Back to Journals » OncoTargets and Therapy » Volume 11
Evidence from an updated meta-analysis of the prognostic impacts of postoperative radiotherapy and chemotherapy in patients with anaplastic thyroid carcinoma
Authors Xia Q, Wang W, Xu J, Chen X, Zhong Z, Sun C
Received 11 October 2017
Accepted for publication 10 February 2018
Published 19 April 2018 Volume 2018:11 Pages 2251—2257
DOI https://doi.org/10.2147/OTT.S153759
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Quansong Xia,1,* Wei Wang,2,* Juan Xu,3 Xue Chen,2 Zhaoming Zhong,2 Chuanzheng Sun2
1Department of Clinical Laboratory, 2Department of Head and Neck Surgery, The Third Affiliated Hospital of Kunming Medical University, 3Department of Internal Medicine, The People’s Hospital of Guandu District, Kunming, People’s Republic of China
*These authors contributed equally to this work
Background: Radiotherapy and chemotherapy are the two important postoperative management approaches for anaplastic thyroid carcinoma (ATC), and several studies have suggested that postoperative radiotherapy and chemotherapy can prolong the survival of patients with ATC. However, the results remain inconsistent.
Objective: A meta-analysis was performed to address whether postoperative radiotherapy and chemotherapy could prolong the survival of patients with ATC.
Methods: Relevant studies were included, and pooled hazard ratios (HRs) together with 95% confidence intervals (CIs) were calculated.
Results: Ten relevant studies on factors that affect the prognosis for ATC were included in this meta-analysis, evaluating a total of 1,163 patients. The pooled HR for overall survival (OS) was calculated using a random-effects model. The pooled results demonstrated that for all patients with resectable ATC, the combination of surgery and radiotherapy significantly reduced the risk of death compared with surgery alone (HR =0.51, 95% CI: 0.36–0.73, Z=3.66, P=0.0002). To investigate the prognostic impacts of chemotherapy in patients with ATC, we also calculated the pooled HR of chemotherapy for OS using a random-effects model; however, the pooled results suggested that chemotherapy did not prolong the survival of ATC patients compared with controls (HR =0.63, 95% CI: 0.33–1.21, Z=1.39, P=0.17).
Conclusion: This study provided evidence that currently, for patients with ATC, postoperative radiotherapy may prolong survival; in contrast, chemotherapy did not improve long-term survival.
Keywords: anaplastic thyroid carcinoma, postoperative radiotherapy, chemotherapy, prognosis, meta-analysis
Introduction
Thyroid cancers include papillary, follicular, medullary, and anaplastic carcinomas. Although anaplastic thyroid carcinoma (ATC) accounts for only 2%–5% of all thyroid cancers,1–5 ATC is responsible for 14%–50% of all thyroid carcinoma-related deaths.6–9 Thus, ATC is a special type of thyroid cancer with high degree of malignancy and poor prognosis. Patients with ATC are typically in their sixth or seventh decade of life. This disease is usually characterized by aggressive growth features and frequently causes extensive local invasion and distant metastases. Therefore, the management of patients with ATC is extremely difficult, and there is little consensus regarding a standard successful treatment protocol.10,11 During the past few years, some studies have demonstrated that multidisciplinary treatment plays an important role for patients with ATC and that postoperative radiotherapy is the main treatment approach rather than chemotherapy.12–14 However, it remains uncertain whether this multimodal treatment truly improves survival because most studies have a small sample size and poor statistical power due to the relatively low incidence of ATC.
Previously, we reported that radiotherapy combined with surgery appears to increase overall treatment efficacy for ATC patients and prolong survival; in contrast, chemotherapy was ineffective.15 However, other studies have reported conflicting results.16–18 Therefore, the objective of this study was to perform a meta-analysis to address whether chemotherapy and radiotherapy could prolong the survival of patients with ATC.
Methods
Literature search and data extraction
Two investigators (WW and QX) searched PubMed, Embase, Web of Science, VIP Database for Chinese Technical Periodicals, Wanfang Data Knowledge Service Platform, and China National Knowledge Infrastructure (CNKI) for articles published up to April 2017, using the terms ATC and anaplastic thyroid carcinoma. A database was then created, with no limits established with respect to language or study design. Studies included in this meta-analysis were required to be 1) original studies and 2) studies reporting hazard ratios (HRs) with 95% confidence intervals (CIs) or sufficient data to calculate HRs and 95% CIs. All studies were independently verified against the inclusion and exclusion criteria for this meta-analysis by two investigators. The first author, publication year, country, language, sample size for ATC patients, and reported HR(s) were extracted from each included study. These processes were performed independently by two investigators (WW and QX), and a consensus was reached.
