Back to Journals » Drug Design, Development and Therapy » Volume 12
Examination of the effect of ovarian radiation injury induced by hysterosalpingography on ovarian proliferating cell nuclear antigen and the radioprotective effect of amifostine: an experimental study
Authors Can B, Atilgan R , Pala S, Kuloğlu T, Kiray S, Ilhan N
Received 11 November 2017
Accepted for publication 29 March 2018
Published 25 May 2018 Volume 2018:12 Pages 1491—1500
DOI https://doi.org/10.2147/DDDT.S156757
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Frank Boeckler
Behzat Can,1 Remzi Atilgan,1 Sehmus Pala,1 Tuncay Kuloğlu,2 Sule Kiray,3 Nevin Ilhan4
1Department of Obstetrics and Gynecology, Firat University School of Medicine, Elazig, Turkey; 2Department of Histology and Embryology, Firat University School of Medicine, Elazig, Turkey; 3Department of Obstetrics and Gynecology, Maltepe University School of Medicine, Istanbul, Turkey; 4Department of Biochemistry, Firat University School of Medicine, Elazig, Turkey
Aim: The aim of this study was to examine the effect of amifostine on cellular injury in the ovarian tissue induced by hysterosalpingography (HSG).
Methods: In total, forty 4-month old female Wistar Albino rats were assigned into 8 groups. Each group contained 5 rats. Group 1 (G1): rats were decapitated without any procedure. Group 2 (G2): rats were decapitated after 3 hours of total body irradiation. Group 3 (G3): rats were decapitated 3 hours after HSG procedure. Group 4 (G4): rats were decapitated 3 hours after HSG procedure performed 30 min after receiving amifostine 200 mg/kg intraperitoneally. Group 5 (G5): rats were decapitated after 1 month without any procedure. Group 6 (G6): rats were decapitated after 1 month of total body irradiation. Group 7 (G7): rats were decapitated 1 month after HSG procedure. Group 8 (G8): rats were decapitated 1 month after HSG procedure performed 30 min after receiving amifostine 200 mg/kg intraperitoneally. After rats were decapitated under general anesthesia in all groups, blood samples were obtained and bilateral ovaries were removed. One of the ovaries was placed in 10% formaldehyde solution for histological germinal epithelial degeneration, apoptosis and proliferating cell nuclear antigen scoring. The other ovary and blood sera were stored at –80°C. TNF-α, total antioxidant status, total oxidant status, and malondialdehyde levels were studied in tissue samples and anti-mullerian hormone levels in blood samples.
Results: At the end of the first month, there was significant ovarian germinal epithelium degeneration. Proliferating cell nuclear antigen immunoreactivity was significantly reduced in all other groups when compared with G1 and G5.
Conclusion: In conclusion, amifostine could significantly reduce the ovarian cellular injury induced by HSG.
Keywords: hysterosalpingography, amifostine, ovarian damage, radiation
Introduction
Hysterosalpingography (HSG) is one of the oldest techniques used for tubal patency testing. It should be quoted that this technique has limited accuracy (positive predictive value <38%), and it is currently replaced by specific ultrasound procedures, especially with air/saline, foam, and Doppler.1 These procedures have significantly higher (2D/3D Doppler HyFoSy) or similar accuracy (air/saline HyCoSy) without exposure to radiation and without significant risk of allergic reactions as in HSG. Diagnosis of other intrauterine lesions is not accurate by HSG, and currently, HSG plays role only in the diagnosis of Asherman’s syndrome. However, in many places, it is still a basic method and recommended as the first-line diagnostic tool because it does not require highly qualified practitioners.2 Patient exposure to ionizing radiation, despite in low doses, represents one drawback of this procedure.3,4 Ionizing radiation administration during radiotherapy (RT) is a known inducer of follicular atresia,5 and in rats, radiation has been shown to cause DNA fragmentation in oocytes.6 Furthermore, radiation may accelerate the natural atresia process via induction of apoptosis of granulosa cells possessing high mitotic activity.7–9 Radioprotectors are compounds that are given immediately prior to or at the time of irradiation to reduce the most radiation-induced damage to normal tissues. Also, the radiation-attenuating compounds are the compounds that even reduce postirradiation toxicity when applied prior to the onset of toxic symptoms. A variety of agents have been investigated for their potential to prevent and cure radiation-induced toxic effects.10 Many compounds showed promising results, but so far only amifostine and palifermin have been clinically approved by the US Food and Drug Administration (FDA).
