Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Evaluation of Urinary Neutrophil Gelatinase Associated Lipocalin and Kidney Injury Molecule-1 as Diagnostic Markers for Early Nephropathy in Patients with Type 2 Diabetes Mellitus
Authors Quang TH , Nguyet MP, Thao DP, Thi MH , Phuong Thi Dam L , Thi HH, Van AP, Luong TC, Tuyet MNT, Duy QD, Nhu BD , Duc TN
Received 17 April 2020
Accepted for publication 5 June 2020
Published 24 June 2020 Volume 2020:13 Pages 2199—2207
DOI https://doi.org/10.2147/DMSO.S258678
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Thuan Huynh Quang,1,2 Minh Pham Nguyet,3 Diep Pham Thao,4 Minh Hoang Thi,1,2 Lan Phuong Thi Dam,1,2 Hang Ho Thi,1,2 Anh Phan Van,1,2 Thang Can Luong,5 Mai Ngo Thi Tuyet,2 Quy Dang Duy,1,2 Binh Do Nhu,1,2 Thuan Nghiem Duc1,2
1Military Hospital 103, Ha Noi, Viet Nam; 2Vietnam Military Medical University, Ha Noi, Vietnam; 3 19-8 Hospital, Ministry of Public Security, Ha Noi, Vietnam; 4Viet Duc Hospital, Ha Noi, Viet Nam; 5Thanh Nhan Hospital, Ha Noi, Viet Nam
Correspondence: Thuan Nghiem Duc Email [email protected]
Purpose: The purpose of this study was evaluating the early diagnostic value of two specific tubular markers neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1) in diabetes nephropathy.
Patients and Methods: Cross-sectional study was carried in three groups of patients from 10/2017 to 10/2018 in Military Hospital 103. Group I included 30 healthy peoples with estimated glomerular filtration rate (eGFR) > 60 mL/min/1.73 m2 and urine albumin creatinine ratio (uACR) < 30 mg/g. Group II included 30 type 2 diabetic patients having uACR < 30 mg/g, eGFR > 60 mL/min/1.73 m2. Group III included 30 type 2 diabetic patients having uACR > 30 mg/g, eGFR > 60 mL/min/1.73 m2.
Results: Urine KIM-1 and NGAL increased progressively from control group (57.29 ± 25.91 pg/mL; 25.71 ± 13.69 ng/mL) to the group of diabetic patients with uACR < 30 mg/g (167.06 ± 44.01 pg/mL; 37.42 ± 10.89 ng/mL) and the group of diabetic patients with uACR ≥ 30 mg/g) (p < 0.05). There were moderate correlations between KIM-1 (r = 0.48, p < 0.05) and NGAL (r = 0.45, p < 0.05) with uACR. There was a mild correlation between KIM-1 and NGAL (r = 0.29, p < 0.05). KIM-1 and NGAL are the independent tests to detect diabetic nephropathy. The sensivity and specificity of KIM-1 with cut-off value of 174.95 pg/mL were 62.37% and 73.48%, respectively; the sensivity and specificity of NGAL with cut-off value of 35.2 ng/mL were 60.45% and 70.37%, respectively.
Conclusion: KIM-1 and NGAL in urine are independent markers for early diagnostic diabetic nephropathy.
Keywords: diabetic kidney disease, urine albumin creatinin ratio, albumin urine
Introduction
Diabetic nephropathy (DN) is a common complication of diabetes, with a high incidence and death rate. In 2019, about 463 million people (representing 9.3% of global population) are estimated to be living with diabetes worldwide. This is predicted to increase to 578 million (10.2%) in 2030 and 700 million (10.9%) in 2045.1,2 Nearly 90% of patients with diabetes will develop complications of micro and macrovascular.3 Diabetic nephropathy is considered to be one of the most serious complications, affecting 20% to 40% of diabetes patients, mostly type 2 diabetes mellitus. The Southeast Asia is on the top five regions in the world with the highest prevalence of DN in both genders and the prevalence of male has even been increasing since 1990.4 Most countries in Southeast Asia are the low- and middle-income countries where diabetes metilius has been increasing over time.5
The disease progresses silently, worsens and leads to irreversible damages.6,7 Mechanism of disease often associates with changes in the structure and function of renal cells due to the effects of prolonged hyperglycemia, the activation of metabolic mechanisms associated with redox imbalance and inflammatory response.8 On the other hand, the kidney injuries have been affected partly by race, ethnicity and socioeconomic status.9 For these reasons, the characteristics of diabetic nephropathy are different between countries in the worldwide.
