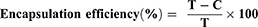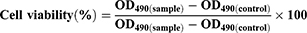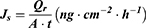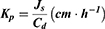Back to Journals » International Journal of Nanomedicine » Volume 17
Examination of Effective Buccal Absorption of Salmon Calcitonin Using Cell-Penetrating Peptide-Conjugated Liposomal Drug Delivery System
Authors Keum T, Noh G, Seo JE, Bashyal S , Sohn DH , Lee S
Received 2 September 2021
Accepted for publication 27 January 2022
Published 15 February 2022 Volume 2022:17 Pages 697—710
DOI https://doi.org/10.2147/IJN.S335774
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Farooq A. Shiekh
Taekwang Keum,1,2,* Gyubin Noh,1,2,* Jo-Eun Seo,1 Santosh Bashyal,1,2 Dong Hwan Sohn,1 Sangkil Lee1,2
1College of Pharmacy, Keimyung University, Daegu, Republic of Korea; 2Center for Forensic Pharmaceutical Science, Daegu, Republic of Korea
*These authors contributed equally to this work
Correspondence: Sangkil Lee, College of Pharmacy, Keimyung University, 1095 Dalgubeol-daero, Dalseo-gu, Daegu, 42601, Republic of Korea, Tel +82-53-580-6655, Fax +82-53-580-5164, Email [email protected]
Introduction: The buccal route has been considered an attractive alternative delivery route for injectable formulations. Cell-penetrating peptides (CPPs) are gaining increased attention for their cellular uptake and tissue permeation effects. This study was aimed to evaluate the in vitro and ex vivo permeation-enhancing effect of penetratin-conjugated liposomes for salmon calcitonin (sCT) in TR146 human buccal cells and porcine buccal tissues.
Methods: Penetratin was conjugated to phospholipids through a maleimide-thiol reaction. Liposomes were prepared and sCT was encapsulated using a thin-film hydration method. Physical properties such as particle size, zeta potential, encapsulation efficiency, and morphological images via transmission electron microscopy were obtained. Cellular uptake studies were conducted using flow cytometry (FACS) and confocal laser scanning microscopy (CLSM). A cell permeation study was performed using a Transwell® assay, and permeation through porcine buccal tissue was evaluated. The amount of sCT permeated was quantified using an ELISA kit and was optically observed using CLSM.
Results: The particle size of penetratin-conjugated liposomes was approximately 123.0 nm, their zeta potential was +29.6 mV, and their calcitonin encapsulation efficiency was 18.0%. In the cellular uptake study using FACS and CLSM, stronger fluorescence was observed in penetratin-conjugated liposomes compared with the solution containing free sCT and control liposomes. Likewise, the amount of sCT permeated from penetratin-conjugated liposomes was higher than that from the free sCT solution and control liposomes by 5.8-fold across TR146 cells and 91.5-fold across porcine buccal tissues.
Conclusion: Penetratin-conjugated liposomes are considered a good drug delivery strategy for sCT via the buccal route.
Keywords: buccal drug delivery, peptide delivery, penetratin, liposomes, TR146 cells, porcine buccal tissues
Introduction
Calcitonin is a hormone consisting of 32 amino acids long secreted from the C cells of the mammalian thyroid. This hormone was discovered by Copp et al, where they found that it lowers blood calcium levels.1 Calcitonin is an endogenous physiological hormone that inhibits bone resorption and is used as a treatment for diseases related to bone resorption, such as postmenopausal osteoporosis, to reduce the adherence of osteoclasts and osteoclast formation.2,3 Other types of synthetic or recombinant calcitonin, such as human calcitonin, porcine calcitonin, and salmon calcitonin (sCT), have been used for medical purposes. Among them, sCT has been the most widely used, as it has a 40–50-fold higher efficacy than human calcitonin.4–6
Oral administration is a drug delivery route preferred in clinical practice and is associated with high patient compliance.7 However, biopharmaceuticals such as sCT have a high molecular weight and hydrophilicity. There are many difficulties facing drug delivery through the gastrointestinal (GI) tract. Protease action, hydrolysis, and hepatic first-pass effects occur in the GI tract. To overcome such limitations, intravenous injection is usually used.8 As biopharmaceuticals are substances with short half-life, they are administered frequently. Hence, patient compliance is low due to needle phobia, pain caused by intravenous injection, and difficulties in self-administration.7,9,10 Various mucous membranes, such as the nasal, rectal, vaginal, ocular, pulmonary, and buccal membranes, are considered alternative drug delivery methods for the delivery of biopharmaceutical substances.10
