Back to Journals » Advances in Medical Education and Practice » Volume 9
Evolving role of pharmaceutical physicians in medical evidence and education
Authors Setia S , Ryan NJ, Nair PS , Ching E , Subramaniam K
Received 29 May 2018
Accepted for publication 6 September 2018
Published 2 November 2018 Volume 2018:9 Pages 777—790
DOI https://doi.org/10.2147/AMEP.S175683
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Md Anwarul Azim Majumder
Sajita Setia,1 Nicola J Ryan,2 Prasad S Nair,3 Elma Ching,3 Kannan Subramaniam4
1Medical Affairs, Pfizer Pte Ltd, Singapore; 2Independent Medical Writing Services, Auckland, New Zealand; 3Department of Pharmacy, National University of Singapore, Singapore; 4Global Medical Affairs, Asia-Pacific Region, Pfizer Australia, West Ryde, NSW, Australia
Abstract: The role of pharmaceutical physicians who are the experts working in pharmaceutical companies has progressed over the last few decades, from supervising research and development (R&D) studies and/or providing support to marketing teams to serving an independent critical function. In this review, we focus on pharmaceutical physicians serving medical affairs functions in the pharmaceutical industry. Historically, members of the medical affairs team mainly provided a bridge between commercial teams and the R&D sector and between the organization and external stakeholders. Such teams may even have been managed by other departments, with an emphasis on acquiring and generating data for regulatory purposes. In recent years, the role of medical affairs has broadened due to a change in focus and the increasingly stringent regulatory landscape. Strict regulations require the detachment of commercial from medical activities within pharmaceutical organizations. This change provides an opportunity for a different type of partnership, allowing scientifically minded and medically driven initiatives. This article summarizes the key role of pharmaceutical industry-based physicians in medical affairs and discusses the emerging and evolving role of medical affairs for value creation in evidence generation and medical education.
Keywords: medical affairs, medical education, pharmaceutical physicians, pharmaceutical industry
Role of medical affairs in the pharmaceutical industry
Internal: bridge between research and development (R&D) and commercial functions
As regulatory bodies increase scrutiny on the promotion of therapeutic products, the medical affairs department has played an important role in separating R&D and commercial functions to reduce the commercial influence on R&D efforts.1,2 Medical affairs serves as a liaison between the two functions, facilitating the transition of drugs from R&D to commercial.3 Although the marketing department designs and provides pharmaceutical sales representatives (PSRs) with visual aids to engage physicians, medical affairs play a vital role in approving and providing feedback on such information tools.4 Pharmaceutical industry-based physicians play an important role in ensuring that key messages are backed by sound scientific evidence and no misleading claims are made.5 The medical affairs department is also involved in training PSRs to be knowledgeable about the company’s products.6 This is relevant because one of the roles of physician–PSR interactions is to inform time-poor physicians about the latest therapeutic area and drug data, although these interactions generally tend to be more promotional.7
External: bridge between the organization and external stakeholders
Although PSRs have historically been considered valuable sources of medical information, their perceived importance to physicians has been steadily decreasing. Many physicians now choose to rely on other sources, such as manufacturer websites and peer-reviewed journals, for their information.8 This may have been one of the reasons why almost half of all new drug launches over the last 8 years have failed to fulfill their financial expectations.9 One approach used by pharmaceutical companies to address this underperformance has been to bolster their medical affairs teams.5 Given that evidence-based information is of high interest to physicians, medical affairs will play an important role in gathering and presenting scientific data alongside information about potential patient outcomes. There has also been a shift in focus from broad therapeutic areas covering large patient groups to therapy involving smaller populations, targeted therapy, and personalized medicine.10 This shift has placed more emphasis on the role of medical affairs in establishing connections with health care professionals and providing education about potential individualized therapeutic options.10 In essence, the external-facing role of medical affairs can be summarized using the medical affairs external communication cycle (Figure 1).10
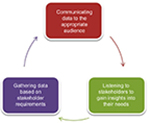  | Figure 1 Overview of medical affairs as a bridge between organization and external stakeholders. |
Companies are increasingly deploying Medical Science Liaisons (MSLs) in the field to communicate scientific information to key stakeholders, including physicians, and to capture relevant medical insights.11 The academic qualifications of MSLs range from medical and pharmacy degrees to PhDs, although most companies prefer postgraduate, doctorate, or specialist qualifications.12 Senior positions in medical affairs, eg, medical manager/medical director roles, generally require at least a basic medical degree and offer dynamic and challenging careers for physicians beyond patient interaction.13 Effective communication skills and commercial acumen as well as management and leadership skills are the key attributes required to be successful in these roles.13
Medical affairs may also be involved in organizing medical advisory boards, Phase IV clinical trials, preparation of medical responses to enquiries, and preapproved early drug access programs.6 Medical affairs departments also play an important function in providing responses to off-label enquiries, which must be handled independently of commercial teams.14
Medical advisory boards provide pharmaceutical companies with valuable insights on various topics, ranging from trial protocols to regulatory submissions.15 Advisory boards can be held at any stage during product development and facilitate the generation of data, the development of products, and the creation of trustworthy educational content.16 However, to make sure that advisory boards provide a return on investment, medical affairs teams need to ensure the optimal allocation of resources.17 This includes having a clear objective for the meeting, selecting the appropriate key opinion leaders (KOLs) or therapeutic area experts, ensuring adequate preparation, and facilitating the implementation of meeting action points.18
Early access programs (EAPs)
EAPs are one way in which pharmaceutical companies can facilitate access to newer, unapproved therapies for patients with rare and/or severe diseases in an ethical manner.19,20 Adoption of EAPs in pharmaceutical companies is becoming increasingly prevalent due to the variety of benefits of these programs.20 In Europe, there are two main types of EAPs: Compassionate Use Programs (CUPs) and Named Patient Programs (NPPs).21 In CUPs, patients in selected health care institutions with severe, debilitating, or life-threatening illnesses who cannot be treated adequately with existing medical treatments are granted access to compassionate (free of cost) use of preapproved drugs by pharmaceutical companies.21 In contrast, NPPs grant access to preapproved drugs to specific “named” patients in response to requests from physicians who ultimately bear the costs of these treatments.21 The emergence of EAPs means that there is a greater need for the mobilization of medical affairs teams to oversee their planning and execution.21 The importance of EAPs cannot be underestimated. For example, the WHO approved the provision of the investigational monoclonal antibody, ZMapp, to six Ebola patients for therapeutic purposes and to prevent the spread of the virus; four of the six patients were saved.20
Investigator-initiated research (IIR)
An IIR is a research led by an independent investigator and that is related to a pharmaceutical company’s area of interest.22 In IIR, the principal investigator is involved in the execution of the study and oversees its regulatory management; the latter is otherwise often the responsibility of pharmaceutical or biotechnology companies.22 However, the investigator may request support from pharmaceutical companies in the form of funding and/or product supply without the company assuming the responsibilities of a “sponsor”, as defined by the International Conference on Harmonization-Good Clinical Practice (ICH-GCP).22,23 Medical affairs teams within pharmaceutical companies are tasked with overseeing IIRs, with an increasingly large proportion of companies using MSLs to support IIR initiatives.24
Evolving role of medical affairs in generating medical evidence
Phase IV real-world clinical studies
Randomized controlled trials (RCTs) are the gold standard for the comparison of different clinical interventions.10,25 Systematic reviews and meta-analyses may also be conducted later to combine results from multiple studies in an effort to increase the statistical power of the results.26 However, RCTs are performed in clinical settings with stringent controls on many variables because they are designed to establish a causal relationship between an intervention and an outcome.25 In addition, RCTs may not be able to detect long-term adverse events.27 Therefore, there is some skepticism about the real-world clinical practice applicability of data from RCTs.