Early diagnosis and treatment play an important role in diabetic nephropathy. Currently, albuminurine is a major marker for diagnosing diabetic nephropathy with cut-off values of 30 mg/24h. However, diabetic patients with lower than cut-off value albuminuria had kidney damage. In addition, most studies have focused on glomerular mutation, but one-third of patients with normal albuminuria actually have histological glomerular disease.6 A growing number of studies have found that renal tubular damage plays an important role in the pathogenesis of diabetic kidney disease.10–13
There are several proteins and tubular enzymes involved in tubular damage such as N-acetyl-β-D-glucosamidase, gamma-glutamyl transferase, neutrophil gelatinase associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1).14–20 In this study, we aimed to evaluate the diagnostic value of both urinary NGAL and KIM-1 for early detection nephropathy in T2DM in Vietnam, a low-income country in Southeast Asia.
Patients and Methods
Subjects
This research included 60 diabetic patients and 30 healthy peoples enrolled in Military Hospital 103 until October 2017 to November 2018. All the diabetic patients were diagnosed according to International Diabetes Federation (IDF) criteria 2004 and fulfilled the following inclusion criteria: age ≥ 18 years, estimated glomerular filtration rate (eGFR) > 60 mL/min/1.73 m2 and stable renal function status without increasing serum creatinine in 3 months continuous, structural normal in ultrasound imaging, no urine sediments, no history of kidney disease and transplantation before, no complications of diabetes. Patients with plasma creatinine > 130 mmol/l; eGRP < 60 mL/min/1.73 m2, urinary albumin creatinine ratio > 300 mg/g, background with renal diseases, hypertension, other complications of diabetes, pregnancy, inflammatory disorders were excluded. The diabetic patients were divided into 2 groups II and III according to urine albumin creatinine ratio (uACR) based on KDIGO 2013.21 Group II contained 30 patients with normoalbuminuria (uACR < 30 mg/g), group III contained 30 patients with microalbuminuria (30 <uACR < 300 mg/g).
Healthy peoples were selected into group I from their medical check-up visit with following inclusion criteria: fasting plasma glucose ≤ 5.6 mmol/l based on 2003 ADA statement, the similar ages and socioeconomic status, no history of kidney damages, normal of kidney structure and function, eGRP > 60 mL/min/1.73 m2.
Sample Collection
The spot urine sample and blood samples were collected from subjects in 3 groups in the morning at their clinic visits. All the clinical characteristics were recorded at the same day.
Spot urine were used to measure: albumin, creatinine, NGAL and KIM-1. Urine albumin was quantitative determined by immuno-turbidimetric method (mg/l) in AU680 system (Beckman Coulter). Urine creatinine was determined by Jaffe method B (g/l) in AU680 system (Beckman Coulter). The eGFR were calculated using Cockcroft – Gault formula (1973): eGFR ={((140–age) x weight)/(72xSCr)}x 0.85 (if female); SCr: serum creatinine. The uACR was calculated by formula: uACR= Albumin/Creatinine (mg/g). KIM-1 was determined in spot urine by double antibody sandwich enzyme-linked immunosorbent assay (ELISA). The kit was human Kim-1 ELISA kit (My BioSource, USA). The formula to calculate urine KIM-1 and creatinine ratio were: uKIM-1/Cre = KIM-1/Creatinine (pg/g). NGAL was measured in spot urine Architect i2000 by chemiluminescent microparticle immunoassay (CMIA) (Abbott Diagnostic kit, USA). The formula to calculate urine NGAL and creatinine ratio were: uNGAL/Cre = NGAL/Creatinine (ng/g).
Glucose, cholesterol, triglyceride, HDL-cholesterol, LDL-cholesterol were determined in plasma in AU 680 system (Beckman Coulter). Plasma glucose level was accessed by hexokinase method. Plasma cholesterol level was accessed by enzyme colorimetric. HbA1c was measured in blood by high-performance liquid chromatography (HPLC) in Premiere 9201 (Trinity Biotec, USA). BMI was calculated as the ratio of the weight to the height (kg/m2).
Ethical Consideration
All participants were dispensed with written informed consents, and the protocol was approved by the Ethical Review Committee of Vietnam Military Medical University (Reference No.70/2017/VMMU-IRB). The study was also conducted using good clinical practice following the Declaration of Helsinki.