Amifostine was approved by the FDA in 1996 due to its ability to reduce the cumulative renal toxicity caused by cisplatin treatment in patients with advanced ovarian cancer or non-small cell lung cancer. At the molecular level, amifostine affects the redox of sensitive transcription factors, gene expression, chromatin stabilization, and enzymatic activity. In cells, it plays a role in the regulation of cell growth and progression of cellular turnover. It is also effective in the proliferation of a variety of cell lines as well as in the protection of normal cells from apoptosis.11 Sulfhydryl moiety in its structure eliminates reactive neutrophiles that are formed due to the production of free radicals after chemotherapy or RT, which are associated with DNA damage.12
Amifostine can significantly reduce the death of normal cells after RT; however, side effects, including hypotension, nausea, vomiting, and other adverse reactions, limit its use.13 In vivo studies have shown that amifostine accumulates more rapidly in various normal tissues than tumors. It is believed that selective preservation of nonmalignant tissues is due to the high alkaline phosphatase activity, higher pH, and vascular microcirculation in normal tissues. These observations led to the concept that amifostine could be used clinically to limit the side effects of RT and chemotherapy in normal tissues, but not affecting the therapeutic efficacy of tumors.14 In an experimental model performed in rats, amifostine was applied before RT, and esophageal damage was evaluated histomorphologically and immunohistochemically after 10 weeks, and radiation-induced esophageal damage was found to decrease with amifostine.15
Objective
To examine the ovarian cell injury due to the radiation doses typically associated with HSG procedure and to investigate the protective effect of amifostine in such an injury.
Materials and methods
This study was conducted at the Laboratory Center for Experimental Studies of Firat University. Forty Wistar albino, 12–14-week-old female rats with regular cycles and weighing 190–220 g were kept under a 12-hour artificial light–dark cycle (08 am to 20 pm) at a temperature of 21°C–23°C, in groups of five per cage, and were fed with standard pellets and tap water. All rats in the estrous cycle were randomly and prospectively divided into eight (n=5) following groups. The number of rats in each group was set as the minimum number that could be statistically significant to prevent animal wastage.
G1 (n=5): early period sham group: the abdomens of the rats were opened and closed under general anesthesia and the rats were decapitated after 3 hours without any procedure.
Surgical procedure
All rats were anesthetized using 70 mg/kg ketamine i.m. (Ketalar, Eczacibaşi, Turkey) and 10 mg/kg xylazine i.m. (Rompun, Bayer, Turkey) and were fixed in supine position. After skin cleaning with 10% Batticone solution, a midline incision was made to access the abdominal cavity. The abdomens of the rats in all groups were closed continuously using 3/0 silk suture after surgical procedures have been completed.
G2 (n=5): early period radiation group: the group was decapitated after 3 hours of total body irradiation three times with 2-min intervals.
Radiation application procedure
Radiation application and HSG procedure were done at the Firat University Veterinary Faculty Animal Hospital. All-body irradiation (Villa-Genius HF-80, Double-table, double-tube x-ray device, 1994; Del Medical, Milan, Italy) was applied at a dose of 15–20 miliRad16 three times with 2-min intervals to the rats after opening and closing of the abdomen.
G3 (n=5): early period HSG group: the group was decapitated 3 hours after HSG procedure under general anesthesia.
HSG procedure
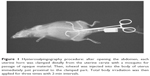
After opening the abdomen, each uterine horn was clamped distally from the uterine cervix with a mosquito for passage of opaque material. Then, 0.1 mL of iohexol (Omnipaque 350 mg/100 mL 1flacon, Opaque, Istanbul, Turkey) was injected into the body of uterus immediately just proximal to the clamped part. Total body irradiation was then applied three times with 2-min intervals (Figure 1).
G4 (n=5): early period amifostine treatment group: the group was decapitated under general anesthesia 3 hours after HSG procedure was performed 30 min17 after receiving amifostine (Ethyol 500 mg 1 flacon, Er-Kim, Istanbul, Turkey) 200 mg/kg intraperitoneally.18
G5 (n=5): late period sham group: the abdomens of the rats were opened and closed under general anesthesia, and the rats were decapitated after 1 month without any procedure.
G6 (n=5): late period radiation group: the group was decapitated after 1 month of total body irradiation three times with 2-min intervals.
G7 (n=5): late period HSG group: the group was decapitated 1 month after HSG procedure under general anesthesia.
G8 (n=5): late period amifostine treatment group: the group was decapitated under general anesthesia 1 month after HSG procedure was performed 30 min after receiving amifostine (Ethyol 500 mg 1 flacon, Er-Kim, Istanbul, Turkey) 200 mg/kg intraperitoneally.