Real-world data (RWD) include information on patients’ health status and outcomes that are obtained regularly from sources such as electronic health records (EHRs) and disease registries. RWD can aid in identifying long-term adverse events and guide the development of specific guidelines in clinical practice.28 Obtaining RWD from pragmatic clinical trials and observational studies is important for demonstrating the “worth” of health care solutions to the various stakeholders.
Medical teams are also increasingly involved in the execution of postlaunch clinical trials, including Phase IV real-world studies.6 The scope of this work is still evolving but is currently the subject of increased attention and may allow the exploration of new questions such as potential new drug indications for smaller patient groups.29
Health economics and outcomes research
As the complexity of the medical landscape increases and the number of treatment options available to patients continues to expand, there is growing demand for the value of medications to be demonstrated.30 This emphasis on the “value” of health care solutions, rather than just the price, has put pressure on pharmaceutical companies to demonstrate the worth of their health care solutions in terms of cost-effectiveness as well as direct and indirect benefits.31 Thus, there is an increased focus on health economics and outcomes research (HEOR) that requires relevant data. Medical affairs, in collaboration with market access colleagues, may conduct HEOR to understand and communicate the value of products to external stakeholders, including Health Technology Assessment (HTA) bodies.31
HTA
There are a number of different HTA bodies around the world, and these are highly relevant to pharmaceutical companies (Figure 2).31–39 The role of HTA organizations is to enable efficient allocation of finite resources to meet the almost infinite demands for better medical technologies, including pharmaceuticals, medical devices, and other medical interventions.34,40
  | Figure 2 Worldwide Health Technology Assessment agencies. |
The HTA-driven global shift toward evidence-based value analysis has seen the expansion of the medical affairs function to consider HEOR aspects. Medical affairs plays an important role in ensuring that HTA requirements are met, sometimes even before the medical therapy has been approved for marketing.41,42 For example, the UK HTA agency, the National Institute for Health and Care Excellence (NICE), receives information about potential new therapies up to 20 months before they have been authorized to enter the market.42 This liaison with HTA bodies ensures that pharmaceutical companies are able to fulfill the criteria required to meet increasingly stringent HTA requirements. This is beneficial because earlier collaboration will decrease delays to market access for products, thereby allowing patients to have timely access to new therapies.
Scientific publications
Medical affairs teams are increasingly being involved in the communication of nonpromotional scientific content via a variety of channels, including scientific publications.3,43 Original articles (eg, from RWD, market research on educational needs, and practice gaps), review articles, pharmaceutical-independent consensus/expert opinions, and letters to editors are key types of publications authored or led by medical affairs personnel.
Several studies have highlighted gaps between actual practice and scientific evidence in the clinical setting.44–47 Narrowing of these gaps has proven beneficial in reducing patient morbidity and mortality and decreasing the cost of health care.48–51 Medical affairs professionals may be involved in identifying and communicating these gaps through cross-sectional research.52–55 They can also collaborate with health providers on narrative review manuscripts that address gaps in current clinical practice or educational needs.56–58 Identifying and communicating practice gaps could provide support to convince government health divisions to take action and update clinical practice guidelines.59
Addressing inconsistencies in trial results by combining data using systematic review and/or meta-analysis techniques is another important approach to facilitate better understating of clinical data.26 Medical affairs has an important, and currently under-utilized, role in leading these publications in collaboration with health providers.
In the absence of local guidelines, pharmaceutical-independent consensus/expert opinion publications play an important role in bridging existing knowledge and practice gaps.60 A “medical consensus” document is generally regarded as a credible and evidence-based publication by health professionals.61 Medical affairs teams can lead the publication of consensus papers (eg, based on medical advisory board meetings and clinical forums) to support the adoption of evidence-based practices in local clinical settings.62
However, it is extremely important for medical affairs personnel to adhere to good publication practice standards while leading such activities. A series of reporting guidelines has been developed and motivated primarily by concerns about the quality of publications (Table 1).63
Evolving role of medical affairs in medical and public education
Continuing medical education (CME) for health providers
CME is a cornerstone of current medical practice.64 Although CME assessments and regulations differ between countries, almost all require physicians to undergo some form of continuous education during their practice.65,66 In countries that mandate CME for physicians, regulatory institutions stipulate the expected standards and practices for such activities. Should a physician fail to meet these requirements, the consequences depend on the regulations of each specific country.66–68
Despite often being mandatory, the costs of attending CME events are usually high and can act as a deterrent to physician participation.69 It is therefore not surprising that commercial support contributes to a large percentage of CME funding.70 While the proportion of total commercial funding has decreased from more than half in 2006 to slightly above a quarter in 2016, it still makes a sizeable contribution ($704 million in 2016).71,72 This commercial funding is vital because many CME events could not proceed without financial backing.73 Pharmaceutical companies see this as a key opportunity to present the latest findings and to educate physicians about new products or even established therapeutic areas.74 Physicians benefit by gaining insight into medications and therapy options that may be useful for their clinical practice.75 However, it is important to note that there is a major difference between industry-led and industry-independent CME events. The former includes events where a pharmaceutical company has substantial influence over the presented content, and these are generally not allowed to contribute to CME credit in most developed countries.76 These events are also often perceived as marketing ventures by companies to introduce new drugs or studies to physicians.68
The pharmaceutical industry supports independent CME events through unrestricted educational grants and sponsorships.77 Most of these may still relate to education about a specific drug or treatment area of commercial relevance.78 Sponsorships differ from educational grants in that they generally provide direct, tangible benefits to the sponsor while grants have no strings attached.78 For example, sponsorship may be provided to subsidize the cost of entry to CME events for physicians, where permitted by law. In addition, conference sponsors are often able to set up booths and promote their brand through the placement of product logos, promotional materials, and handouts directly outside the event venue. In contrast, independent grants come with no requirement for the payer’s direct benefit.79,80 Many companies offer independent grants to a variety of official institutions.81–83 These include universities, medical schools, patient associations, and professional bodies.82,84 Grants are given with the goal of ensuring continuous improvement in health care provision and patient outcomes.85 The defining difference between independent grants and sponsorship is that grant recipients have full control over the content of their communications.86 This means that grant-supported events are less likely to be under industry influence and more likely to promote the communication of unbiased information. As a result, grant-funded events are often perceived as having greater credibility than sponsored events.87 In fact, many have suggested that industry grants should be overseen and managed by independent committees that would be responsible for decisions about which specific course or event this funding would be disseminated to.88–90
In the pharmaceutical industry, there has been a recent trend for responsibility for CME activities to be transferred from the marketing department to medical affairs.91 This reflects the shift in pharmaceutical strategy driven by stakeholder demand for credible, accurate, and evidence-based information. The growing role of medical affairs in the management of CME activities is an important step toward mitigating the “stigma” associated with industry-sponsored events and building trust between the pharmaceutical industry and its key stakeholders.