In contrast to oral administration, alternative drug delivery methods offer a milder environment for the absorption of biopharmaceuticals. Compared to other non-oral administration routes, the buccal route may lead to high patient compliance due to its easy administration and lower incidence of irritation.8,9 The buccal route is abundant in blood vessels, and the administered drug can directly enter the jugular veins via the blood vessels, allowing its absorption into the systematic circulation. In emergencies, formulations can also be immediately removed,10 repetitive administration is possible, and the buccal tissues can quickly recover.11
Liposomes are artificial lipid bilayer vesicles made from non-toxic lipids and are biocompatible12 with their characteristics depending on their lipid composition.13 Liposomes can increase the permeation of incorporated drugs and simultaneously encapsulate both hydrophilic and hydrophobic drugs.14,15 It is possible to change their vesicle size, modify their surface properties (to be positively or negatively charged), and decorate them with various ligands such as antibodies, polymers, and cell-penetrating peptides.13,16,17 Various studies on buccal drug delivery using liposomes are currently underway. Liposomal buccal mucoadhesive films containing vitamin B6 were prepared to have improved stability,16 deformable liposomes containing sodium deoxycholate have been prepared to increase the bioavailability of insulin18 and the bioavailability of silymarin and rifampicin was increased through liposome encapsulation.19,20 Elastic liposomes containing bile salt increased the permeation of insulin in TR 146 cells and porcine buccal tissues.21,22
Cell-penetrating peptides (CPPs), which are short (less than 30 amino acids long) cationic, amphipathic peptides, have attracted attention in the past few decades as an effective cellular uptake tool.23,24 CPPs can deliver various cargo molecules (DNA, siRNAs, peptides, proteins, oligonucleotides, and nanoparticles) in vitro and in vivo with high efficiency and low toxicity.25,26 By using CPPs, surface modification and covalent and noncovalent bonding of cargo molecules have been done to deliver biopharmaceuticals.27 CPPs have also been considered a strategic choice to overcome the limitations of delivering high-molecular-weight and hydrophilic biopharmaceuticals through cell membranes and tissues. Among non-invasive routes, the delivery of biopharmaceuticals via the nasal, pulmonary, transdermal, and ocular pathways using CPPs has been actively studied. However, the delivery of biopharmaceuticals via the buccal route has been less studied than other delivery routes. A recent study reported that LMWP-conjugated insulin and a physical mixture of LMWP with insulin were effectively delivered via the buccal pathway.28 A physical mixture of Penetratin and sCT was evaluated in the buccal pathway. Penetratin increased the permeation of sCT by acting as an effective permeation enhancer.29
In this study, we designed penetratin-conjugated liposomes to enhance sCT penetration via the buccal route. The physical properties of typical liposomes and penetratin-conjugated liposomes were measured, imaging studies were performed using Alexa 647-sCT-incorporated liposomes via flow cytometry (FACS) and confocal laser scanning microscopy (CLSM), and the amount of sCT permeated in TR146 cells and buccal tissues were measured using an sCT ELISA kit.
Materials and Methods
Materials
Salmon calcitonin (sCT) was purchased from Bachem AG (Bubendorf, Switzerland). The peptide CRQIKIWFQNRRMKWKK (Cys-penetratin) was synthesized and purchased from Peptron Co., Ltd. (Daejeon, Republic of Korea). The lipid 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidophenyl) butyramide] (MPB-PE) was purchased from Avanti Polar Lipids (Alabaster, AL). L-α-lecithin (97.7% phosphatidylcholine) was purchased from Merck Millipore (Billerica, MA, USA). Tween 80® was purchased from Duksan (Ansan, Republic of Korea). An sCT ELISA kit was purchased from Phoenix Pharmaceuticals (Burlingame, CA, USA). A Ham-F-12 Nutrient mixture (Ham’s F-12) was purchased from Welgene (Gyeongsan-si, Republic of Korea). A CellTiter 96® Aqueous One Solution Cell Proliferation Assay kit (MTS) was purchased from Promega (Madison, WI, USA). Alexa Fluor 647 NHS Ester, SnakeSkinTM dialysis tubing (3.5 K MWCO), fetal bovine serum (FBS), 0.25% trypsin-EDTA, and penicillin/streptomycin were purchased from Thermo Fisher Scientific (Waltham, MA, USA). All other chemicals and solvents were of reagent grade.
Preparation of Liposomes
Preparation of Control Liposomes
Control liposomes were prepared using the film hydration method.13 Briefly, L-α-Lecithin and Tween 80® were blended at a molar ratio of 9:1 and dissolved in chloroform. An organic solution containing lipids was evaporated using a rotary evaporator. After purging with nitrogen to remove the residual organic solvent, the thin film was dried overnight. The dried thin film was rehydrated using 40 μg/mL of a sCT PBS solution (pH 7.4) at a final lipid concentration of 10 mg/mL. Liposomes were extruded using an Avanti Mini-Extruder with a 0.2-μm filter.
