which were not treated with peroxide. The data also indicate that the slight increase in the average RFU values seen at times T(3) and T(4), which are six hours and eight hours post-peroxide treatment, respectively, while appearing slightly elevated, are not statistically different from the original baseline measurements.
 |
Figure 4 Raw ATP biofluorescent relative fluorescent unit (RFU) averages for each arm site and statistical analyses comparing the baseline measurements versus hydrogen peroxide-treated sites at each time point and the H2O2-Treated (blue) versus Untreated Control (orange) sites at each time point. The 24-Hour time point is discussed in greater detail in Figure 6. Only the H2O2-Treated baseline versus 1-hour and 3-hour sites show statistically significant differences indicating that the 3% hydrogen peroxide treatment has reduced the overall level of ATP biofluorescence at these sites versus the Untreated Control sites. N=30, single asterisks p≤0.05. |
The results of the baseline-normalized ATP RFU measurements post-hydrogen peroxide treatment for various time points, T(1)-T(5), are shown in Figure 5. These data show more clearly the indication that the 1-Hour and 3-Hour hydrogen peroxide-treated sites (asterisked) show an overall suppression of ATP bioluminescence compared against their corresponding Control (non-peroxide treated) sites.
Examining the average Relative Fluorescence Units (RFU) of the initial baseline measurements versus similar measurements 24 hours later demonstrated that, within all measurable parameters, the initial measurements were identical to the testing results taken 24 hours after the skin had been treated with 3% hydrogen peroxide, Figure 6.
There is a very important potential source of error that can occur when using ATP bioflourescence to detect human skin microbes: human skin cells also make ATP. It is very likely that ATP coming from the epidermis can find its way to the surface of the skin’s stratum corneum and thus complicate measuring microbial ATP versus what is known as somatic skin cell ATP.18 This problem became acute when 70% aqueous 2-propanol was employed to try and sanitize the skin’s surface. It was found that skin sanitized with 70% aqueous 2-propanol provided inconsistent and contradictory results (data not shown). It was felt that to employ this technique the method of sanitization of the skin must kill the surface microbes while not significantly disrupting the skin’s lipid bilayer. One possible solution was to consider using mild surfactants. However, work published in 2016 by Two et al demonstrated that mild surfactants did not seem to impact the skin microbiome when measured using expression of LL37, an antimicrobial peptide expressed in the skin.19 In the conclusion of these surfactant studies, it was suggested that the “deeper bacteria” in the skin might be able to “rapidly repopulate the surface” of the skin. However, in this interesting microbiome work, the timeframe for this repopulation was not discussed.
In an interesting study published in 2017 through Becker’s Hospital Review, Liceaga et al examined the effectiveness of various aqueous sanitizing ingredients including antiseptic sanitizers containing chlorhexidine, and ozonized water, both in the presence and absence of soap and using simple tap water with soap alone as a control.20 The authors employed an ATP analyzer [Hygiena’s SystemSURE plus ATP Cleaning Verification System] that examined the levels of ATP on the hands after the treatments. The studies employed ten people. The authors of this study noted the following in their study review:
The skin as a living organ has naturally occurring levels of ATP that should not [be] attributed to exogenous microorganisms, eg. virus, fungi, spores, or bacteria. It is believed impossible to eliminate all ATP from skin cells to achieve a zero ‘0’ RLU result.
They recognized that the skin produces natural ATP but felt that the impact of the somatic ATP was likely small. The study focused principally on the cleansing properties of the cleansers and not on how the microbiota might respond to the treatments.
It was then felt that perhaps treatment of the skin with a commonly used sanitizing spray containing 3% hydrogen peroxide might be able to effectively remove surface microorganisms while maintaining the viable pool of deeper microorganisms that would repopulate the skin. An initial study using the product as a spray demonstrated that it was tricky to control the spray and so it was decided that application of the 3% hydrogen peroxide with saturated cotton balls would allow for more precise control of sanitizer application. In addition, while a single treatment with 3% hydrogen peroxide did appear to offer some reductions in skin ATP biofluorescence, three treatments spaced five minutes apart appeared to work very well to reduce ATP biofluorescence to statistically significant levels. It is noted that the physical effects of cotton balls might remove some surface microorganisms. However, for the purposes of these studies, this is not a detrimental problem for the techniques described. The advantage of the 3% hydrogen peroxide is that it would not as seriously disrupt the skin’s lipid bilayer as 70% aqueous 2-propanol and would minimize migration of somatic ATP present in the lower layers of the stratum corneum to the skin’s surface. It is, however, impossible to differentiate somatic ATP from microbial ATP with this testing technique as both molecules are chemically identical.
It has been shown recently in very interesting work done by Hillebrand et al that the microbial and fungal compositional of the skin microbiome is very stable. The work examined changes in the skin microbial composition over a 2-year period in an in-depth and lengthy longitudinal study and noted that over the course of the two years that the subjects participated in the study, the skin microbiome remained generally unchanged.21 Two’s work mentioned above also indicates that the skin microbiome is relatively resistant to surfactant treatments.19 In a recently published study by Peng et al, from the University of Maryland’s Department of Animal and Avian Science it was demonstrated that while the skin’s microbiome can be stable, it can also be influenced significantly by a person’s environmental exposures.22 In Peng’s work, they investigated the relationship of the microbiome of farm workers’ inner volar forearms to the microbial diversity of various farms in which they worked. It was found that the microbial populations can change depending on the individual’s environment.