Statistical Analysis
The data were analyzed in Microsoft Excel 2013 and Spss 20.0. Data were present as numbers of patients, mean ± standard deviation or median, maximum, minimum for quantitative variables with normal and abnormal distribution. Difference between groups was tested with t-test and Mann–Whitney’s test, according to distribution. The correlation between variables was tested Pearson correlation. The results were considered to be significant at p<0.05. The diagnostic accuracy was determined by using receiver operating characteristic (ROC) curves. Cut-off values were chosen by maximizing the Youden index. Based on cut-off values, the sensitivity and specificity were calculated.
Results
Clinical Characteristic of the Subjects
The results showed that there were no significant differences within 3 groups in BMI, age and gender. There were no significant differences between 2 diabetes patient’s groups in duration of disease, FPG, HbA1c, lipid disorder status. Especially, the plasma urea, creatinine concentration and eGFR were not significantly different between 2 diabetic patient’s groups (Table 1).
 |
Table 1 Characteristic of the Population |
The results in Table 2 showed the difference in urine albumin, creatinine and uACR. The urine albumin concentration in 2 diabetic patient’s groups was higher than control group (p < 0.05). The urine creatinine concentration in 2 diabetic patient’s groups was lower than control group (p significantly). And the uACR were decreased from group III to II and I (p significantly) (Table 2).
 |
Table 2 Urine Albumin, Urine Creatinine, Urine Albumin Creatinine Ratio of the Subjects |
The Level of Two Markers in Urine and Their Diagnostic Values in Diabetic Nephropathy
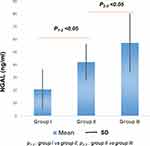
The concentration of NGAL and KIM-1 in both 2 diabetes groups was higher than control group (p significantly). Both NGAL and KIM-1 in group III were higher than group II (p < 0.05) (Figures 1 and 2).
ROC curves were carried out to access the nephropathy diagnostic values of urinary NGAL, KIM-1 in type 2 diabetes patients (Figure 3A–D). The cut-off values of urinary NGAL, KIM-1, NGAL/Creatinine, KIM-1/Creatinine were 35.3 ng/mL; 174.95 pg/mL; 44.39 ng/g; 182.06 pg/g, respectively.
Table 3 shows the sensitivity (Se), specificity (Sp) at the cut-off value of uNGAL, uKIM-1, uNGAL/Cr, uKIM-1/Cr. With the cut off value of uNGAL>35.3 ng/mL, the sensitivity and specificity were 60.45% and 70.31%, respectively. With the cut off value of NGAL/Cr >44.39 ng/g, the Se and Sp were 57.29% and 69.75%, respectively. The Se, Sp of NGAL/Cr were lower than NGAL. With the cut off value of KIM-1/Cr > 174.95 pg/mL, the Se and Sp were 62.37% and 73.48%, respectively. With the cut off value of KIM-1/Cr > 182.06 pg/g, the Se and Sp were 59.46% and 75.69%, respectively. KIM-1/Cr increased the Sp in prediction the nephropathy in diabetes patients (Table 3).
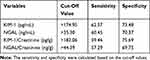 |
Table 3 Diagnostic Values of All Markers |
The Correlations Between Two Markers and Some Clinical Characteristics
The correlations of studied markers to age, duration of disease, eGFR, albuminuria, plasma glucose, cholesterol, HDL – cholesterol, LDL – cholesterol, triglyceride, HbA1c are showed in Table 4. There was no significant correction between uNGAL and u KIM-1 to them except uACR.
 |
Table 4 The Correlations Between Some Characteristic to KIM-1 and NGAL |
Figure 4 shows the positive light correlation between uNGAL and u KIM-1 (r=0.29; p significantly) (Figure 4).