Conjugation of Penetratin and MPB-PE
Penetratin-conjugated liposomes were prepared using MPB-PE and Cys-penetratin. For the specific binding of penetratin and liposomes, Cys-penetratin with Cysteine added to the N-terminus of Penetratin and MPB-PE containing a Maleimide group were used. MPB-PE was conjugated with Cys-penetratin via a maleimide-thiol reaction.30,31 To produce MPB-PE liposomes, L-α-Lecithin, Tween 80®, and MPB-PE were mixed at a fixed molar ratio of 89:10:1 (MPB-PE was fixed at 121 μM) and dissolved in chloroform in a round flask. MPB-PE liposomes were prepared according to the method described in Section 2.2.1. For the thiol-maleimide reaction, Cys-penetratin was added to the MPB-PE liposomes, the pH was adjusted to 7.4, and then reacted for 12 h at 4 °C. The chemical structure and a schematic diagram of the maleimide-thiol reaction are shown in Figure 1.
 |
Figure 1 Schematic diagram of the chemical structure and the reaction of Cys-penetratin and MPB-PE used to prepare penetratin-conjugated liposomes. |
Characterization of Penetratin-Conjugated Liposomes
Size and Zeta Potential of the Liposomes
The size and zeta potential of the liposomal formulations were measured using ZetaPlus (Brookhaven Instruments Corp., Holtsville, NY, USA) after a 100-fold dilution with distilled water. The measurements were repeated three times.
Encapsulation Efficiency
The encapsulation efficiency of sCT in the liposomes was measured using a Beckman Coulter Optima LE-80K ultracentrifuge (Beckman Coulter Corp., Pasadena, CA). Liposomes were centrifuged at 100,000 × g and 4 °C for 1 h. The supernatant was collected and analyzed using an ELISA kit. The encapsulation efficiency was calculated using the following formula:
where T is total initial amount of sCT added and C is the amount of sCT in the supernatant.
Transmission Electron Microscopy
The morphology of the liposomes was determined via TEM (H-7600, HITACHI Ltd., Tokyo, Japan). Liposomes were negatively stained using 2% phosphotungstic acid and spread on a carbon-coated copper grid. The carbon-coated copper grid was then dried, and the samples were observed at 10,000× magnification.
TR146 Cell Culture
The TR146 cell line (ECACC 10032305) was purchased from Public Health England (London, UK). TR146 cells were incubated in Ham’s F-12 medium supplemented with 10% FBS, 2 mM glutamine, penicillin (10,000 units/mL), and streptomycin (10,000 μg/mL), and incubated at 37 °C in 5% CO2. The medium was replaced every 2–3 days. When the cultures reached 70–80% confluency, the cells were harvested for subculture using a 0.25% trypsin–EDTA solution.
Cytotoxicity Assay
Cell viability was determined by comparing with control (no treated group) using MTS assay. Briefly, 1.0×104 TR146 cells were seeded in a 96-well plate and incubated for 24 h. After 24 h, the media was removed and 100 μL of a liposome solution at different concentrations (10, 5, 2.5 and 1.25 mg/mL) were added and the cells were incubated for another 24 h. Then, the samples were removed and 100 μL of fresh culture media and 20 μL of CellTiter 96® solution were added and incubated with the cells for 2 h. The optical density was then measured at 490 nm using a microplate reader. Cell viability was calculated using the following formula:
where OD490 is the optical density at 490 nm.
In vitro Cellular Uptake Study
Preparation of Liposomes for the Cell Uptake Study
Alexa 647-sCT was synthesized for cellular uptake studies. To prepare this, sCT was dissolved in 0.1 M sodium bicarbonate buffer. Alexa 647 was dissolved in DMSO and allowed to react for 2 h with the sCT solution at room temperature. After 2 h, a large quantity of ammonium chloride was added to terminate the reaction, and the resulting solution was purified via dialysis. The synthesized Alexa 647-sCT was encapsulated into the control liposomes and penetratin-conjugated liposomes for subsequent imaging studies.
Flow Cytometry
A cellular uptake study was conducted using TR146 cells via flow cytometry. TR146 cells were seeded in a 6-well culture plate at a density of 5.0×105 cells per well and incubated for 24 h at 37 °C in an atmosphere of 5% CO2. Liposomes encapsulating Alexa 647-sCT were incubated for 2 h. After incubation, the cells were washed three times with DPBS, and 0.25% trypsin-EDTA was added for 10 min to harvest the cells. After obtaining the cells, they were collected in FACS buffer and analyzed immediately using FACS.