Recently, a book publication has appeared that delves into the science surrounding the skin microbiome and how it is impacting the development of therapeutic and cosmetics products.23 All of this recent work suggests that understanding the role of the skin’s microbiome on skin aging and health remains an area of considerable interest and research. However, this has not stopped companies offering cosmetic ingredients from suggesting that topical treatment may balance the skin microbiome or improve the growth of beneficial commensal microorganisms by application of pre- or postbiotic ingredients. The marketing claims for ingredients appear to be preceded the science around what the skin’s microbiome is doing and why it is important in aging and skin disease. It is also not uncommon to see cosmetic formulations touting probiotics when the formulations contain microbial or fungal lysates, which are not living microorganisms and so are not, technically, probiotics.24
Conclusions
With the work presented here, the principal idea was to demonstrate in a small pilot study that ATP biofluorescence might work as a method to rapidly examine skin that has had a microbial disruption imparted to it via a mild sanitizing treatment and to investigate how quickly the normal microbiota might respond to a sanitizing treatment. It may be that one of the major impacts of the skin’s microbiota and a unique measure of the skin’s health is how quickly the skin’s surface microbiota can return to normal functionality. Data presented from these initial studies suggest that ATP biofluorescence can offer a way to investigate the global skin microbiota without attention to specific microorganism populations. The results presented here suggest that after a topical disruption, the skin’s microbiota may be able to return to normality in as little as six hours. And, importantly, as the data in Figure 6 demonstrates, within 24 hours of application of the sanitizing treatment, the skin has completely returned to original baseline measurements indicating that, regardless of whether there may be subtle somatic ATP influences, the skin microbiota has returned to pre-treatment levels. This suggests that the skin’s microbiota is a robust and aggressively adapting system that does not remain disrupted for long in healthy skin. This does raise the question of how this rapid return to normal might become dysfunctional in abnormal or aging skin.
From a more pragmatic cosmetic and therapeutic perspective, attention to topical treatments that can help to accelerate this microbial repopulating might be a unique area of interest in developing novel topical treatments that can influence the skin’s microbiome and health. In addition, the technique may offer a superior way to examine in vivo the concepts around “microbiome-safe” which are currently becoming popular cosmetic claims. As noted, for example, in the work by Gallez et al, the use of ATP bioluminescence to examine gingival microbial populations suggests that it may have applications in numerous areas of research.15 The mildness of the hydrogen peroxide sanitizing treatments will likely also allow for testing to occur around the face or other sensitive skin areas where the composition of the microbiome is quite different compared to the relatively oil-free surface of the arms.
Abbreviations
ATP, Adenosine Triphosphate; RNA, Ribonucleic Acid; H2O2, Hydrogen Peroxide; CFU, Colony Forming Units; RFU, Relative Fluorescence Units.
Ethics Approval and Consent to Participate
The studies reported here were conducted using non-invasive, cosmetic testing methods and are exempt from oversight by an IRB. The laboratory conducting the studies follows the protocols established within the Declaration of Helsinki. All the participants in the study signed Informed Consent prior to participating in the studies, had the nature of the study explained to them prior to participating and were free to leave the study at any time.
Author Contributions
All authors made a significant contribution to the work reported, whether it was in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas. Both authors also took part in drafting, revising or critically reviewing the article and gave final approval for the version to be published. Both authors agreed on the journal to which the article has been submitted and agreed to be accountable for all aspects of the work.
Funding
All funding for the study was provided by BotanicalsPlus and Jeen International. No additional funding sources are noted.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gruber JV, Riemer J. Measuring the skin microbiome after a 3% aqueous hydrogen peroxide (H2O2) dysbiosis using ATP biofluorescence. Res Square Preprint. 2022. doi:10.21203/rs.3.rs-1325039/v1
2. Grice EA, Kong HH, Renaud G, et al. A diversity profile of the human skin microbiota. Genome Res. 2008;18:1043–1050. doi:10.1101/gr.075549.107
3. Flowers L, Grice EA. The Skin Microbiota: balancing Risk and Reward. Cell Host Microbe. 2020;28:190–200. doi:10.1016/j.chom.2020.06.017
4. Myles IA, Earland NJ, Anderson ED, et al. First-in-human transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight. 2018;3:e120608. doi:10.1172/jci.insight.120608
5. Wang Y, Kuo S, Shu M, et al. Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of Propionibacterium acnes: implications of probiotics in acne vulgaris. Appl Microbial Cell Physiol. 2014;98:411–424.
6. Fourniere M, Latire T, Souak D, Feuilloley MGJ, Bedous G. Staphylococcus epidermidis and Cutibacterium acnes: two major sentinels of skin microbiota and the influence of cosmetics. Microorganisms. 2020. 8:1752.