 |
Figure 4 The correlation between NGAL and KIM-1. Notes: r, p-value with pearson correlation between urine NGAL and urine KIM-1 were showed on the figure. |
Discussion
KIM-1, kidney injury molecule 1, is a transmembrane protein type 1, with an immunoglobulin and mucin domain. NGAL, neutrophil gelatinase-associated lipocalin, is secreted by epithelial and neutrophil. KIM-1 and NGAL had found to appear early in acute renal tubular injury.22–26 Many researches had improved them as markers for injury in chronic kidney disease.15,22,27,28 Urinary NGAL was a noninvasive biomarker of normoalbuminuria renal in type 2 diabetes.29,30 Serum KIM-1 had performed as a marker for rapid decline renal functions in type 2 diabetes.16,31
Histopathological types of T2DM nephropathy include disproportionate tubulo-interstitial, glomerulosclerotic and vascular changes. Due to the reabsorption a large amounts of protein of the renal tubules, inflammatory response and fibrosis are developed in renal tubular cells. Inflammatory interstitial infiltrates appeared with lymphocytes and macrophages.32 Moreover, proteinuria causes tubular cell death. Recent evidence has focused on the importance of identifying renal tubular injury and its association with kidney damage in diabetes patients.10,11,33,34 With the appearance of inflammatory and cell death, the amount of NGAL and KIM-1 would have appeared earlier than albumin. Because in the early stage, glomerular basement membrane is thickened only so that albuminuria would not appear. Our study researched the concentration of urinary NGAL and KIM-1 in the T2DM had normal eGRP more than 60 mL/min/1.73m2. We found that both urinary NGAL and KIM-1 of microalbuminurin group had increased higher than normal albuminurin group. So that both KIM-1 and NGAL increased and appeared in the urine earlier than albumin. Although our diabetic subject had different average age, duration of disease, race and socioeconomic status, the result had high consistency with other studies and also demonstrated that NGAL and KIM-1 appeared earlier in nephropathy.15,16,27,28,31,35-39
Sensitivity and specificity of urine NGAL and KIM-1 in early diagnostic diabetic nephropathy of our study were similar to other researches.39,40 Kapoula et al had showed the pooled sensitivity and specificity among the different settings analyzed ranged from 0.42 (95% CI, 0.22–0.66) to 1.00 (95% CI, 0.99–1.00) and 0.72 (95% CI, 0.62–0.80) to 0.98 (95% CI, 0.50–1.00) in T2DM patients, respectively.40 Zylka et al had found sensitivity of NGAL and KIM-1 were 80%, 79%, respectively, and specificity of NGAL, KIM −1 was 61%, 51%, respectively.39 Based on these values, urinary NGAL and KIM-1 could be used as independent markers for diagnostic and prediction for diabetic nephropathy.
Our results show that KIM-1 has a moderate correlation with uACR index (r = 0.48, p <0.05), NGAL was positive correlated with uACR index (r = 0.45), p <0.05. KIM-1 and NGAL were not correlated with other biochemical tests such as plasma glucose, urea, creatinine, cholesterol, HDL-C, LDL-C, triglycerides, HbA1c. We found some common points in our research results with the one carried by Zylka et al in 2018, which studied on 80 type 2 diabetic patients divided into 2 groups: 61 patients with uACR <30 mg/g, 19 patients with uACR> 30 mg/g, normal eGRF. The authors found that there was no correlation between KIM-1 and NGAL with other tests and duration of diabetes but there was a moderate correlation between KIM-1 and NGAL with uACR index (r = 0.39, p <0.001 and r = 32, p = 0.005). When the correlation between KIM-1 and NGAL was adjusted for urinary creatinine, the authors also found that NGAL/Cr and KIM-1/Cr were positively correlated with uACR (r = 0.36, p = 0.02 and r = 0.45, p <0.05), there was no correlation between KIM-1 and NGAL with duration of diabetes.39 Research made by de Carvalho et al also found that there was a positive correlation between uKIM-1 and uNGAL with uACR index (r = 0.64, p <0.001; r = 0.72, p <0.001).41
Although NGAL and KIM-1 had been considered as an early marker for DN since 2000 years,25,42 our study had some differences to earlier studies such as: the socioeconomic status, the average age and the known disease duration, the background diseases of the diabetic subjects. Vietnam is a developing country with the low income so the life and health care system for treatment and control the complications are still limited. Moreover, since 1990, the Southeast Asia region has always been one of five regions had highest DN prevalence.4 The geographic region, race, life style, the health care system and socioeconomic status may affect the diabetic kidney disease. On the other hand, our exclusion criteria for diabetic subject were the background of hypertension, other renal diseases which diseases the patients often affected together with diabetes could eliminate other causes of the kidney disease. The other studies39,41,42 selected the microalbumurine patients group had the average known disease duration 5 years longer than the normoalbuminurine patients group. Our study selected these two groups had similar disease duration (average 5 years) means these complications may appear and could be detected more earlier, especially in the diabetic patients in the high-risk world region as Southeast Asia.