Confocal Laser Scanning Microscopy
CLSM was used to visualize the cellular uptake of the liposomes. Approximately 5.0×105 TR146 cells were seeded and incubated for 24 h at 37 °C in an atmosphere of 5% CO2. Alexa 647-sCT-loaded liposomes were then added to the cells and were incubated for 24 h. After incubation, the cells were washed three times with DPBS and fixed for 10 min in 4% formalin. DAPI (4’,6’-diamidino-2-phenylindole) staining was conducted and the cells were immediately observed using CLSM.
In vitro Cell Permeation Study
To observe the cell permeation efficacy of the liposomes, a cell permeation study using Transwell® inserts was conducted. TR 146 cells (5×104) were seeded in 12-well Transwell® inserts, and the media was changed every other day for 28 days. Culturing TR146 cells in Transwell® inserts for 3–4 weeks formed 4 to 7 layers of flattened cells and showed a constant TEER value.32 Aliquot of liposomes solution (500 μL) containing 40 μg/mL sCT were added to the apical chamber of the Transwell® insert for cell permeation study. After 0.5, 1, 2, 4, and 8 h, 500 μL sample was obtained from the basolateral chamber and the same amount of HBSS-HEPES buffer (pH 7.4) was added. The amount of sCT that permeated the TR146 cell layers was quantified using an sCT ELISA kit.
Pretreatment of Porcine Buccal Tissues
Buccal pretreatment was performed according to the experimental method of Oh et al.33 After sacrificing pigs at a slaughterhouse, fresh porcine buccal tissues were immediately obtained, and the fatty and connective tissue were removed. The collected tissues were placed in a PBS solution (pH 7.4) at 60 °C for 1 min to obtain the buccal epithelium. The prepared epithelial tissues were then used for the in vitro tissue permeation experiments.
Ex vivo Buccal Tissue Permeation Study
The permeation efficacy of the penetratin-conjugated liposomes was determined using porcine buccal tissues. The amount of sCT permeated was confirmed using a Franz diffusion cell. The area of the donor part was maintained at 2.0 cm2 and the volume of media in the receptor was 12.5 mL. The receptor was filled with PBS (pH 7.4) and was equilibrated for the next 30 min after mounting the buccal tissues. After 1 mL of each liposome solution was added to the donor, samples (0.5 mL) were obtained from the receptor after 1, 2, 4, and 8 h. The permeated sCT was analyzed using an sCT ELISA kit.
Permeation Parameters
The flux (Js) was calculated using the following formula:
where 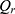 is the amount of sCT permeated (ng), A is the permeation area (
is the amount of sCT permeated (ng), A is the permeation area (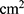 ), and t is the permeation time of sCT (h).
), and t is the permeation time of sCT (h).
Further, 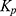 was calculated using the following formula:
was calculated using the following formula:
where Js is the flux (ng⋅cm−2⋅h−1) and Cd is the initial concentration in the donor chamber (ng⋅cm−3). Finally, the enhancement ratio (ER) was obtained by dividing the Kp value of each formulation with that of the control.
Confocal Laser Scanning Microscopy Imaging Study of Buccal Tissues
CLSM was used to observe the penetration profile of sCT through buccal tissues optically. Approximately 40 μg/mL Alexa 647-sCT and 1 mL each of the control liposomes encapsulating Alexa 647-sCT and penetratin-conjugated liposomes were added to the donor chambers of the Franz diffusion cells and were treated for 8 h. After 8 h, the buccal tissues were separated from the Franz diffusion cells. The isolated tissues were then frozen in OCT compound. After freezing, the tissues were cut into 12-μm segments using a cryostat microtome and fixed on slides. The Alexa 647-sCT-permeated tissues were then observed using CLSM.
Statistical Analysis
Statistical analysis was performed using Student’s t-test. Data are presented as means ± standard deviations (SDs). A single, double, or triple asterisk was used for all the data if the p-values were less than 0.05, 0.01, or 0.001, respectively.