Sampling and preparation
Of the two ovaries removed, one was fixed in 10% formaldehyde solution for histological examination, while the second was dry stored at −80°C after wrapping in aluminum foil for biochemical assays. Tumor necrosis factor-alpha (TNF-α), total antioxidant status (TAS), total oxidant status (TOS), and malondialdehyde (MDA) levels were determined in the supernatant following their respective methodology. Blood samples were centrifuged at 3,000 rpm for 10 min to separate the serum, which were then placed into Eppendorf tubes and stored at −80°C. Anti-mullerian hormone (AMH) assay was performed using rat AMH ELISA kits (Elabscience, catalogue no: E-EL-R0640). For TNF-α estimations, rat TNF-α ELISA kit (Boster Immuno leader, Boster Biological Technology Co., Ltd. Pleasanton, CA, USA, catalogue no; EK0526) was utilized. The ovarian tissues were prepared for homogenization by washing each tissue with cold saline. The resultant supernatants were removed and stored in sterile tubes at −80°C for tissue TAS and TOS analyses. An autoanalyzer (Siemens Advia 2400 Chemistry System, Siemens, Tokyo, Japan) and Reel Assay Total Antioxidant Status Test Kits were used for TAS and TOS determinations in accordance with standard kit procedures at the Central Laboratory of Firat University Hospital. All blood and tissue samples were stored and analyzed in the Firat University Biochemical Laboratory.
Terminal deoxynucleotidyl transferase dUTP nick end labeling staining
Sections of 5–6 μm thickness obtained from paraffin blocks were mounted on polylysine glass slides. Following the instructions by the manufacturer, ApopTagPlus Peroxidase In Situ Apoptosis Detection Kit (Chemicon, cat no: S7101, Temecula, CA, USA) was used to detect apoptotic cells. Preparations were evaluated through examination using microscope (Novel N-800M). In the evaluation of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining, blue-stained nuclei by Harris hematoxylin were evaluated as normal, while cells displaying brown-stained nuclei were considered as apoptotic. At least 500 cells, normal and apoptotic, were detected in the randomly selected regions of the sections at ×10 magnification. Apoptotic index (AI) was calculated from the proportion of apoptotic cells to total (normal+apoptotic) cells,19 and statistical analyses were performed.
Immunohistochemical examination
Sections of 5–6 mm thickness obtained from paraffin blocks were taken to glass slides with polylysine. Deparaffinized tissues were passed through graded alcohol series and boiled in citrate buffer solution at pH 6 in microwave oven (750 W) for 7+5 min for antigen retrieval. In order to prevent surface staining, tissues were incubated with primary antibodies (Monoclonal Mouse Anti-Proliferating Cell Nuclear Antigen, M0879, Dako, Baltimore, MD, USA, Anti-ab6463 antibody) for 60 min following treatment with Ultra V Block (TA-125-UB, the Lab Vision Corporation, Fremont, CA, USA) solutions. After the application of primary antibodies, tissues were incubated with secondary antibodies (30 min; biotinized anti-mouse/rabbit IgG, Diagnostic BioSystems, KP 50A, Pleasanton, CA, USA), Streptavidin Alkaline Phosphatase (30 min; TS-060-AP, the Lab Vision Corporation), and Fast Red Substrate System (TA-125-AF, the Lab Vision Corporation). Tissues that were conducted a contrasting staining with Mayer’s hematoxylin were treated with PBS and distilled water, and then, closed with the appropriate shutdown solution. The preparations were examined and evaluated under Olympus BX50 microscope and photographed. Extensity of the staining was taken as the basis when evaluating immunohistochemical staining. A histoscore was derived from the distribution (0.1: <25%, 0.4: 26%–50%, 0.6: 51%–75%, 0.9: 76%–100%) and intensity (0: no staining; +0.5: very little staining; +1: little staining; +2: medium; +3: very strong) of staining immune reactivity20 (Histoscore=distribution×intensity). Histological and immunohistochemical evaluations were performed in the Firat University Histology Laboratory.
Statistical analyses
Statistical analyses were performed using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA, ABD) software package. The results were expressed as mean±SD. Test of normality was carried out for each variable. For data with normal distribution, one way analysis of variance was performed followed by Tukey post-hoc test. For data without normal distribution, nonparametric Kruskal–Wallis analysis of variance was used, followed by Mann–Whitney U-test. Also for associated valuables, Wilcoxon’s test was utilized.