Public education
The internet has become one of the primary sources for consumers to access medical information.92,93 It provides a cost- and time-efficient avenue for seeking medical advice without having to consult a physician. One study found that about 35% of people who search for medical information online use this as a tool to self-diagnose their ailments.94 More alarmingly, over a third of these people do not double-check their self-diagnoses with a health care professional.94
While there are many credible sources of information on the internet,95 a large portion of online resources provide low quality information and can thus pose harm to consumers’ health.96,97 The widespread availability of noncredible medical information is a danger to society, and pharmaceutical companies have a civic duty to protect consumers by being sources of trustworthy information. The European Union (EU) took this into account when they decided to loosen the policy against allowing prescription medication information to be posted online by their manufacturing companies.98 In addition to posting information about their products online, pharmaceutical companies provide health education to the public and carry out real-world public health initiatives.99,100 The importance of medical affairs personnel in these ventures will only grow as more and more patients go online to source health and medical information.
Another pertinent issue is direct-to-consumer advertising (DTCA) of prescription medicines. This is prohibited in most countries, with notable exceptions being Hong Kong, New Zealand, and the USA.101–103 In the USA, the Food and Drug Administration (FDA) imposes restrictions on advertising and mandates that no misleading or misrepresented information is to be provided to consumers.104 While DTCA provides a potential avenue for pharmaceutical companies to educate the general public about their products, many also see a potential risk for public harm.105,106 In fact, the perceived risk is so high that in 2008, 22 of the 27 EU members voted to prohibit even the provision of limited information to consumers via DTCA.107 These fears are largely due to the perception that pharmaceutical companies do not consider public education as the main aim of their advertising but, instead, strive to gain a larger profit margin by acquiring more paying customers.108 As the importance of consumers as stakeholders increases (aptly demonstrated by the rising prominence of patient associations in Europe), medical affairs will have an ever-expanding role in ensuring that patient centricity is at the forefront of any initiative that their company undertakes.109
Collaborations with patient associations
More recently, companies have extended medical engagement activities to patient associations in order to fully leverage the valuable patient insights they can provide.6 The medical affairs function plays an important role in understanding and integrating patient perspectives into the organization. According to the US FDA, patient involvement in drug development is a priority and, therefore, medical affairs should aim to involve patients earlier in the drug development process to better facilitate the identification of meaningful clinical trial endpoints.110 Medical affairs departments also help to educate patients and health care professionals on the organization’s R&D pipeline and current clinical trials. In addition, reaching out to patients can increase the possibility of obtaining funding for orphan drugs from charitable patient organizations given the shift in focus to smaller therapeutic areas.111 Such engagements provide pharmaceutical companies with the opportunity to develop more scientific, accurate, and patient-centric programs to engage their key stakeholders, giving them a competitive edge in the changing pharmaceutical landscape.
Unique conflicts of interest (COIs) for medical affairs professionals
Medical affairs physicians themselves face many challenges in terms of COIs. These arise when a physician’s ability to make decisions in the best interests of their patients becomes affected by their relationship with the pharmaceutical company that employs them.112 Their position within the company is noncommercial in nature and could potentially conflict with marketing objectives.113 However, as both an employee of a company and a physician who has a duty to act in a patient’s best interest, a pharmaceutical physician needs to ensure that his/her obligations to both parties have been fulfilled.114 Therefore, it is important to understand and adhere to the key internal and external check systems that control the quality and credibility of internal compliance governance by medical affairs personnel and their external stakeholder interactions.
Control systems in pharmaceutical industry
In today’s ever-changing world, controls on pharmaceutical industry practices have to evolve to keep pace with its dynamic nature. The four main pillars of control systems are regarded to be internal company protocols, industry codes of practice, regulations, and laws.115 Table 2 summarizes the different control systems governing pharmaceutical communication around the world. These are of increasing importance to medical affairs departments given that they have an expanding role in communicating both directly and indirectly with physicians, patients, and other key stakeholders.
Laws and regulations
Laws and regulations relating to the communications between the industry and their stakeholders are governed by national and potentially even regional regulatory organizations. For example, member nations of the EU are required to follow European Medicines Agency (EMA) laws.116 However, individual nations within the EU may also impose additional local laws or regulations that they deem necessary to meet the main directives set by the EMA.117
Local laws and regulations are upheld by the regulatory bodies of individual countries. Both the laws and their enforcement can vary from country to country. For example, the advertising of medicines is regulated by the FDA in the United States and by the Therapeutic Goods Administration in Australia.104,118 Furthermore, laws that govern other commercial entities within the country also apply to pharmaceutical companies. For example, the Foreign Corrupt Practices Act in the USA and the Bribery Act in the UK affect the commercial behavior of local companies’ subsidiaries that are abroad.119,120 Laws and regulations in individual countries can differ greatly, as seen in the above example of DTCA. Relevant laws are usually taken as the basis for which industry and internal policies or guidelines are enacted, although these policies can often be more comprehensive than the rules and regulations of governmental agencies.115
Industry codes
Local trade associations specific to the pharmaceutical industry have been established all over the world and fall under the jurisdiction of the International Federation of Pharmaceutical Manufacturers & Associations (IFPMA).121 Members of the IFPMA adhere to a strict set of regulatory measures that ensure high standards of quality and promote ethical practices among pharmaceutical companies.122 Examples of member associations include the Pharmaceutical Research and Manufacturers of America in the USA, the Association of the British Pharmaceutical Industry in the UK, the Singapore Association of Pharmaceutical Industries, Medicines Australia, and the European Federation of Pharmaceutical Industries and Associations, which represents EU nations.123 These member associations determine regulations based on the IFPMA Codes of Practice. All member associations and companies implement these codes. In particular, the IFPMA regulates companies’ interactions with health care professionals, medical institutions, and patient organizations.124
Internal policies
International pharmaceutical companies have a set of global internal policies that govern their communications with stakeholders worldwide.125–127 These are the first point of reference because employees are expected to adhere to company policies by following industry-, national-, or international-level regulations.128,129 These policies often incorporate higher-level regulations and are, thus, more stringent and detailed.115
Additional control systems for medical affairs
The goal of the medical affairs department is to communicate medical data about products with a high level of integrity, so they are seen by key stakeholders and the general public as a credible and transparent source of medical information.6 This could contradict with the promotion-minded approach of marketing colleagues and needs to be managed effectively because both departments have to work together to ethically and informatively educate physicians about their products.113 In order to achieve this balance, there has to be a clear separation between medical affairs and commercial functions. Approaches such as having separate reporting structures (ie, medical affairs does not report to commercial) and separating the funding of both departments (ie, medical affairs does not rely on commercial sales for their funding) help to ensure that medical affairs remains independent of commercial pressures and is able to focus on working in an ethical and patient-centric manner.130 Medical affairs physicians needed to be commercially independent and be able to function without consideration of commercial objectives.