Results
Physical Characterization of Liposome Formulations
Table 1 shows the physical characteristics of the control liposomes and the penetratin-conjugated liposomes. The particle size of the two liposomes was approximately 120 nm, which was also shown in the TEM images in Figure 2. The zeta potentials of the control liposomes and penetratin-conjugated liposomes were −15.7 mV and +29.6 mV, respectively. The encapsulation efficiencies were 27.1% and 18.0% for the control liposomes and the penetratin-conjugated liposomes, respectively.
 |
Table 1 Physical Characteristics of the Liposome Formulations |
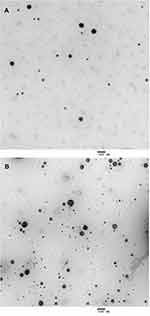 |
Figure 2 TEM images of the control liposomes (A) and the penetratin-conjugated liposomes (B). |
Cytotoxicity Assay
Cytotoxicity was determined by treating TR146 cells with various concentrations of liposomes (Figure 3). The cytotoxicity of each liposome was compared with control (no treated group). The control liposomes and penetratin-conjugated liposomes did not show cytotoxicity at concentrations below 2.5 mg/mL. However, as the liposome concentration increased, cytotoxicity also increased, and both liposomes showed cytotoxicity at concentrations of 10 and 5 mg/mL. Since the liposomes were not cytotoxic at 2.5 mg/mL, this concentration was used in the permeation and cell uptake studies.
 |
Figure 3 Cytotoxicity of various liposome concentrations in TR146 cells after 24 hours of incubation. Error bars represent SD (n = 5). ***p < 0.001 versus control (no treated group). |
In vitro Cell Uptake Study
The cellular uptake efficiency of the liposomes was investigated using relative mean fluorescence intensity and CLSM. Compared to the control liposomes, the fluorescence intensity of Alexa 647-sCT was higher in the penetratin-conjugated liposome-treated group (Figure 4). In the CLSM experiment, the intracellular uptake of Alexa 647-sCT was also higher in the cells treated with penetratin-conjugated liposomes (Figure 5).
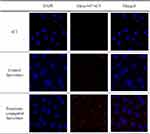 |
Figure 5 Confocal laser scanning microscopy (CLSM) images of the control liposomes containing Alexa 647-sCT and penetratin-conjugated liposomes in TR146 cells. |
In vitro TR146 Cell Permeation Study
TEER is a parameter that determines cell layer integrity. In this study, the maximum TEER value of the TR 146 cell layer cultured for 28 days was 69.07 Ω·cm2. In all the experimental groups, TEER recovery was > 90%. As shown in Table 2, there was no significant change in TEER values before and after the experiment. In the cell permeation study, the amount of sCT penetrating the cells from the penetratin-conjugated liposomes was 1.93 times higher compared to the control liposomes and 5.8 times higher than that of the free sCT solution (Figure 6 and Table 3).
 |
Table 2 TEER Values of the Formulations Before and After Permeability Experiments Using TR 146 Cell Layers |
 |
Figure 6 In vitro TR146 cell permeation profiles of sCT, control liposomes, and penetratin-conjugated liposomes. |
Ex vivo Buccal Tissue Permeation Study
A permeation study was conducted using porcine buccal tissue to investigate the permeation-enhancing effect of penetratin-conjugated liposomes. Each liposome group was used to treat buccal tissues for 8 h. The permeation profile at 8 h of the different liposomes is shown in Figure 7. The Js of sCT solution, control liposomes, and penetratin-conjugated liposomes after 8 h of permeation were 0.173, 6.384, and 15.802 ng·cm−2·h−1, respectively. In the penetratin-conjugated liposomes, the amount of sCT permeated increased 91.5-fold compared to the free sCT solution group and 2.47-fold compared to the control liposomes (Table 4).
 |
Table 3 Permeation Parameters Calculated from the TR146 Cell Permeation Study |
 |
Table 4 Permeation Parameters Calculated from the Buccal Tissue Permeation Study |
 |
Figure 7 Ex vivo buccal tissue permeation profiles of sCT, control liposomes, and penetratin-conjugated liposomes. |
CLSM of Buccal Tissues
CLSM was used to confirm the permeation-enhancing effect of the penetratin-conjugated liposomes in the buccal tissues. Liposomes containing Alexa 647-sCT were used to treat buccal tissues, which were observed after 8 h. The strongest fluorescence was observed from the tissues treated with penetratin-conjugated liposomes (Figure 8).
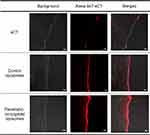 |
Figure 8 Confocal laser scanning microscopy (CLSM) images of the control liposomes containing Alexa 647-sCT and the penetratin-conjugated liposomes after permeation through the buccal tissue at 8 h. |
Discussion
CPPs have been widely applied to various cargo molecules in drug delivery systems for several years because of their ability to penetrate cell membranes. CPPs have also been successfully used to deliver biomacromolecules via alternative administration routes.34,35 In this study, penetartin-conjugated liposomes encapsulating sCT were prepared, and their effects in TR146 cells and buccal tissues were evaluated.