7. Leong C, Wang J, Toi MJ, et al. Effect of zinc pyrithione shampoo treatment on skin commensal Malassezia. Med Mycol. 2021;59:201–213. doi:10.1093/mmy/myaa068
8. Dawson TL. Malassezia globosa and restricta: breakthrough Understanding of the Etiology and Treatment of Dandruff and Seborrheic Dermatitis through Whole-Genome Analysis. J Invest Dermatol. 2007;12:15–19. doi:10.1038/sj.jidsymp.5650049
9. Grogan MD, Bartow-McKenney C, Flowers L, Knight SAB, Uberoi A, Grice EA. Research techniques made simple: profiling the skin microbiota. J Invest Dermatol. 2019;139:747–752. doi:10.1016/j.jid.2019.01.024
10. Ferretti P, Farina S, Cristofolini M, Girolomoni G, Tett A, Segata N. Experimental metagenomics and ribosomal profiling of the human skin microbiome. Exp Dermatol. 2017;26:211–219. doi:10.1111/exd.13210
11. Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nat Rev Microbiol. 2018;16:143–155. doi:10.1038/nrmicro.2017.157
12. Biermann NM, McClure JT, Sanchez J, Saab M, Doyle AJ. Prospective, randomized clinical trial of four different presurgical hand antiseptic techniques in equine surgery. Equine Vet. 2019;51:600–605. doi:10.1111/evj.13060
13. Omidbakhsh N, Ahmadpour F, Kenny N. How reliable are ATP bioluminescence meters in assessing decontamination of environmental surfaces in healthcare settings? PLoS One. 2014;9:e99951. doi:10.1371/journal.pone.0099951
14. Ihssen J, Jovanovic N, Sirec T, Spitz U. Real-time monitoring of extracellular ATP in bacterial cultures using thermostable luciferase. PLoS One. 2021;16:e0244200. doi:10.1371/journal.pone.0244200
15. Gallez F, Fadel M, Scruel O, Cantraine F, Courtois P. Salivary biomass assessed by bioluminescence ATP assay related to (bacterial and somatic) cell counts. Cell Biochem Funct. 2000;18:103–108. doi:10.1002/(SICI)1099-0844(200006)18:2<103::AID-CBF860>3.0.CO;2-N
16. Johnson A, Papnicki P, Farnsworth R, et al. Measuring effectiveness of teat preparation. Univ. Minn. Coll. Vet. Med. Minn. Dairy Heath Conf; 2003. Available from: https://conservancy.umn.edu/bitstream/handle/11299/108987/1/Johnson.pdf.
17. Siragusa GR, Dorsa WJ, Cutter CN, Perino LJ, Koohmaraie M. Use of a newly developed rapid microbial ATP bioluminescence assay to detect microbial contamination on poultry carcasses. J Biolumin Chemilumin. 1996;11:297–301. doi:10.1002/(SICI)1099-1271(199611)11:6<297::AID-BIO422>3.0.CO;2-C
18. Kwan SE, Peccia J, Simonds J, Haverinen-Shaughnessy U, Shaughnessy RJ. Comparing bacterial, fungal, and human cell concentrations with rapid adenosine triphosphate measurements for indicating microbial surface contamination. Am J Infect Cont. 2019;47:671–676. doi:10.1016/j.ajic.2018.11.011
19. Two AM, Nakatsuji T, Kotol PF, et al. The cutaneous microbiome and aspects of skin antimicrobial defense system resists acute treatment with topical cleansers. J Invest Dermatol. 2016;136:1950–1954. doi:10.1016/j.jid.2016.06.612
20. Liceaga A, Mercado E, Narcaroti M, Liceaga L Hand hygiene skin ATP study: hand washing with tap water and soap vs ozonated water and soap vs antiseptic and tap water vs antiseptic and ozonated water vs antiseptic and tap water. Becker’s Hospital Review (2017). Available from: https://www.beckershospitalreview.com/quality/hand-hygiene-skin-atp-study-hand-washing-with-tap-water-and-soap-vs-ozonated-water-and-soap-vs-antiseptic-and-ozonated-water-vs-antiseptic-and-tap-water.html.
21. Hillebrand GG, Dimitriu PD, Malik K, et al. Temporal variation of the facial skin microbiome: a 2-year longitudinal study in healthy adults. Plast Reconstr Surg. 2021;147:50S. doi:10.1097/PRS.0000000000007621
22. Peng M, Biswas D. Environmental influences of high-density agricultural animal operation on human forearm skin microflora. Microorganisms. 2020;8:1481. doi:10.3390/microorganisms8101481
23. Dayan N. Skin Microbiome Handbook: From Basic Research to Product Development. Hoboken, NJ: John Wiley & Sons, Pub; 2020.
24. Yu Y, Dunaway S, Champer J, Kim J, Alikhan A. Changing our microbiome: probiotics in dermatology. Brit J Dermatol. 2020;182:39–46. doi:10.1111/bjd.18659
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.