Continuous professional development for medical affairs professionals
The rapidly changing pharmaceutical industry and the health care sector landscape have increased the importance of medical affairs in pharmaceutical companies. This is reflected by the growing proportion of clinicians entering the pharmaceutical industry,131 with some companies viewing a specialty medical qualification, a PhD, and/or a business-related qualification such as an MBA as helpful additions to a primary medical qualification.131 This shift calls for medical affairs personnel to undertake their own continuing education to address knowledge or experience gaps to be able to respond effectively and efficiently to the dynamic nature of the industry. While traditional roles such as MSL interaction with stakeholders and publishing scientific material remain important, several evolving responsibilities (such as pharmacoeconomic considerations, communicating with patient associations, and planning for prelaunch obligations) are gaining increasing prominence. Pharmaceutical industry physicians need to be trained in these important aspects of medical affairs, necessitating participation in robust training to prepare for the dynamic nature of their roles. To meet these challenges, many educational programs are in place for pharmaceutical physicians to enhance career progression and prepare for their increasingly complex roles.131–133
One example is the International Federation of Associations of Pharmaceutical Physicians and Pharmaceutical Medicine (IFAPP)-Kings College Medical Affairs in Medicines Development Certification Program. This year-long e-learning program was designed by the IFAPP academy in collaboration with industry, professional association, and academic personnel.134,135 The aim of the program is to enhance the skills of medical affairs professionals who have at least a year of experience working in their specific function.135,136 The Duke-National University of Singapore (NUS) Medical School in Singapore also offers the Joint Alliance Duke-NUS Education (JADE).133 JADE provides a similar e-learning platform to the IFAPP-Kings College Program and is designed to develop medical affairs professionals to suit the evolving needs of their roles.133 This program caters not only to medical affairs professionals but also to health care professionals who are keen to develop a career in medical affairs.133
Although the modular, e-learning model is popular in today’s internet age, many medical affairs professionals have limited time to pursue such ventures. Thus, programs offered in the form of short, traditional lecture models do have a role. The Center for Executive Leadership for the Pharmaceutical Industry (C.E.L.forpharma) offers short workshops (1–2 days) with expert speakers and educators.132 These programs are specifically for the betterment of medical affairs executives, enable personnel to develop new skills, and stay relevant in a changing environment.132
The Pharmaceutical Medicine Specialty Training in the UK offers a more intensive, selective, and rigorous program. This 4-year training program for pharmaceutical physicians is overseen by the Joint Royal Colleges of Physicians Training Board and offers physicians the opportunity to be listed on the General Medical Council’s specialist register for pharmaceutical medicine. This program is specifically offered to upgrade licensed medical doctors with positions within the pharmaceutical industry and is more selective than most other programs on offer.131
Conclusion
Pharmaceutical companies are allocating more resources to the medical affairs function in order to increase new product development and postlaunch execution activities. This is vital in addressing the need to improve R&D productivity within the scrutiny of ever-increasing HTA standards and the tightening of laws and regulations globally. The traditional role of pharmaceutical physicians in the medical affairs function is evolving well beyond the usual support to internal commercial functions and collaboration with KOLs. In the future, the focus of medical affairs may be on generating and communicating medical evidence, leading medical education, and empowering patient associations. Increasing responsibility for various facets of the industry, such as CME, public health initiatives, and HEOR, make the field of medical affairs a dynamic venture. A career in medical affairs may offer the right niche, progression, and fulfillment to many health professionals, including physicians, by enabling them to challenge global unmet needs in medical and health communication and education in this exciting and expanding field, with the ultimate goal of improving patient care and outcomes.
Acknowledgments
The authors would like to thank Ms Sonia Menezes, consultant, scientific communications, Pfizer, for her editorial support for this manuscript and Dr Chandrashekhar N Potkar, regional medical affairs lead, Pfizer, for his valuable comments during design of the manuscript. This publication contains personal views and opinions of the authors, and no inference should be derived related to their current or previous employers. Pfizer Pte Ltd paid the publishing fee for this paper.
Author contributions
All authors were involved in conception, design, analysis, and interpretation of data. All authors were also involved in the preparation of the manuscript, revising it for scientific content, final approval before its submission for publication, gave final approval of the manuscript version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Dr Sajita Setia was an employee of Pfizer Pte Ltd at the time of submission of this manuscript and Dr Kannan Subramaniam is an employee of Pfizer Australia. Mr Prasad S Nair and Ms Elma Ching underwent indirect patient care pharmacy training for 3 months at Pfizer, Singapore. The authors report no other conflicts of interest in this work.
References
PharmaForum. Medical Affairs – the heart of a data-driven, patient-centric pharma; 2017. Available from: https://pharmaphorum.com/views-and-analysis/medical-affairs-heart-data-driven-patient-centric-pharma/. Accessed April 28, 2018. | ||
Jain S. Bridging the Gap Between R&D and commercialization in pharmaceutical industry: role of medical affairs and medical communications. Int J Biomed Sci. 2017;3(3):44–49. | ||
Grom T. Medical affairs: beyond the science. Showcase feature; 2013. Available from: http://www.pharmavoice.com/article/medical-affairs-beyond-the-science/. Accessed April 24, 2018. | ||
Rollins BL, Perri M. Pharmaceutical Marketing. Burlington: Jones & Bartlett Learning; 2014. | ||
Plantevin L, Schlegel C, Gordian M. Reinventing the Role of Medical Affairs; 2017. Available from: http://www.bain.com/publications/articles/reinventing-the-role-of-medical-affairs.aspx. Accessed April 24, 2018. | ||
Evers M, Fleming E, Ghatak A. Pharma Medical Affairs: 2020 and beyond. New York: McKinsey & Company; 2014. | ||
Patwardhan AR. Physicians-pharmaceutical sales representatives interactions and conflict of interest: challenges and solutions. Inquiry. 2016;53:0046958016667597. | ||