Ethical standards
Approval from the local Animal Ethics Committee, Firat University, was obtained for conducting this study (dated: 17.09.2014, session no: 2014/19, decision no: 179). This article does not contain any studies with human participants performed by any of the authors. All experimental manipulations were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Results
Biochemistry results
MDA levels
There were no significant differences in terms of tissue MDA levels between early period sham, radiation, HSG, and amifostine groups (p>0.05). MDA was significantly higher in late period radiation and HSG groups (p<0.01) when compared with late period sham group. The difference between late period sham and late period amifostine groups was insignificant (p>0.05; Table 1).
TOS levels
There were no significant differences in terms of ovarian TOS levels in between early period sham, radiation, HSG, and amifostine groups (p>0.05). TOS levels were significantly higher in late period radiation and late period HSG groups (p<0.01) when compared with late period sham group. Also, TOS in late period HSG group was significantly higher than late period radiation group (p<0.05). The difference between late period sham and late period amifostine groups was insignificant (p>0.05; Table 1).
TAS levels
There were no significant differences in terms of ovarian TAS levels between early period sham, radiation, HSG, and amifostine groups (p>0.05). TAS levels were comparable between late period sham and late period amifostine groups, while these two groups had significantly higher TAS level than late period radiation and late period HSG groups (p<0.05; Table 1).
TNF-α levels
Tissue TNF-α levels in early period radiation and early period HSG groups were significantly higher when compared with early period control group (p<0.05). While the difference between early period sham and early period amifostine groups was insignificant, TNF-α in early period amifostine group was significantly lower than those in early period radiation and early period HSG groups (p<0.05). TNF-α in late period HSG group was significantly higher than that of the late period sham group (p<0.05). The difference between late period radiation and late period sham groups was not significant (p>0.05). Late period amifostine and late period sham groups had comparable TNF-α levels (p>0.05; Table 1).
AMH levels
There was no significant difference observed between any groups regarding AMH levels (p>0.05; Table 1).
Histological findings
Light microscopic examination of Masson’s trichrome stained specimens in all groups revealed the following:
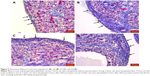
At 3 hours: no change was determined in early period radiation, HSG, and amifostine groups when compared with early period sham group (p>0.05; Figure 2; Table 2).
At 1 month: marked germinal epithelial degeneration (red arrow) was present in late period radiation and late period HSG groups when compared with late period sham group (p<0.05). Germinal epithelial degeneration in late period amifostine group was significantly lower than that of late period radiation and late period HSG groups (p<0.05) and was comparable to G5 (p>0.05; Figure 3; Table 2).
TUNEL results
When compared with early period sham group, an insignificant result was obtained in G2 early period radiation group (p>0.05), while there was a significant increase in early period HSG group (p<0.05). Also, when compared with early period HSG group, there was a significant reduction in early period amifostine group (p<0.01) and the results were comparable to those in early period sham group (p>0.05; Table 2). Although there were no significant alterations in late period radiation group when compared with late period sham group (p>0.05), a significant increase was found in late period HSG group (p<0.05). The results were significantly lower in late period amifostine group when compared with late period HSG group (p<0.05), and comparable results were observed between late period HSG group and late period sham group (p>0.05; Table 2). In the overall study groups, the comparison between 3 hours and 1 month with regard to TUNEL positivity showed a significant increase at 1 month when compared with 3-hour time-point assessment only in late period HSG group (p<0.05).
Immunohistochemical findings
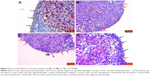
Proliferating cell nuclear antigen (PCNA) immunoreactivity was seen in stromal cells in ovarian tissue. When compared with early period sham group, there was a significant decrease in early period radiation, HSG, and amifostine groups (p<0.05). When compared with late period sham group, there was a significant decrease in late period radiation, HSG, and amifostine groups (p<0.05). When all groups in third hour (G1, G2, G3, G4) and first month (G5, G6, G7, G8) were compared, there was no significant difference regarding immunoreactivity (p>0.05; Figure 4; Table 2).