A maleimide-thiol reaction was used to prepare the penetratin-conjugated liposomes. The zeta potentials of the control liposomes and the penetratin-conjugated liposomes were −15.7 mV and +29.6 mV, respectively. This change was due to the presence of arginine and lysine residues, which are cationic amino acids present in penetratin, suggesting that penetratin was successfully conjugated to the surface of MPB-PE liposomes.30 The size of the penetratin-conjugated liposomes was 123.0 nm, with a PDI value of 0.151. The PDI value determines size homogeneity, which suggests that the prepared liposomes have good homogeneity. In lipid-based carriers such as liposomes, PDI values lower than 0.3 indicate homogeneous vesicle distribution.36,37
Figure 2 shows the cytotoxicity of the penetratin-conjugated liposomes at various lipid concentrations. Both liposomes showed no cytotoxicity at concentrations below 2.5 mg/mL but showed cytotoxicity above 5.0 mg/mL. Similar cytotoxicity results observed for the two liposomes indicated that the binding of penetratin did not affect liposome cytotoxicity.
Compared to the control liposomes, the fluorescence intensity of Alexa 647-sCT was higher in the penetratin-conjugated liposome-treated tissue. The increased fluorescence intensity of Alexa 647-sCT in the tissues treated with penetratin-conjugated liposomes was due to penetratin binding onto the liposome surface. The arginine residues in penetratin bind to negatively charged components on the cell membrane.38,39 Specifically, the guanidinium moieties of arginine bind to the sulfate and carboxylate moieties in GAGs and the phosphates of the phospholipid head group of the membrane lipids while the hydrophobic residue tryptophan is known as an important amino acid for internalization into cells.38,40,41 In addition, tryptophan is also known to induce lipid bilayers to form negative curvatures.42
The TEER value indicates the integrity of the cell layers and tight junctions.43 The recovery of the TEER values before and after the liposome formulation experiment was more than 90% without any significant decrease. Penetratin did not affect the TEER value of TR 146 cell layers. Similarly, treatment with penetratin-conjugated liposomes did not affect the TEER value, suggesting that it does not change the integrity of the cell layers and the tight junctions.21,43 The amount of sCT that penetrated the TR 146 cell layer was higher in the penetratin-conjugated liposomes than in the control liposomes, which indicates that penetratin conjugated to the liposomes enhanced cell permeation. Penetratin acted as a permeation enhancer in the Buccal pathway and effectively enhanced the permeation of sCT when physically mixed with sCT.29 Penetratin-conjugated liposomes also increased the amount of sCT permeation. This means that penetratin acts as an effective permeation enhancer in the physical mixture and conjugating to the liposome. The FACS and CLSM results supported that penetratin-conjugated liposomes enhanced permeation through transcellular routes. Likewise, penetratin-conjugated liposomes also had increased cell permeability compared to control liposomes in the porcine tissue permeation study (Figures 6 and 7). Results of the permeation experiments in TR 146 cells and porcine buccal tissues showed that penetratin-conjugated liposomes could enhance the permeation of sCT in cell layers and tissues. CLSM results revealed that penetratin-conjugated liposomes showed an increase in fluorescence intensity compared to control liposomes.