van Bisen T, Weisbrod J, Brookshire M, Coffman J, Pasternak A. Front Line of Healthcare Report 2017: Why involving doctors can help improve US healthcare; 2017. Available from: http://www.bain.com/publications/articles/front-line-of-healthcare-report-2017.aspx. Accessed April 24, 2018. | ||
Natanek R, Schlegel C, Retterath M, Eliades G. How to Make Your Drug Launch a Success. Available from: https://www.bain.com/insights/how-to-make-your-drug-launch-a-success/. Accessed April 26, 2018. | ||
FirstWorld. The Future of Medical Affairs; 2016. Available from: http://www.fwreports.com/dossier/the-future-of-medical-affairs/#.W8UdbGgzY2w. Accessed October 18, 2018. | ||
Rutherford P, Smith N.J. Medical Science Liaisons: A Key to Driving Patient Access to New Therapies. QuintilesIMS 2016. Available from: https://www.iqvia.com/-/media/library/white-papers/medical-science-liaisons.pdf. Accessed April 26, 2018. | ||
Medical Science Liaison Society. What Is a Medical Science Liaison? 2018. Available from: http://www.themsls.org/what-is-an-msl/. Accessed April 26, 2018. | ||
Kausa IS. A day in the life of a pharmaceutical physician; 2007. Available from: http://careers.bmj.com/careers/advice/view-article.html?id=2461. Accessed April 24, 2018. | ||
International Federation of Pharmaceutical Manufacturers & Associations. IFPMA Code of Practice Geneva, Switzerland: International Federation of Pharmaceutical Manufacturers & Associations; 2012. Available from: https://www.ifpma.org/subtopics/code-of-practice-2/. Accessed April 26, 2018. | ||
Presson J. Advisory Board Functions Expanding in Pharma Industry; 2018. Available from: https://www.cuttingedgeinfo.com/2013/advisory-board-functions-expanding-pharma-industry/. Accessed April 24, 2018. | ||
Evens R. Drug and Biological Development: From Molecule to Product and Beyond. Verlag: Springer; 2007:240–274. | ||
Best Practices. New Study Presents Insights to Advisory Board Effectiveness in the Pharmaceutical Sector. Available from: https://www.prnewswire.com/news-releases/new-study-presents-insights-to-advisory-board-effectiveness-in-the-pharmaceutical-sector-300076091.html. Accessed April 24, 2018. | ||
American Express Open Access. 10 Steps to forming an effective advisory board; 2011. Available from: https://www.americanexpress.com/us/small-business/openforum/articles/10-steps-to-forming-an-effective-advisory-board-1/. Accessed April 24, 2018. | ||
Balasubramanian G, Morampudi S, Chhabra P, Gowda A, Zomorodi B, Arun Gowda BZ. An overview of Compassionate Use Programs in the European Union member states. Intractable Rare Dis Res. 2016;5(4):244–254. | ||
Patil S. Early access programs: benefits, challenges, and key considerations for successful implementation. Perspect Clin Res. 2016;7(1):4–8. | ||
Yazdani M, Boggi F. Initiating Early Access Programs: 5 Things to Consider; 2018. Available from: http://www.executiveinsight.ch/early_access_programs. Accessed April 24, 2018. | ||
Berro M, Burnett BK, Fromell GJ, et al. Support for investigator-initiated clinical research involving investigational drugs or devices: the Clinical and Translational Science Award experience. Acad Med. 2011;86(2):217–223. | ||
Suvarna V. Investigator initiated trials (IITs). Perspect Clin Res. 2012;3(4):119–121. | ||
Cutting Edge Information. Medical Science Liaisons Aim to Support Investigator Initiated Trials; 2012. Available from: https://www.businesswire.com/news/home/20121016005277/en/Medical-Science-Liaisons-Aim-Support-Investigator-Initiated. Accessed April 24 2018. | ||
Sullivan GM. Getting off the “gold standard”: randomized controlled trials and education research. J Grad Med Educ. 2011;3(3):285–289. | ||
Haidich AB. Meta-analysis in medical research. Hippokratia. 2010;14(Suppl 1):29–37. | ||
Chan EW, Liu KQ, Chui CS, Sing CW, Wong LY, Wong IC. Adverse drug reactions: examples of detection of rare events using databases. Br J Clin Pharmacol. 2015;80(4):855–861. | ||
McKesson. Real-World Evidence from Electronic Health Record Data; 2017. Available from: http://www.mckesson.com/blog/real-world-evidence-from-electronic-health-record-data/. Accessed April 24, 2018. | ||
Grudzinskas C. Atkinson J. Design of Clinical Development Programs. In: Arthur J. Atkinson J, Abernethy Darrell R, Daniels Charles E, Dedrick Robert L, Markey Sanford P, editors. Principles of Clinical Pharmacology. 2nd ed. 2007:501–517. | ||
Dekoven M, Hazard EH, Goldberg E, Pokras S. Got value? Determine it. Demonstrate it. Communicate it. Realise it. J Commerc Biotechnol. 2008;14(4):299–306. | ||
Canadian Agency for Drugs and Technologies in Health. What Does the Evidence Say? 2018. Available from: https://www.cadth.ca/about-cadth. Accessed April 24, 2018. | ||
ISPOR. Directory of Health Technology Assessment Organizations Worldwide; 2018. Available from: https://www.ispor.org/HTADirectory/Index.aspx. Accessed April 26, 2018. | ||
INHATA. INAHTA Members List; 2018. Available from: http://www.inahta.org/members/members_list/. Accessed April 24, 2018. | ||
European Federation of Pharmaceutical Industries and Associations. The Use of Health Technology Assessments to Evaluate Medicines; 2005. Available from: https://www.efpia.eu/media/25170/the-use-of-health-technology-assessments-hta-to-evaluate-medicines-key-principles-2007.pdf. Accessed April 24, 2018. | ||
Ministry of Health Singapore. About Us; 2018. Available from: https://www.moh.gov.sg/content/dam/moh_web/ACE-HTA/about-us.html#who-we-are. Accessed April 24, 2018. | ||
Shiroiwa T, Fukuda T, Ikeda S, Takura T. New decision-making processes for the pricing of health technologies in Japan: The FY 2016/2017 pilot phase for the introduction of economic evaluations. Health Policy. 2017;121(8):836–841. | ||
Barber JM, Sheehy KP. Uptake of new medicines in New Zealand: evidence of a waiting list. N Z Med J. 2015;128(1412):10–20. | ||
European Network for Health Technology Assessment. EUnetHTA and the HTA Network; 2018. Available from: https://www.eunethta.eu/about-eunethta/our-network/. Accessed April 26, 2018. | ||
Brockis E, Marsden G, Cole A, Devlin N. A Review of NICE Methods Across Health Technology Assessment Programmes: Differences, Justifications and Implications: Office of Health Economics; 2016. https://www.ohe.org/publications/review-nice-methods-across-health-technology-assessment-programmes-differences. Accessed September 28, 2018. | ||
Pichon-Riviere A, Augustovski F, Rubinstein A, Martí SG, Sullivan SD, Drummond MF. Health technology assessment for resource allocation decisions: are key principles relevant for Latin America? Int J Technol Assess Health Care. 2010;26(4):421–427. | ||
PMLive. Novo Nordisk’s Klaus Henning Jensen on trends in medical affairs; 2018. Available from: http://www.pmlive.com/pharma_thought_leadership/novo_nordisks_klaus_henning_jensen_on_trends_in_medical_affairs_607002. Accessed April 24, 2018. | ||
Ciani O, Jommi C. The role of health technology assessment bodies in shaping drug development. Drug Des Devel Ther. 2014;8:2273–2281. | ||
PCC. Medical Affairs Compliance: Analyze the Involvement of Medical Affairs in Commercial Activities; 2017. Available from: http://www.cbinet.com/sites/default/files/files/Connor_Brian_Pres.pdf. Accessed April 26, 2018. | ||
Grol R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care. 2001;39(8 Suppl 2):46–54. | ||