Discussion
There is no realistic prediction of the diversity and size of health problems that will occur in living organisms at low doses (≤10 cGy) of ionizing radiation. A number of factors, such as environmental conditions, and dietary and biological variabilities between living things, also play a role in the damage that will occur. In addition, the presence of many factors such as the total radiation dose received, the dose taken in each session and the duration of exposure, linear energy transfer, the defense mechanisms demonstrated by the organism, simultaneous chemical carcinogens, and other toxins that may lead to proto-oncogen activation increases ionizing radiation-related damage. In this respect, the low-dose ionizing radiation should not be viewed as safe or tolerable under any circumstances. Because, due to radiation independent of the dose, somatic mutations may develop that may lead to neoplastic and nonneoplastic diseases. In addition, life-threatening heritable mutations can also be triggered as a result of interaction with other toxins. Therefore, the interaction between ionizing radiation and the organism should be considered as a very variable process.21 The total radiation exposure to the patient during HSG withdrawal was calculated as 713 cGy/cm2 (range: 247–1.623 cGy/cm2)22. In another study, the mean radiation exposure of ovaries during HSG treatment was reported as 500–1,000 miliRad.23 In an experimental study, Pala et al16 demonstrated that this dose is ~14–19 miliRad when applied to the radiation dose used during HSG withdrawal in rats. In our experience, we have applied the average radiation dose around 15–20 miliRad. We have not found any literature reporting that HSG has harmful effects on ovarian tissue. For this reason, we investigated in our experimental study whether the radiation dose used during HSG treatment had a detrimental effect on ovaries, even at very low doses. However, it is obvious that HSG in women does not require general anesthesia and surgery as it is in our experimental model. Also, the rat counts in the groups are limited to prevent animal wastage. It is also clear that the results of the animal model cannot directly mimic the same effect in humans. These are limitations of our study. However, for the first time, this is an experimental model of HSG process in which the effects on ovarian histopathology and reserve are studied, and this is the first pilot study in this area. This is the strength of our study.
Lee et al9 investigated the primary and primordial follicular damage after exposure to gamma radiation in rats and found that the most significant damage occurred after 3 hours with a reduction after 6–12 hours following radiation exposure. Pala et al16 showed that the HSG treatment caused a significant increase in epithelial degeneration in the rat endometrium at 3 hours after HSG withdrawal. For this reason, we have chosen a period of 3 hours to examine possible radiation damage in the early period and a 1-month period to investigate possible late period radiation damage. The development of the first follicular wave in the rodents to the antral follicle occurs in about 3 weeks.24 Well-developed secondary follicles are observed on the seventh day. Minimal ovarian cell apoptosis occurs but on day 18.25,26 In this period, early antral follicles are also observed. The developmental stage of primordial follicle to secondary follicular may take >30 days. The developmental period from secondary stage to gradual ovulation can be 28±2 to 3 days.24 For these reasons, we planned to investigate the ovaries 1 month after HSG in order to be able to evaluate the effects of all follicles in the stages ranging from primordial follicular to ovulation on ovarian reserve after exposure to radiography.
Ionized radiation induces cell damage by inducing metabolic oxidative stress through the formation of free radicals.27,28 Total antioxidant capacity measurements reflect the total antioxidant capacity of the body against the effects of strong free radicals.29,30 The usefulness of methods to measure the TAS of body fluids in determining the antioxidant response in clinical settings has been well established.31 Thus, TAS was the method chosen to assess the antioxidant capacity in our study. In our study, TOS and MDA in ovarian tissue samples were significantly higher in late period radiation group and late period HSG group when compared with late period sham group at 1-month assessments. Similarly, there was a decrease in TOS and MDA in late period amifostine treatment group, close to those observed in late period sham group. In these groups, radiation has altered the oxidant/antioxidant balance in favor of oxidative stress, with a resultant decrease in total antioxidant capacity. Increased TAS in late period amifostine treatment group when compared with late period radiation group and late period HSG group suggests that amifostine may reduce the toxic effects associated with oxidative injury through augmentation of the antioxidant effects. MDA, TOS, and TAS, which were utilized to gage the oxidative stress in late period radiation group and late period HSG groups, exhibited a significant change at 1 month rather than at 3-hour time point in comparison to early period sham and early period amifostine treatment groups. Pala et al16 also showed that administration of vitamin C and vitamin E before HSG in rats caused a significant decrease in epithelial degeneration with MDA and Ki-67 immunoreactivity scores in the endometrium. This radioprotective effect also attributed to the antioxidative properties of vitamin C and vitamin E. This suggests that endogenous defense mechanisms reduced in the long term are unable to overcome the oxidative stress and that maintenance of adequate endogenous defense may be possible only through administration of antioxidative agents such as antioxidant vitamins and amifostine.
Approximately 60 minutes after radiation-induced cellular damage, TNF-α becomes detectable in the circulation, reaching a peak at 4–6 hours, activating the release of other cytokines at 15–18 min, and remains in the circulation for almost 10 days.32,33 In our study, TNF-α levels in the ovarian tissue samples were elevated in early period radiation and HSG groups at 3 hours, with lower TNF-α concentrations at 1 month. This elevation is most likely due to the acute radiation induced by tissue injury.34 Also, accessing the abdominal cavity surgically and its subsequent closure represent a kind of surgical trauma, which may also partially account for the acute increase in TNF-α levels. Furthermore, the more marked elevation of TNF-α at 3 hours in early period radiation group and HSG group suggests that the tissue injury induced by radiation may have contributed to this observation. On the other hand, amifostine might have offset the effect of the depleted endogenous antiinflammatory and antioxidant molecules, and therefore, could have provided a cytoprotective effect through maintaining significantly lower TNF-α levels in early period amifostine treatment group when compared with early period HSG group.