Statistical analyses
Pooled HRs and 95% CIs were calculated.19 In addition, χ2-based Q statistics and I2 metrics were used to assess the heterogeneity between studies. When I2<50%, a fixed-effects model was used to calculate pooled HR; otherwise, a random-effects model was used for this purpose. The prognostic impacts of chemotherapy and postoperative radiotherapy in ATC patients were assessed by meta-analysis. All statistical analyses were performed using the Review Manager software (v.5.2; The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) and STATA (v. 12.0; StataCorp LP, College Station, TX, USA).
Results
A database that included each article’s first author, publication year, country, language, sample size, and other important information was established based on the information extracted from 10 relevant studies15–18,20–25 that satisfied the inclusion criteria (Table 1). The original search yielded a total of 2,563 articles related to the keywords. Figure 1 summarizes the selection process of this study. After titles, keywords, and abstracts were screened, 2,478 of these articles were excluded. The full texts of 85 articles were reviewed, and an additional 75 articles were excluded (with 74 articles excluded for not providing usable data and one article excluded due to the duplication of the same article in different languages); thus, 10 studies (a total of 1,163 patients) remained for further review.
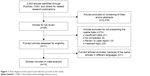  | Figure 1 Flow diagram of the search and selection process in this study. |
The pooled HR for overall survival (OS) was calculated using a random-effects model. The pooled results demonstrated that for all patients with resected ATC, the combination of surgery and radiotherapy significantly reduced the risk of death compared with surgery alone (HR =0.51, 95% CI: 0.36–0.73, Z=3.66, P=0.0002) (Figure 2). In addition, to investigate the prognostic impact of chemotherapy in ATC patients, we calculated the pooled HR of chemotherapy for OS using a random-effects model; however, the pooled results demonstrated that ATC patients treated with chemotherapy did not exhibit prolonged survival relative to patients who did not receive chemotherapy (HR =0.63, 95% CI: 0.33–1.21, Z=1.39, P=0.17) (Figure 3).
  | Figure 3 Forest plots for patients treated with chemotherapy and patients treated without chemotherapy. |
To assess the stability of our results, a sensitivity analysis was performed by removing one study at a time. The pooled HR for patients who received a combination of surgery and radiotherapy or chemotherapy compared with control patients was not significantly changed, suggesting that our results were stable. Funnel plots for this meta-analysis were symmetric (Figure 4), indicating the absence of publication bias. Finally, we used STATA software to perform Egger’s test to calculate publication bias. No publication bias was detected via Egger’s test, which was performed to provide statistical evidence for funnel plot symmetry (P=0.183 for surgery combined with radiotherapy; P=0.441 for chemotherapy).
Discussion
ATC is one of the most aggressive types of malignant tumor in humans,26 and the prognosis of patients with ATC is extremely poor; in particular, only 20% of affected patients survive for 1 year, and the median survival duration is 3–9 months after diagnosis.1–5,11,27 There is currently no standardized therapeutic regimen for ATC patients. Multimodal therapy has been reported to achieve better results than unimodal treatment;12–14 however, no firm conclusions have been reached and no individualized treatment regimens have been established.10,11 Although most studies have demonstrated that postoperative radiotherapy can prolong the survival of ATC patients while chemotherapy is ineffective for this purpose, it remains unclear which treatment truly improves survival because most studies have a small sample size and poor statistical power due to the relatively low incidence of ATC. In this study, we performed a meta-analysis to address whether postoperative radiotherapy or chemotherapy could prolong the survival of ATC patients and attempted to determine the best treatment strategy to guide therapeutic decisions.