Cochrane LJ, Olson CA, Murray S, Dupuis M, Tooman T, Hayes S. Gaps between knowing and doing: understanding and assessing the barriers to optimal health care. J Contin Educ Health Prof. 2007;27(2):94–102. | ||
Buchan H. Gaps between best evidence and practice: causes for concern. Med J Aust. 2004;180(6 Suppl):S48. | ||
Mcglynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635–2645. | ||
Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172. | ||
Komajda M, Lapuerta P, Hermans N, et al. Adherence to guidelines is a predictor of outcome in chronic heart failure: the MAHLER survey. Eur Heart J. 2005;26(16):1653–1659. | ||
Mccullough ML, Patel AV, Kushi LH, et al. Following cancer prevention guidelines reduces risk of cancer, cardiovascular disease, and all-cause mortality. Cancer Epidemiol Biomarkers Prev. 2011;20(6):1089–1097. | ||
Shapiro DW, Lasker RD, Bindman AB, Lee PR. Containing costs while improving quality of care: the role of profiling and practice guidelines. Annu Rev Public Health. 1993;14:219–241. | ||
Setia S, Subramaniam K, Tay JC, Teo BW. Hypertension and blood pressure variability management practices among physicians in Singapore. Vasc Health Risk Manag. 2017;13:275–285. | ||
Setia S, Subramaniam K, Teo BW, Tay JC. Ambulatory and home blood pressure monitoring: gaps between clinical guidelines and clinical practice in Singapore. Int J Gen Med. 2017;10:189–197. | ||
Setia S, Fung SS, Waters DD. Doctors’ knowledge, attitudes, and compliance with 2013 ACC/AHA guidelines for prevention of atherosclerotic cardiovascular disease in Singapore. Vasc Health Risk Manag. 2015;11:303–310. | ||
National Institute of Clinical Studies. Evidence-Practice Gaps Report Volume 2; 2005. Available from: https://www.nhmrc.gov.au/guidelines-publications/nic47. Accessed April 25 2018. | ||
Gwee KA, Goh V, Lima G, Setia S. Coprescribing proton-pump inhibitors with nonsteroidal anti-inflammatory drugs: risks versus benefits. J Pain Res. 2018;11:361–374. | ||
Lansberg P, Lee A, Lee ZV, Subramaniam K, Setia S. Nonadherence to statins: individualized intervention strategies outside the pill box. Vasc Health Risk Manag. 2018;14:91–102. | ||
Chadachan VM, Ye MT, Tay JC, Subramaniam K, Setia S. Understanding short-term blood-pressure-variability phenotypes: from concept to clinical practice. Int J Gen Med. 2018;11:241–254. | ||
Shekelle P, Eccles MP, Grimshaw JM, Woolf SH. When should clinical guidelines be updated? BMJ. 2001;323(7305):155–157. | ||
Joshi C. Consensus Guidelines: A Tool for Establishing Evidence-Based Practices; 2017. Available from: https://www.sciformix.com/medical-affairs-blog/consensus-guidelines-tool-establishing-evidence-based-practice/. Accessed April 25, 2018. | ||
Council of Europe. Developing a Methodology for Drawing Up Guidelines on Best Medical Practices; 2001. Available from: https://www.leitlinien.de/mdb/edocs/pdf/literatur/coe-rec-2001-13.pdf. Accessed April 25, 2018. | ||
Marie-Odile F. Medical affairs writing: a key role to relay medical information to everyone. Medical Writing. 2016;25(2):6–8. | ||
Johansen M, Thomsen SF. Guidelines for reporting medical research: a critical appraisal. Int Sch Res Notices. 2016;1346026. | ||
Varetto T, Costa DC. Continuing Medical Education Committee and UEMS-EACCME. Eur J Nucl Med Mol Imaging. 2013;40(3):470–474. | ||
World Health Organization (SEAR). Regional Guidelines for Continuing Medical Education (CME)/Continuing Professional Development (CPD) Activities; 2010. Available from: http://apps.searo.who.int/PDS_DOCS/B4489.pdf. Accessed April 25, 2018. | ||
Murgatroyd GB. Continuing Professional Development: The International Perspective; 2011. Available from: http://www.gmc-uk.org/CPD___The_International_Perspective_Jul_11.pdf_44810902.pdf. Accessed April 25, 2018. | ||
Mcleod PJ, Mcleod AH. If formal CME is ineffective, why do physicians still participate? Med Teach. 2004;26(2):184–186. | ||
Delsignore JL. Current guidelines regarding industry-sponsored continuing medical education. Clin Orthop Relat Res. 2003;412(412):21–27. | ||
Venkataraman R, Ranganathan L, Ponnish AS, Abraham BK, Ramakrishnan N. Funding sources for continuing medical education: An observational study. Indian J Crit Care Med. 2014;18(8):513–517. | ||
Steinbrook R. Financial support of continuing medical education. JAMA. 2008;299(9):1060–1062. | ||
ACCME. Annual Report Data. Accreditation Council for Continuing Medical Education; 2006. Available from: http://www.accme.org/sites/default/files/null/395_2006_Annual_report_2006_20070706.pdf. Accessed April 26, 2018. | ||
ACCME. ACCME Data Report: Growth and Evolution in Continuing Medical Education; 2016. Available from: http://www.accme.org/news-releases/accme-data-report-shows-growth-and-evolution-accredited-continuing-medical-education. Accessed April 25, 2018. | ||
Wieting MW, Mevis H, Zuckerman JD. The role of industry in Internet education. Clin Orthop Relat Res. 2003;412(412):28–32. | ||
Wilson FS. Continuing medical education: ethical collaboration between sponsor and industry. Clin Orthop Relat Res. 2003;412(412):33–37. | ||
Holmer AF. Industry strongly supports continuing medical education. JAMA. 2001;285(15):2012–2014. | ||
US Department of Health and Human Services. Final Guidance on Industry-Supported Scientific and Educational Activities. Food and Drug Administration; 1997. Available from: https://www.gpo.gov/fdsys/pkg/FR-1997-12-03/pdf/97-31741.pdf. Accessed April 26, 2018. | ||
Mehta PN. Drugmakers and continuing medical education. Indian Pediatr. 2000;37(6):626–630. | ||
American College of Emergency Physicians. Grants and Sponsorships; 2016. Available from: https://www.acep.org/Content.aspx?id=42670#sm.0001nd5f2nup5ebxuh32804wind6b. Accessed April 25, 2018. | ||
Pfizer. Transparency in Grants; 2018. Available from: https://www.pfizer.com/purpose/medical-grants/grant-transparency. Accessed April 25, 2018. | ||
Celgene Corporation. Grant Application Requirements; 2018. Available from: http://www.celgene.com/research-development/celgene-medical-affairs/educational-grant-requests/grant-application-requirements/. Accessed April 25, 2018. | ||
Boehringer Ingelheim GmbH. Applying for an Independent Medical Education Grant; 2018. Available from: https://www.boehringer-ingelheim.us/funding-opportunities/medical-education-grants/applying-grant. Accessed April 25, 2018. | ||
Pfizer. Descriptions of Funding Types and Recipient Organizations; 2018. Available from: https://www.pfizer.com/responsibility/grants_contributions/description_funding_recipient_organizations. Accessed April 25, 2018. | ||
Mallinckrodt Pharmaceuticals. Independent Medical Education Grants. Available from: http://www.mallinckrodt.com/research/medical_affairs/independent_medical_education. Accessed April 25, 2018. | ||
Janssen Scientific Affairs. Requirements for Grants Requests; 2018. Available from: https://www.janssenime.com/requirements-for-grant-requests. Accessed April 25, 2018. | ||
Pfizer. Organizations Eligible to Apply Directly for Grants and Types of Initiatives Supported; 2018. Available from: http://www.pfizer.com/files/IGLC_OrganizationEligibility_effJuly2015.pdf. Accessed April 25, 2018. | ||