Conclusions
We designed penetratin-conjugated liposomes to deliver sCT through the buccal tissues. Flow cytometry and CLSM studies showed that penetratin-conjugated liposomes had an improved cellular sCT uptake effect. In addition, penetratin-conjugated liposomes showed enhanced permeation effect in vitro and ex vivo experiment model. Although further in vivo studies are needed to demonstrate the permeation mechanism of penetratin-conjugated liposomes, these studies fully demonstrate the potential of penetratin-conjugated liposomes as a platform for buccal delivery of peptide drugs.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Sciences, ICT, and Future Planning (NRF-2016R1D1A1B01015369 and NRF-2016R1A6A1A03011325).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Copp DH, Cameron EC. Demonstration of a hypocalcemic factor (calcitonin) in commercial parathyroid extract. Science. 1961;134(3495):2038. doi:10.1126/science.134.3495.2038.a
2. Chesnut CH
3. Chesnut CH
4. Calis S, Jeyanthi R, Tsai T, et al. Adsorption of salmon calcitonin to PLGA microspheres. Pharm Res. 1995;12(7):1072–1076. doi:10.1023/A:1016278902839
5. Guggi D, Kast CE, Bernkop-Schnürch A. In vivo evaluation of an oral salmon calcitonin-delivery system based on a thiolated chitosan carrier matrix. Pharm Res. 2003;20(12):1989–1994. doi:10.1023/B:PHAM.0000008047.82334.7d
6. Lee HE, Lee MJ, Park CR, et al. Preparation and characterization of salmon calcitonin-sodium triphosphate ionic complex for oral delivery. J Control Release. 2010;143(2):251–257. doi:10.1016/j.jconrel.2009.12.011
7. Junginger HE, Hoogstraate JA, Verhoef JC. Recent advances in buccal drug delivery and absorption–in vitro and in vivo studies. J Control Release. 1999;62(1–2):149–159. doi:10.1016/S0168-3659(99)00032-2
8. Veuillez F, Kalia YN, Jacques Y, et al. Factors and strategies for improving buccal absorption of peptides. Eur J Pharm Biopharm. 2001;51(2):93–109. doi:10.1016/S0939-6411(00)00144-2
9. Caon T, Jin L, Simões CMO, et al. Enhancing the buccal mucosal delivery of peptide and protein therapeutics. Pharm Res. 2015;32(1):1–21. doi:10.1007/s11095-014-1485-1
10. Senel S, Kremer M, Katalin N, et al. Delivery of bioactive peptides and proteins across oral (buccal) mucosa. Curr Pharm Biotechnol. 2001;2(2):175–186. doi:10.2174/1389201013378734
11. Salamat-Miller N, Chittchang M, Johnston TP. The use of mucoadhesive polymers in buccal drug delivery. Adv Drug Deliv Rev. 2005;57(11):1666–1691. doi:10.1016/j.addr.2005.07.003
12. Okafor NI, Nkanga CI, Walker RB, et al. Encapsulation and physicochemical evaluation of efavirenz in liposomes. J Pharm Investig. 2020;50(2):201–208. doi:10.1007/s40005-019-00458-8
13. Akbarzadeh A, Rezaei-Sadabady R, Davaran S, et al. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8(1):102. doi:10.1186/1556-276X-8-102
14. Kiio TM, Park S. Physical properties of nanoparticles do matter. J Pharm Investig. 2021;51(1):35–51. doi:10.1007/s40005-020-00504-w
15. Zeb A, Cha J-H, Noh AR, et al. Neuroprotective effects of carnosine-loaded elastic liposomes in cerebral ischemia rat model. J Pharm Investig. 2020;50(4):373–381. doi:10.1007/s40005-019-00462-y
16. Abd El Azim H, Nafee N, Ramadan A, et al. Liposomal buccal mucoadhesive film for improved delivery and permeation of water-soluble vitamins. Int J Pharm. 2015;488(1–2):78–85. doi:10.1016/j.ijpharm.2015.04.052
17. Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160. doi:10.1038/nrd1632
18. Yang TZ, Wang X-T, Yan X-Y, et al. Phospholipid deformable vesicles for buccal delivery of insulin. Chem Pharm Bull. 2002;50(6):749–753. doi:10.1248/cpb.50.749
19. El-Samaligy MS, Afifi NN, Mahmoud EA. Increasing bioavailability of silymarin using a buccal liposomal delivery system: preparation and experimental design investigation. Int J Pharm. 2006;308(1–2):140–148. doi:10.1016/j.ijpharm.2005.11.006
20. Lankalapalli S, Tenneti VS. Formulation and evaluation of rifampicin liposomes for buccal drug delivery. Curr Drug Deliv. 2016;13(7):1084–1099. doi:10.2174/1567201813666151221145617
21. Bashyal S, Seo J-E, Keum T, et al. Facilitated permeation of insulin across TR146 cells by cholic acid derivatives-modified elastic bilosomes. Int J Nanomed. 2018;13:5173–5186. doi:10.2147/IJN.S168310
22. Bashyal S, Seo J-E, Keum T, et al. Development, characterization, and ex vivo assessment of elastic liposomes for enhancing the buccal delivery of insulin. Pharmaceutics. 2021;13(4):565. doi:10.3390/pharmaceutics13040565
23. Kurrikoff K, Gestin M, Langel Ü. Recent in vivo advances in cell-penetrating peptide-assisted drug delivery. Expert Opin Drug Deliv. 2016;13(3):373–387. doi:10.1517/17425247.2016.1125879