DNA alterations occur in cells exposed to ionizing radiation. Double-strand DNA breaks lead to the formation of DNA-PK histon H2Ax. Activation of phosphorylase and kinases prevent DNA repair, and consequently G1, S, G2 cell cycles cannot proceed, leading to cellular death models including apoptosis, mitotic catastrophy, and terminal binding.35 Oocyte atresia is induced in the early stages of follicular growth following DNA damage.36 We believe that the elevated apoptosis in early period radiation and HSG groups and late period HSG and amifostine treatment groups is due to oxidative injury, and amifostine may account for the decreased apoptosis in early period and late period amifostine treatment groups via its antioxidant properties. The results of the study by Yoon et al,37 one of the few publications showing the efficacy of amifostine in the prevention of radiation-induced apoptosis, are consistent with our observations.
AI and epithelial degeneration were significantly higher in late period HSG group than in all other groups. This is probably due to more prominent and prolonged effect of radiation on ovarian tissues due to the absorption of radiation by the contrast medium used. Also, extravasation of the contrast medium into utero ovarian vessels might have punctuated the degenerative effects. When compared with late period HSG group, there was a significant decrease in germinal epithelial degeneration in ovarian tissue specimens obtained at 1 month. In some experimental rat models, amifostine given prior to RT was found to confer functional and histopathological protection.38 Similarly, there was a significant reduction in MDA and apoptosis with amifostine in our study. This might reflect the fact that the degenerative changes observed in the germinal epithelium may be associated with increased generation of ROS.39,40 Furthermore, radiation vasculitis41 might have developed in utero ovarian vasculature, subsequently resulting in ischemia-reperfusion injury in the germinal epithelium and increased MDA.
If the cell is exposed to stress with radiation or drugs and DNA damage occurs, then the cell responds to this stimulation by increasing the level of p53. By activating p21, the cell cycle is stopped by preventing further phosphorylation of the Rb protein at the G1 control point, and thus, PCNA is inhibited.42 PCNA has been shown to be a sensitive marker of early events in follicular growth. Elevated PCNA expression is the earliest manifestation of granulosa cell proliferation. In addition, oocytes start exposing PCNA in early follicular growth before they begin to grow. PCNA immunoreactivity increases significantly in granulosa and theca cells during follicular growth and progressively decreases with increased atresia.43 Atilgan et al44 have shown that total salpingectomy causes a decrease in PCNA immunoreactivity in secondary follicles. Pala et al16 showed that HSG procedure significantly increased Ki-67 immunoreactivity scores, a marker of proliferation in endometrium, while vitamin C and vitamin E administration resulted in a decrease in Ki-67 immunoreactivity. In our study, PCNA immunoreactivity in all irradiated groups was lower than control groups (early period sham group and late period control group), suggesting that cell proliferation ceased in response to cellular damage caused by radiation. In our study, we showed that amifostine had no significant effects on the PCNA immunoreactivity score.
The fact that AMH levels do not differ between groups despite increased ovarian epithelial degeneration at the end of the first month during HSG treatment suggests that this dose of radiation does not cause a loss of follicles at levels that affect over reserve.
In our study, we showed that the ovarian cellular injury was present in rats during HSG. Studies with assessment of ovarian injury in women should be developed by using AMH and ultrasound to measure ovarian reserve.45 Amifostine should be considered for testing in women if studies show ovarian cellular injury in humans during HSG. Probably, it is better to use other minimally invasive methods for tubal patency testing – for example, HyFoSy/Doppler-HyFoSy – which are accurate and reliable,46 and without risk of radiation. This seems to be a more reasonable resolution of the problem than the use of amifostine+HSG because, independent from the use of radioprotection, the risk of ovarian damage will always be present.47
Conclusion
Although amifostine has been found to significantly reduce the ovarian cellular injury due to radiation exposure during HSG in rats, it may be reasonable to confirm impact of radiation during HSG on ovaries before testing amifostine in women. Though amifostine seems to be attractive for clinical radioprotection, it is worth to consider using other new methods for tubal patency testing without risk of radiation.
Acknowledgments
The present study was funded by Firat University, Scientific Research Grant (Project number: 15.10.2014-TF.14.58).