Boston Scientific Corporation. Independent Medical Education Program Grants; 2018. Available from: http://www.bostonscientific.com/en-US/corporate-citizenship/giving/grants-donations/request-types/educational-conference.html. Accessed April 25, 2018. | ||
ESC Board. The future of continuing medical education: the roles of medical professional societies and the health care industry: position paper prepared with contributions from the European Society of Cardiology Committees for Advocacy, Education and Industry Relations, Endorsed by the Board of the European Society of Cardiology. Eur Heart J. 2018. | ||
Morris L, Taitsman JK. The agenda for continuing medical education: limiting industry’s influence. N Engl J Med. 2009;361(25):2478–2482. | ||
Rothman DJ, Mcdonald WJ, Berkowitz CD, et al. Professional medical associations and their relationships with industry: a proposal for controlling conflict of interest. JAMA. 2009;301(13):1367–1372. | ||
Brennan TA, Rothman DJ, Blank L, et al. Health industry practices that create conflicts of interest: a policy proposal for academic medical centers. JAMA. 2006;295(4):429–433. | ||
Practices B. Continuing Medical Education: Trends in Structure and Functional Management; 2018. Available from: https://www.best-in-class.com/bestp/domrep.nsf/products/continuing-medical-education-trends-in-structure-and-functional-management?opendocument. Accessed April 25, 2018. | ||
Hesse BW, Nelson DE, Kreps GL, et al. Trust and sources of health information: the impact of the Internet and its implications for health care providers: findings from the first Health Information National Trends Survey. Arch Intern Med. 2005;165(22):2618–2624. | ||
Eysenbach G, Kohler C. What is the prevalence of health-related searches on the World Wide Web? Qualitative and quantitative analysis of search engine queries on the Internet. AMIA Annu Symp Proc. 2003;2003:225–229. | ||
Fox S, Duggan M. Health Online; 2013. Available from: http://www.pewinternet.org/2013/01/15/health-online-2013/. Accessed April 25, 2018. | ||
Cline RJ, Haynes KM. Consumer health information seeking on the Internet: the state of the art. Health Educ Res. 2001;16(6):671–692. | ||
Doupi P, van der Lei J. Rx medication information for the public and the WWW: quality issues. Med Inform Internet Med. 1999;24(3):171–179. | ||
Latthe M, Latthe PM, Charlton R. Quality of information on emergency contraception on the Internet. Br J Fam Plann. 2000;26(1):39–43. | ||
Directorate-General for Health and Food Safety. Information to Patients: Legislative Approach; 2018. Available from: https://ec.europa.eu/health/human-use/information-to-patient/legislative_en. Accessed April 25, 2018. | ||
Pfizer. Medicine Safety Education; 2018. Available from: https://www.pfizer.com/products/patient-safety. Accessed April 25, 2018. | ||
Davies N. Public-Private Partnerships Essential for Public Health Education; 2015. Available from: https://social.eyeforpharma.com/market-access/public-private-partnerships-essential-public-health-education. Accessed April 25 2018. | ||
Donohue JM, Cevasco M, Rosenthal MB. A decade of direct-to-consumer advertising of prescription drugs. N Engl J Med. 2007;357(7):673–681. | ||
Ventola CL. Direct-to-consumer pharmaceutical advertising. Therapeutic or toxic? P. T. 2011;36(10):669–684. | ||
Cheng KW, Chong DWK. Direct-to-consumer advertising in Hong Kong: possible impact and regulation. Hong Kong Pharma J. 2014;21(4):130–136. | ||
US Food and Drug Administration. CFR - Code of Federal Regulations Title 21. Code of Federal Regulations; 2018. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=202.1. Accessed April 25, 2018. | ||
Parekh A, Marcus R, Roberts M, Raisch DW. Risks and benefits of direct to consumer advertising on patient - provider relationships. Report of the ISPOR Direct to Consumer Advertisements Working Group. Available from: https://www.ispor.org/news/articles/June12/Risks-and-Benefits-of-Direct-To-Consumer.asp. Accessed April 25, 2018. | ||
Graziano L. Direct to Consumer Drug Advertisements: A Dangerous Game of Pitching Products to Parents. Wright State University; 2009. Available from: https://corescholar.libraries.wright.edu/oamh_presentations/4/. Accessed April 26, 2018. | ||
World Health Organization. Direct-to-consumer advertising under fire. Bull World Health Organ. 2009;87(8):565–644. | ||
Hollon MF. Direct-to-consumer advertising: a haphazard approach to health promotion. JAMA. 2005;293(16):2030–2033. | ||
Baggott R, Forster R. Health consumer and patients’ organizations in Europe: towards a comparative analysis. Health Expect. 2008;11(1):85–94. | ||
US Food and Drug Administration. CDER Patient-Focused Drug Development; 2018. Available from: https://www.fda.gov/Drugs/DevelopmentApprovalProcess/ucm579400.htm. Accessed April 26, 2018. | ||
Rachul C, Toews M, Caulfield T. Controversies with Kalydeco: Newspaper coverage in Canada and the United States of the cystic fibrosis “wonder drug”. J Cyst Fibros. 2016;15(5):624–629. | ||
Muth CC. Conflict of interest in medicine. JAMA. 2017;317(17):1812. | ||
Myshko D. The Medical Affairs-Marketing Connection; 2004. Available from: http://www.pharmavoice.com/article/137/. Accessed August 1, 2018. | ||
Goldacre B. Evil ways of the drug companies. Bad Science; 2007. Available from: https://www.theguardian.com/science/2007/aug/04/sciencenews. Accessed August 1, 2018. | ||
Francer J, Izquierdo JZ, Music T, et al. Ethical pharmaceutical promotion and communications worldwide: codes and regulations. Philos Ethics Humanit Med. 2014;9(1):7. | ||
European Medicines Agency. About Us; 2018. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/about_us/document_listing/document_listing_000426.jsp&mid. Accessed April 26 2018. | ||
European Union. Regulations, Directives and Other Acts; 2018. Available from: https://europa.eu/european-union/eu-law/legal-acts_en. Accessed April 26, 2018. | ||
Australian Government Department of Health. Therapeutic goods advertising code. Therapeutic Goods Administration; 2018. Available from: https://www.tga.gov.au/publication/therapeutic-goods-advertising-code. Accessed April 26, 2018. | ||
US Securities and Exchange Commission. Foreign Corrupt Practices Act; 2018. Available from: https://www.sec.gov/spotlight/foreign-corrupt-practices-act.shtml. Accessed April 26, 2018. | ||
Transparency International UK. The Bribery Act; 2018. Available from: http://www.transparency.org.uk/our-work/business-integrity/bribery-act/#.WuFuxIhubIV. Accessed April 26, 2018. | ||
International Federation of Pharmaceutical Manufacturers & Associations. Our Membership; 2018. Available from: https://www.ifpma.org/who-we-are/our-membership/full-members/companies/#!/. Accessed April 26, 2018. | ||
International Federation of Pharmaceutical Manufacturers & Associations. Corporate Brochure: Committed to a Healthier Future. Geneva 2016. Available from: https://www.ifpma.org/resource-centre/ifpma-corporate-brochure/. Accessed April 26, 2018. | ||
International Federation of Pharmaceutical Manufacturers & Associations. Global Code Comparison: A Snapshot of IFPMA Member Associations’ Self-Regulation Mechanisms. International Federation of Pharmaceutical Manufacturers & Associations; 2018. Available from: https://www.ifpma.org/wp-content/uploads/2018/02/IFPMA_GCC_V7.pdf. Accessed April 26, 2018. | ||