The first limitation of this study is the small sample size, only 30 T2DM patients with normal albuminuria and 30 T2D patients with microalbuminuria were enrolled in this study. This might be not reflected for all disease groups because the numbers of diabetic patients have been increased rapidly. If we had studied in larger number of patients, we would have divided the population into many groups with the level of albuminuria or uACR to understand exactly the correlation and changes of urine NGAL, KIM-1 and ACR. Secondly, we had to collect the spot urine of all patients to quality the markers. Some researchers had improved the diagnostic value of urine ACR.43 But in some cases, that might reflect insufficient the total amount of albuminuria in 24 hours and the status of kidney damage. Thus, the main limitation of our research was the cross-sectional setting so that we could not follow up the patients. So the assessment of kidney damage progress and increasing the markers were not carried out by the time. The indication for biopsy in diabetic nephropathy was still limited. So that the relationship between these markers to histopathonic injury has not been found. Besides, NGAL depends on many factors such as body mass index, age,36 the re-absorbance itself in renal.30
Conclusion
KIM-1 and NGAL in urine are independent markers for early diagnostic diabetic nephropathy in Vietnamese population. Prospective researches and studies with larger population or multicenter studies are required to clarify the value of two markers.
Acknowledgments
This is the first study on evaluation of the diagnostic value of urinary NGAL and KIM 1 in type 2 diabetic nephropathy in Vietnam; We would like to thank Military Hospital 103 for creating favorable conditions for the research to be conducted. We also would like to send our deepest thanks to the research team members for their dedication and hard work to complete the study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes Atlas, 9th edition. Elsevier BV. 2019;57:1–10.
2. Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep. 2016;16(1):7. doi:10.1007/s11892-015-0699-x
3. Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62(1):3–16.
4. Thomas B. The global burden of diabetic kidney disease: time trends and gender gaps. Curr Diab Rep. 2019;19(4):18. doi:10.1007/s11892-019-1133-6
5. Dagenais GR, Gerstein HC, Zhang X, et al. Variations in diabetes prevalence in Low-, Middle-, and High-Income Countries: results from the prospective urban and rural epidemiological study. Diabetes Care. 2016;39(5):780–787. doi:10.2337/dc15-2338
6. Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43(2):487–493. doi:10.2337/dci19-0066
7. Ezzati M, Zhou B, Riley L, et al. Challenges of monitoring global diabetes prevalence. Lancet Diabetes Endocrinol. 2017;5(3):162. doi:10.1016/S2213-8587(17)30036-0
8. Ivanac-Jankovic R, Lovcic V, Magas S, Sklebar D, Kes P. The novella about diabetic nephropathy. Acta Clin Croat. 2015;54(1):83–91.
9. Patzer RE, McClellan WM. Influence of race, ethnicity and socioeconomic status on kidney disease. Nat Rev Nephrol. 2012;8(9):533–541. doi:10.1038/nrneph.2012.117
10. Zheng JM, Jiang ZH, Chen DJ, Wang SS, Zhao WJ, Li LJ. Pathological significance of urinary complement activation in diabetic nephropathy: a full view from the development of the disease. J Diabetes Investig. 2019;10(3):738–744. doi:10.1111/jdi.12934
11. Afroz T, Sagar R, Reddy S, Gandhe S, Rajaram KG. Clinical and histological correlation of diabetic nephropathy. Saudi J Kidney Dis Transpl. 2017;28(4):836–841.
12. Jiang H, Shao X, Jia S, et al. The mitochondria-targeted metabolic tubular injury in diabetic kidney disease. Cell Physiol Biochem. 2019;52(2):156–171.
13. Satirapoj B. Tubulointerstitial biomarkers for diabetic nephropathy. J Diabetes Res. 2018;2018:2852398. doi:10.1155/2018/2852398
14. Cai L, Rubin J, Han W, Venge P, Xu S. The origin of multiple molecular forms in urine of HNL/NGAL. Clin J Am Soc Nephrol. 2010;5(12):2229–2235. doi:10.2215/CJN.00980110
15. Castillo-Rodriguez E, Fernandez-Prado R, Martin-Cleary C, et al. Kidney injury marker 1 and neutrophil gelatinase-associated lipocalin in chronic kidney disease. Nephron. 2017;136(4):263–267. doi:10.1159/000447649
16. Colombo M, Looker HC, Farran B, et al. Serum kidney injury molecule 1 and beta2-microglobulin perform as well as larger biomarker panels for prediction of rapid decline in renal function in type 2 diabetes. Diabetologia. 2019;62(1):156–168. doi:10.1007/s00125-018-4741-9
17. Furuya F, Ishii T, Kitamura K. Chronic inflammation and progression of diabetic kidney disease. Contrib Nephrol. 2019;198:33–39.