24. Langel Ü. Handbook of Cell-Penetrating Peptides. CRC press; 2006.
25. Kim CH, Lee SG, Kang MJ, et al. Surface modification of lipid-based nanocarriers for cancer cell-specific drug targeting. J Pharm Investig. 2017;47(3):203–227. doi:10.1007/s40005-017-0329-5
26. Wang F, Wang Y, Zhang X, et al. Recent progress of cell-penetrating peptides as new carriers for intracellular cargo delivery. J Control Release. 2014;174:126–136. doi:10.1016/j.jconrel.2013.11.020
27. Gao H, Zhang Q, Yu Z, et al. Cell-penetrating peptide-based intelligent liposomal systems for enhanced drug delivery. Curr Pharm Biotechnol. 2014;15(3):210–219. doi:10.2174/1389201015666140617092552
28. Xu Y, Zhang X, Wang N, et al. Cell-penetrating peptide enhanced insulin buccal absorption. Int J Pharm. 2020;584:119469. doi:10.1016/j.ijpharm.2020.119469
29. Keum T, Noh G, Seo J-E, et al. In vitro and ex vivo evaluation of penetratin as a non-invasive permeation enhancer in the penetration of salmon calcitonin through TR146 buccal cells and porcine buccal tissues. Pharmaceuticals. 2020;13(11):408. doi:10.3390/ph13110408
30. Kwon SS, Kim SY, Kong BJ, et al. Cell penetrating peptide conjugated liposomes as transdermal delivery system of Polygonum aviculare L. extract. Int J Pharm. 2015;483(1–2):26–37. doi:10.1016/j.ijpharm.2015.01.030
31. Yang Y, Yang Y, Xie X, et al. PEGylated liposomes with NGR ligand and heat-activable cell-penetrating peptide-doxorubicin conjugate for tumor-specific therapy. Biomaterials. 2014;35(14):4368–4381. doi:10.1016/j.biomaterials.2014.01.076
32. Nielsen HM, Verhoef JC, Ponec M, et al. TR146 cells grown on filters as a model of human buccal epithelium: permeability of fluorescein isothiocyanate-labelled dextrans in the presence of sodium glycocholate. J Control Release. 1999;60(2–3):223–233. doi:10.1016/S0168-3659(99)00081-4
33. Oh DH, Chun K-H, Jeon S-O, et al. Enhanced transbuccal salmon calcitonin (sCT) delivery: effect of chemical enhancers and electrical assistance on in vitro sCT buccal permeation. Eur J Pharm Biopharm. 2011;79(2):357–363. doi:10.1016/j.ejpb.2011.05.010
34. Dietz GP, Bähr M. Delivery of bioactive molecules into the cell: the Trojan horse approach. Mol Cell Neurosci. 2004;27(2):85–131. doi:10.1016/j.mcn.2004.03.005
35. Tang J, Zhang L, Fu H, et al. A detachable coating of cholesterol-anchored PEG improves tumor targeting of cell-penetrating peptide-modified liposomes. Acta Pharm Sin B. 2014;4(1):67–73. doi:10.1016/j.apsb.2013.12.004
36. Chen M, Liu X, Fahr A. Skin penetration and deposition of carboxyfluorescein and temoporfin from different lipid vesicular systems: in vitro study with finite and infinite dosage application. Int J Pharm. 2011;408(1–2):223–234. doi:10.1016/j.ijpharm.2011.02.006
37. Danaei M, Dehghankhold M, Ataei S, et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10(2):57. doi:10.3390/pharmaceutics10020057
38. Rothbard JB, Jessop TC, Lewis RS, et al. Role of membrane potential and hydrogen bonding in the mechanism of translocation of guanidinium-rich peptides into cells. J Am Chem Soc. 2004;126(31):9506–9507. doi:10.1021/ja0482536
39. Walrant A, Bauzá A, Girardet C, et al. Ionpair-π interactions favor cell penetration of arginine/tryptophan-rich cell-penetrating peptides. Biochim Biophys Acta Biomembr. 2020;1862(2):183098. doi:10.1016/j.bbamem.2019.183098
40. Rothbard JB, Jessop TC, Wender PA. Adaptive translocation: the role of hydrogen bonding and membrane potential in the uptake of guanidinium-rich transporters into cells. Adv Drug Deliv Rev. 2005;57(4):495–504. doi:10.1016/j.addr.2004.10.003
41. Sakai N, Matile S. Anion-mediated transfer of polyarginine across liquid and bilayer membranes. J Am Chem Soc. 2003;125(47):14348–14356. doi:10.1021/ja037601l
42. Berlose JP, Convert O, Derossi D, et al. Conformational and associative behaviours of the third helix of antennapedia homeodomain in membrane-mimetic environments. Eur J Biochem. 1996;242(2):372–386. doi:10.1111/j.1432-1033.1996.0372r.x
43. Srinivasan B, Kolli AR, Esch MB, et al. TEER measurement techniques for in vitro barrier model systems. J Lab Autom. 2015;20(2):107–126. doi:10.1177/2211068214561025
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.