Our pooled results demonstrated that for all patients with resectable ATC, the combination of surgery and postoperative radiotherapy significantly reduced the risk of death compared with surgery alone; in contrast, chemotherapy did not prolong the survival of ATC patients. In fact, American Thyroid Association (ATA) guidelines already recommend that definitive radiotherapy should be offered after complete or near complete surgical resection (R0/R1) in patients without metastatic disease.28 In addition, it has been suggested that for patients whose performance statuses permit such treatment, multimodal regimens that include chemotherapy result in the longest median survival.5,11,29,30 The aforementioned findings raise the following question: if surgery followed by radiotherapy and chemotherapy truly improves the long-term survival of ATC patients, why would prior studies have reported differing curative effects for this therapeutic approach?17,20,22,25 To address this question, we performed a literature review; based on this review, we considered the following potential explanations for differences in reported curative effects. First, there is no standard protocol for either chemotherapy or radiotherapy for ATC, and these treatments cannot be continued because rapid enlargement occurs, particularly in elderly patients.31 Second, for radiotherapy, the dose appears to be important; several studies have demonstrated a clear improvement in survival among patients given higher doses of radiotherapy.32 Furthermore, there is currently no general consensus regarding which chemotherapy regimens are best, although several studies have compared different agents and demonstrated that toxic regimens are poorly tolerated.5,11,33,34 Although the pooled result in our study suggested that chemotherapy is ineffective, we still cannot completely exclude its potential for producing beneficial effects.
In contrast, several limitations of the current meta-analysis should be noted. First, despite our best attempts to gather evidence from the literature, we were unable to perform a methodological assessment of certain studies due to a lack of usable data. Additional work must be performed in the future. Second, there is potential publication bias in this study because we did not consider several unpublished articles and abstracts because these unpublished works were not available. In addition, our meta-analysis only included studies published in English or Chinese, with other publications excluded due to our language criteria; this restriction may also have introduced bias and affected our findings. Finally, our meta-analysis may have been too underpowered to acquire original data from the included studies. Despite all of the aforementioned limitations, this study provided evidence that for ATC patients, surgery combined with radiotherapy may offer prolonged survival; in contrast, postoperative chemotherapy did not improve long-term survival.
Conclusion
This study provided evidence that surgery combined with radiotherapy may prolong survival in ATC patients; in contrast, postoperative chemotherapy did not improve long-term survival.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (81560470 and 81773127) and the Special Foundation of Young and Middle-aged Academic Leaders Reserve Talent in Yunnan Province (2015HB086).
Disclosure
The authors report no conflicts of interest in this work.
References
Jereb B, Stjernsward J, Lowhagen T. Anaplastic giant-cell carcinoma of the thyroid. A study of treatment and prognosis. Cancer. 1975;35(5):1293–1295. | ||
Spires JR, Schwartz MR, Miller RH. Anaplastic thyroid carcinoma. Association with differentiated thyroid cancer. Arch Otolaryngol Head Neck Surg. 1988;114(1):40–44. | ||
Lo CY, Lam KY, Wan KY. Anaplastic carcinoma of the thyroid. Am J Surg. 1999;177:337–339. | ||
Nilsson O, Lindeberg J, Zedenius J, et al. Anaplastic giant cell carcinoma of the thyroid gland: treatment and survival over a 25-year period. World J Surg. 1998;22(7):725–730. | ||
Tan RK, Finley RK 3rd, Driscoll D, Bakamjian V, Hicks WL Jr, Shedd DP. Anaplastic carcinoma of the thyroid: a 24-year experience. Head Neck. 1995;17(1):41–48. | ||
Landis SH, Murray T, Bolden S, et al. Cancer statistics, 1998. CA Cancer J Clin. 1998;48:6–29. | ||
Gilliland FD, Hunt WC, Morris DM, Key CR. Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973–1991. Cancer. 1997;79(3):564–573. | ||
Are C, Shaha AR. Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Ann Surg Oncol. 2006;13(4):453–464. | ||
Bhatia A, Rao A, Ang KK, et al. Anaplastic thyroid cancer: clinical outcomes with conformal radiotherapy. Head Neck. 2010;32(7):829–836. | ||