Disclosure
The authors report no conflicts of interest in this work.
References
Ludwin I, Ludwin A, Wiechec M, et al. Accuracy of hysterosalpingo-foam sonography in comparison to hysterosalpingo-contrast sonography with air/saline and to laparoscopy with dye. Hum Reprod. 2017;32:758–769. | ||
NICE Citizens Council Report on Age [Internet]. London: National Institute for Health and Care Excellence (NICE); 2003. | ||
Baakdah H, Tulandi T. Adhesion in gynecology complication, cost, and prevention: a review. Surg Technol Int. 2005;14:185–190. | ||
Maheux-Lacroix S, Boutin A, Moore L, et al. Hysterosalpingosonography for diagnosing tubal occlusion in subfertile women: a systematic review with meta-analysis. Hum Reprod. 2014;29:953–963. | ||
Lee CJ, Yoon Y. γ-Radiation-induced follicular degeneration in the repubertal mouse ovary. Mutat Res. 2005;578:247–255. | ||
Gibbons AFE, Chang MC. The effects of X-irradiation of the rat ovary on implantation and embryonic development. Biol Reprod. 1973;9:343–349. | ||
Lee YK, Chang H, Kim W, Kim J, Yoon Y. Effects of gamma-radiation on ovarian follicles. Arh Hig Rada Toksikol. 1998;49:147–153. | ||
Kim JK, Lee CJ, Song KW, Do BR, Yoon YD. Gamma-radiation accelerates ovarian follicular atresia in immature mice. In Vivo. 1999;13:21–24. | ||
Lee CJ, Park HH, Do BR, Yoon Y, Kim JK. Natural and radiation-induced degeneration of primordial and primary follicles in mouse ovary. Anim Reprod Sci. 2000;59:109–117. | ||
Patyar RR, Patyar S. Role of drugs in the prevention and amelioration of radiation induced toxic effects. Eur J Pharmacol. 2018;819:207–216. | ||
Kang Y, Kai-Yan Y, Xing-Zhou R, Yi C. Amifostine protects bone marrow from benzene-induced hematotoxicity in mice. Int J Toxicol. 2007;26:315–323. | ||
Culy CR, Spencer CM. Amifostine: an update on its clinical status as a cytoprotectant in patients with cancer receiving chemotherapy or radiotherapy and its potential therapeutic application in myelodysplastic syndrome. Drugs. 2001;61:641–684. | ||
Hospers GA, Eisenhauer EA, de Vries EG. The sulfhydryl containing compounds WR-2721 and glutathione as radio-and chemoprotective agents. A review, indications for use and prospects. Br J Cancer. 1999;80:629–638. | ||
Buntzel J, Schuth J, Kuttner K, Glatzel M. Radiochemotherapy with amifostine cytoprotection for head and neck cancer. Support Care Cancer. 1998;6:155–160. | ||
Vujaskovic Z, Thrasher BA, Jackson IL, Brizel MB, Brizel DM. Radioprotective effects of amifostine on acute and chronic esophageal injury in rodents. Int J Radiat Oncol Biol Phys. 2007;69:534–544. | ||
Pala Ş, Atilgan R, Kuloğlu T, et al. Protective effects of vitamin C and vitamin E against hysterosalpingography-induced epithelial degeneration and proliferation in rat endometrium. Drug Des Devel Ther. 2016;10:4079–4089. | ||
Peters GJ, Van der Vijgh WJ. Protection of normal tissues from the cytotoxic effects of chemotherapy and radiation by amifostine (WR-2721): preclinical aspects. Eur J Cancer. 1995;31:1–7. | ||
Yuhas JM. Active versus passive absorption kinetics as the basis for selective protection of normal tissues by S-2-(3-aminopropylamino)-ethylphosphorothioic acid. Cancer Res. 1980;40:1519–1524. | ||
Lipponen P, Aaltomaa S, Kosma VM, Syrajänen K. Apoptosis in breast cancer as related to histopathological characteristics and prognosis. Eur J Cancer. 1994;30:2068–2073. | ||
Mallenby J, Dunyer J, Hawkins C, Hitchen C. Effects of experimental limbic on the estrus cycle and reproductive success in rats. Epilepsia. 1991;34(2):220–227. | ||
Prasad KN. Rationale for using multiple antioxidants in protecting humans against low doses of ionizing radiation. Br J Radiol. 2005;78:485–489. | ||
Fernández JM, Vañó E, Guibelalde E. Patient doses in hysterosalpingography. Br J Radiol. 1996;69:751–754. | ||
Shirley LH. Ovarian radiation dosage during hysterosalpingogram. Fertil Steril. 1971;22:83–85. | ||
McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. | ||