International Federation of Pharmaceutical Manufacturers & Associations. IFPMA Code of Practice; 2012. Available from: https://www.lif.se/globalassets/etik/dokument/ifpma_code_of_practice_2012.pdf. Accessed April 26, 2018. | ||
Pfizer. Sales and Marketing Compliance. 2018. Available from: Pfizer. Sales and Marketing Compliance. Accessed April 26, 2018. | ||
Takeda Pharmaceutical Company Limited. Takeda Global Code of Conduct; 2017. Available from: https://www.takeda.com/siteassets/system/who-we-are/corporate-governance/compliance/global-code-of-conduct_en.pdf. Accessed April 26, 2018. | ||
Astellas Pharma Inc. Policies & Position Statements; 2018. Available from: https://www.astellas.com/en/about/policies-and-position-statements. Accessed April 26, 2018. | ||
AstraZeneca. AstraZeneca Global Policy Communications; 2015. Available from: https://www.astrazeneca.com/content/dam/az/PDF/Global-Communications%20Policy_v6.0_December_2015.pdf. Accessed April 26, 2018. | ||
GlaxoSmithKline plc. Our Code of Practice for Promotion of Prescription Medicines and for Scientific Engagement; 2018. Available from: https://www.gsk.com/media/2831/code-of-practice.pdf. Accessed April 26, 2018. | ||
Werling K, Carnell H, McCormick D. Focus on Life Science Compliance: The Evolution of Medical Affairs Departments; 2011. Available from: https://www.mcguirewoods.com/news-resources/publications/health_care/focus-life-science-compliance-nov-2011.pdf. Accessed August 1, 2018. | ||
British Medical Association. The Pharmaceutical Physician; 2013. Available from: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0ahUKEwiVm43n6J3aAhVCso8KHSLxBYEQFggsMAA&url=https%3A%2F%2Fwww.bma.org.uk%2F-%2Fmedia%2Ffiles%2Fpdfs%2Fabout%2520the%2520bma%2Fhow%2520we%2520work%2Fmedical%2520academics%2Fpharmaceuticalphysican_sept2013_update.pdf&usg=AOvVaw0pemmjh2E-xGyAz54SO7Zf. Accessed April 26, 2018. | ||
C.E.L. for Pharma. Medical Affairs Training Courses; 2018. Available from: https://www.celforpharma.com/medical-affairs-training-courses. Accessed April 26, 2018. | ||
Duke NUS Medical School. Joint Alliance Duke-NUS Education Certificate Programme in Medical Affairs. Available from: https://www.duke-nus.edu.sg/core/page/jade-certification-programme-medical-affairs-brochure. Accessed April 26, 2018. | ||
IFAPP Academy. The IFAPP-Kings College Medical Affairs in Medicines Development Certification Timelines; 2018. Available from: https://ifappacademy.org/index.php/timelines/. Accessed April 26, 2018. | ||
IFAPP Academy. IFAPP Academy; 2018. Available from: https://ifappacademy.org/. Accessed April 26, 2018. | ||
IFAPP Academy. IFAPP Academy Student Requirements; 2018. Available from: https://ifappacademy.org/index.php/ifapp-academy-student-requirements/. Accessed April 26, 2018. | ||
International Committee of Medical Journal Editors. Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals; 2017. Available from: http://www.icmje.org/icmje-recommendations.pdf. Accessed April 25, 2018. | ||
Battisti WP, Wager E, Baltzer L, et al. Good publication practice for communicating company: sponsored medical research: GPP3. Ann Intern Med. 2015;163(6):461–464. | ||
CONSORT. Consolidated Standards of Reporting Trials. Available from: http://www.consort-statement.org. Accessed April 26, 2018. | ||
von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–808. | ||
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. | ||
Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. | ||
Des Jarlais DC, Lyles C, Crepaz N, TREND Group. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health. 2004;94(3):361–366. | ||
US Food and Drug Administration. What We Do; 2018. Available from: https://www.fda.gov/AboutFDA/WhatWeDo/default.htm. Accessed April 26, 2018. | ||
UK Government. Services and information. Medicines and Healthcare products Regulatory Agency. Available from: https://www.gov.uk/government/organisations/medicines-and-healthcare-products-regulatory-agency/services-information. Accessed April 26, 2018. | ||
Government of Canada. Health Canada. Available from: https://www.canada.ca/en/health-canada.html. Accessed April 26, 2018. | ||
Health Sciences Authority. Corporate Profile; 2018. Available from: http://www.hsa.gov.sg/content/hsa/en/About_HSA/Corporate_Profile.html. Accessed April 26, 2018. | ||
New Zealand Medicines and Medical Devices Safety Authority. About Medsafe. Available from: http://www.medsafe.govt.nz/other/about.asp. Accessed April 26, 2018. | ||
Japan Pharmaceutical Manufacturers Association. Regulatory Information Task Force: Information on Japanese Regulatory Affairs. Pharmaceutical Administration and Regulations in Japan; 2017. Available from: http://www.jpma.or.jp/english/parj/pdf/2017_contents.pdf. Accessed April 26, 2018. | ||
Central Drugs and Standard Control Organization. About Us. Available from: http://www.cdsco.nic.in/forms/contentpage1.aspx?lid=962. Accessed April 26, 2018. | ||
China Food and Drug Administration. Main Responsibilities. Available from: http://eng.sfda.gov.cn/WS03/CL0756/. Accessed April 26, 2018. | ||
Rothstein NA. Protecting privacy and enabling pharmaceutical sales on the Internet: a comparative analysis of the United States and Canada. Fed Commun Law J. 2001;53(2)Article 6. | ||
UK Government Legislation. The Medicines (Advertising) Regulations; 1994. Available from: https://www.legislation.gov.uk/uksi/1994/1932/contents/made. Accessed April 26, 2018. | ||
European Commission Health and Food Safety Directorate-General. Procedures for Marketing Authorisation; 2018. Available from: https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-2/vol2a_chap1_rev7_201712.pdf. Accessed July 2, 2018. | ||
Singapore Statutes Online. Health Products Act; 2008. Available from: https://sso.agc.gov.sg/Act/HPA2007. Accessed April 26, 2018. | ||
Federal Register of Legistlation. Therapeutic Goods Act; 1989. Available from: https://www.legislation.gov.au/Details/C2017C00226. Accessed April 26, 2018. | ||
Pharmaceutical Affairs Law. Japan. Available from: http://www.jouhoukoukai.com/repositories/source/pal.htm. Accessed April 26, 2018. | ||
International Comparative Legal Guides. Pharmaceutical Advertising 2017: Japan. Available from: https://iclg.com/practice-areas/pharmaceutical-advertising/pharmaceutical-advertising-2017/japan. Accessed April 26, 2018. | ||
International Comparative Legal Guides. Pharmaceutical Advertising 2017: India. Available from: https://iclg.com/practice-areas/pharmaceutical-advertising/pharmaceutical-advertising-2017/india. Accessed April 26, 2018. | ||
Ma F, Lou N. Chinese regulation of off-label use of drugs. Food Drug Law J. 2013;68(2):189–200. | ||
China Food and Drug Administration. Provisions for Drug Advertisement Examination; 2007. Available from: http://eng.sfda.gov.cn/WS03/CL0768/61649.html. Accessed April 28, 2018. | ||
Database of Laws and Regulations: National People’s Congress. Economic and Advertisement Law of the People’s Republic of China; 2018. Available from: http://www.npc.gov.cn/englishnpc/Law/2007-12/12/content_1383782.htm. Accessed April 28, 2018. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