Akaishi J, Sugino K, Kitagawa W, et al. Prognostic factors and treatment outcomes of 100 cases of anaplastic thyroid carcinoma. Thyroid. 2011;21(11):1183–1189. | ||
Haigh PI, Ituarte PH, Wu HS, et al. Completely resected anaplastic thyroid carcinoma combined with adjuvant chemotherapy and irradiation is associated with prolonged survival. Cancer. 2001;91(12):2335–2342. | ||
Chen J, Tward JD, Shrieve DC, Hitchcock YJ. Surgery and radiotherapy improves survival in patients with anaplastic thyroid carcinoma: analysis of the surveillance, epidemiology, and end results 1983–2002. Am J Clin Oncol. 2008;31(5):460–464. | ||
Yau T, Lo CY, Epstein RJ, Lam AK, Wan KY, Lang BH. Treatment outcomes in anaplastic thyroid carcinoma: survival improvement in young patients with localized disease treated by combination of surgery and radiotherapy. Ann Surg Oncol. 2008;15(9):2500–2505. | ||
Pierie JP, Muzikansky A, Gaz RD, Faquin WC, Ott MJ. The effect of surgery and radiotherapy on outcome of anaplastic thyroid carcinoma. Ann Surg Oncol. 2002;9(1):57–64. | ||
Sun C, Li Q, Hu Z, et al. Treatment and prognosis of anaplastic thyroid carcinoma: experience from a single institution in China. PLoS One. 2013;8(11):e80011. | ||
Nachalon Y, Stern-Shavit S, Bachar G, Shvero J, Limon D, Popovtzer A. Aggressive palliation and survival in anaplastic thyroid carcinoma. JAMA Otolaryngol Head Neck Surg. 2015;141(12):1128–1132. | ||
Roche B, Larroumets G, Dejax C, et al. Epidemiology, clinical presentation, treatment and prognosis of a regional series of 26 anaplastic thyroid carcinomas (ATC). Comparison with the literature. Ann Endocrinol (Paris). 2010;71(1):38–45. | ||
Brignardello E, Palestini N, Felicetti F, et al. Early surgery and survival of patients with anaplastic thyroid carcinoma: analysis of a case series referred to a single institution between 1999 and 2012. Thyroid. 2014;24(11):1600–1606. | ||
Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. | ||
Baek SK, Lee MC, Hah JH, et al. Role of surgery in the management of anaplastic thyroid carcinoma: Korean nationwide multicenter study of 329 patients with anaplastic thyroid carcinoma, 2000 to 2012. Head Neck. 2017;39(1):133–139. | ||
Ito K, Hanamura T, Murayama K, et al. Multimodality therapeutic outcomes in anaplastic thyroid carcinoma: improved survival in subgroups of patients with localized primary tumors. Head Neck. 2012;34(2):230–237. | ||
Kebebew E, Greenspan FS, Clark OH, Woeber KA, McMillan A. Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer. 2005;103(7):1330–1335. | ||
Kobayashi T, Asakawa H, Umeshita K, et al. Treatment of 37 patients with anaplastic carcinoma of the thyroid. Head Neck. 1996;18(1):36–41. | ||
Liu TR, Xiao ZW, Xu HN, et al. Treatment and prognosis of anaplastic thyroid carcinoma: a clinical study of 50 cases. PLoS One. 2016;11(10):e0164840. | ||
Palestini N, Brignardello E, Freddi M, et al. Surgical treatment of anaplastic thyroid carcinoma. Our experience. G Chir. 2010;31(6–7):282–285. | ||
McIver B, Hay ID, Giuffrida DF, et al. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery. 2001;130(6):1028–1034. | ||
Ain KB. Anaplastic thyroid carcinoma: behavior, biology, and therapeutic approaches. Thyroid. 1998;8(8):715–726. | ||
Smallridge RC, Ain KB, Asa SL, et al. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22:1104–1139. | ||
Swaak-Kragten AT, de Wilt JH, Schmitz PI, Bontenbal M, Levendag PC. Multimodality treatment for anaplastic thyroid carcinoma – treatment outcome in 75 patients. Radiother Oncol. 2009;92(1):100–104. | ||
De Crevoisier R, Baudin E, Bachelot A, et al. Combined treatment of anaplastic thyroid carcinoma with surgery, chemotherapy, and hyperfractionated accelerated external radiotherapy. Int J Radiat Oncol Biol Phys. 2004;60:1137–1143. | ||
Nagaiah G, Hossain A, Mooney CJ, Parmentier J, Remick SC. Anaplastic thyroid cancer: a review of epidemiology, pathogenesis, and treatment. J Oncol. 2011;2011:542358. | ||
Wang Y, Tsang R, Asa S, Dickson B, Arenovich T, Brierley J. Clinical outcome of anaplastic thyroid carcinoma treated with radiotherapy of once- and twice-daily fractionation regimens. Cancer. 2006;107(8):1786–1792. | ||
Ain KB, Egorin MJ, DeSimone PA. Treatment of anaplastic thyroid carcinoma with paclitaxel: phase 2 trial using ninety-six-hour infusion. Collaborative Anaplastic Thyroid Cancer Health Intervention Trials (CATCHIT) Group. Thyroid. 2000;10(7):587–594. | ||
Troch M, Koperek O, Scheuba C, et al. High efficacy of concomitant treatment of undifferentiated (anaplastic) thyroid cancer with radiation and docetaxel. J Clin Endocrinol Metab. 2010;95(9):E54–E57. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