Mazaud S, Guigon CJ, Lozach A, et al. Establishment of the reproductive function and transient fertility of female rats lacking primordial follicle stock after fetal gamma-irradiation. Endocrinology. 2002;143:4775–4787. | ||
Gaytan F, Morales C, Bellido C, Aguilar E, Sanchez-Criado JE. Ovarian follicle macrophages: is follicular atresia in the immature rat a macrophage-mediated event? Biol Reprod. 1998;58:52–59. | ||
Azzam EI, Jay-Gerin JP, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012;327:48–60. | ||
Park YS, You SY, Cho S, et al. Eccentric localization of catalase to protect chromosomes from oxidative damages during meiotic maturation in Mouse oocytes. Histochem Cell Biol. 2016;146:281–388. | ||
Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37:112–119. | ||
Erel O. A new automated colorimetric method for measuring total exident status. Clin Biochem. 2005;38:1103–1111. | ||
Liu X, Zhao J, Zheng R. DNA damage of tumor-associated lymphocytes and total antioxidant capacity in cancerous patients. Mutat Res. 2003;539:1–8. | ||
Friedmann MC, Migone TS, Russell SM, Leonard WJ. Different interleukin 2 receptor beta-chain tyrosines couple to at least two signaling pathways and synergistically mediate interleukin 2-induced proliferation. Proc Natl Acad Sci U S A. 1996;93:2077–2082. | ||
Baumgartner SW. Tumor necrosis factor inactivation in the management of rheumatoid arthritis. South Med J. 2000;93:753–759. | ||
Rubin P, Johnston CJ, Williams JP, McDonald S, Finkelstein JN. A perpetual cascade of cytokines post irradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys. 1995;33:99–109. | ||
Faulhaber O, Bristow RG. Basis of cell kill following clinical radiotherapy. In: Sluyser M, editor. Application of Apoptosis to Cancer Treatment. Dordrecht: Springer. 2005;125:293–320. | ||
Suh EK, Yang A, Kettenbach A, et al. p63 protects the female germ line during meiotic arrest. Nature. 2006;444:624–648. | ||
Yoon YD, Kim JH, Lee KH, Kim JK. Amifostine has an inhibitory effect on the radiation-induced p53-branched cascade in the immature mouse ovary. In Vivo. 2005;19:509–514. | ||
Kaldir M, Cosar-Alas R, Cermik TF, et al. Amifostine use in radiation-induced kidney damage. Preclinical evaluation with scintigraphic and histopathologic parameters. Strahlenther Onkol. 2008;184:370–375. | ||
Beltran-Garcia MJ, Espinosa A, Herrera N, Perez-Zapata AJ, Beltran-Garcia C, Ogura T. Formation of copper oxychloride and reactive oxygen species as causes of uterine injury during copper oxidation of Cu-IUD. Contraception. 2000;61:99–103. | ||
Ávila J, González-Fernández R, Rotoli D, Hernández J, Palumbo A. Oxidative stress in granulosa-lutein cells from in vitro fertilization patients. Reprod Sci. 2016;23:1656–1661. | ||
Fajardo LF. Acta Oncologica Lecture. The pathology of ionizing radiation as defined by morphologic patterns. Acta Oncol. 2005;44:13–22. | ||
Harper J, Adami G, Wei N. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. | ||
Oktay K, Schenken RS, Nelson JF. Proliferating cell nuclear antigen marks the initiation of follicular growth in the rat. Biol Reprod. 1995;53:295–301. | ||
Atilgan R, Kuloğlu T, Boztosun A, et al. Investigation of the effects of unilateral total salpingectomy on ovarian proliferating cell nuclear antigen and follicular reserve: experimental study. Eur J Obstet Gynecol Reprod Biol. 2015;188:56–60. | ||
Coelho Neto MA, Ludwin A, Borrell A, et al. Counting ovarian antral follicles by ultrasound: a practical guide. Ultrasound Obstet Gynecol. 2018;51(1):10–20. | ||
Ludwin I, Ludwin A, Nastri CO, Coelho Neto MA, Kottner J, Martins WP. Inter-rater reliability of air/saline HyCoSy, HyFoSy and HyFoSy combined with power doppler for screening tubal patency. Ultraschall Med. Epub 2017 Dec 12. | ||
Winship AL, Stringer JM, Liew SH, Hutt KJ. The importance of DNA repair for maintaining oocyte quality in response to anti-cancer treatments, environmental toxins and maternal ageing. Hum Reprod Update. Epub 2018 Jan 25. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.