18. Jagadesan I, Agarwal I, Chaturvedi S, Jose A, Sahni RD, Fleming JJ. Urinary neutrophil gelatinase associated lipocalin - a sensitive marker for urinary tract infection in children. Indian J Nephrol. 2019;29(5):340–344. doi:10.4103/ijn.IJN_276_18
19. Kamianowska M, Szczepanski M, Kulikowska EE, Bebko B, Wasilewska A. The tubular damage markers: neutrophil gelatinase-associated lipocalin and kidney injury molecule-1 in newborns with intrauterine growth restriction. Neonatology. 2019;115(2):169–174. doi:10.1159/000494102
20. Komosinska-Vassev K, Olczyk P, Kuznik-Trocha K, et al. Circulating C1q/TNF-related protein 3, omentin-1 and NGAL in obese patients with type 2 diabetes during insulin therapy. J Clin Med. 2019;8(6):805. doi:10.3390/jcm8060805
21. Members) ISoNKB. Kidney international supplements. Off J Int Soc Nephrol. 2013;3(1):19–62.
22. Zhao X, Chen X, Zhang Y, et al. Kidney injury molecule-1 is upregulated in renal lipotoxicity and mediates palmitate-induced tubular cell injury and inflammatory response. Int J Mol Sci. 2019;20(14):14. doi:10.3390/ijms20143406
23. Tanase DM, Gosav EM, Radu S, et al. The predictive role of the biomarker Kidney Molecule-1 (KIM-1) in Acute Kidney Injury (AKI) cisplatin-induced nephrotoxicity. Int J Mol Sci. 2019;20(20):5238. doi:10.3390/ijms20205238
24. Sun IO, Shin SH, Cho AY, Yoon HJ, Chang MY, Lee KY. Clinical significance of NGAL and KIM-1 for acute kidney injury in patients with scrub typhus. PLoS One. 2017;12(4):e0175890. doi:10.1371/journal.pone.0175890
25. Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14(10):2534–2543. doi:10.1097/01.ASN.0000088027.54400.C6
26. Khawaja S, Jafri L, Siddiqui I, Hashmi M, Ghani F. The utility of neutrophil gelatinase-associated Lipocalin (NGAL) as a marker of acute kidney injury (AKI) in critically ill patients. Biomark Res. 2019;7(1):4. doi:10.1186/s40364-019-0155-1
27. Khan FA, Fatima SS, Khan GM, Shahid S. Evaluation of kidney injury molecule-1 as a disease progression biomarker in diabetic nephropathy. Pak J Med Sci. 2019;35(4):992–996. doi:10.12669/pjms.35.4.154
28. Kim SY, Jeong TD, Lee W, et al. Plasma neutrophil gelatinase-associated lipocalin as a marker of tubular damage in diabetic nephropathy. Ann Lab Med. 2018;38(6):524–529. doi:10.3343/alm.2018.38.6.524
29. Li A, Yi B, Liu Y, et al. Urinary NGAL and RBP are biomarkers of normoalbuminuric renal insufficiency in type 2 diabetes mellitus. J Immunol Res. 2019;2019:5063089. doi:10.1155/2019/5063089
30. Kuwabara T, Mori K, Mukoyama M, et al. Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int. 2009;75(3):285–294. doi:10.1038/ki.2008.499
31. Satirapoj B, Pooluea P, Nata N, Supasyndh O. Urinary biomarkers of tubular injury to predict renal progression and end stage renal disease in type 2 diabetes mellitus with advanced nephropathy: a prospective cohort study. J Diabetes Complications. 2019;33(9):675–681. doi:10.1016/j.jdiacomp.2019.05.013
32. Tervaert TW, Mooyaart AL, Amann K, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21(4):556–563. doi:10.1681/ASN.2010010010
33. Zhu X, Xiong X, Yuan S, et al. Validation of the interstitial fibrosis and tubular atrophy on the new pathological classification in patients with diabetic nephropathy: a single-center study in China. J Diabetes Complications. 2016;30(3):537–541. doi:10.1016/j.jdiacomp.2015.12.002
34. Singh S, Sonkar SK, Sonkar GK, Mahdi AA. Diabetic kidney disease: a systematic review on the role of epigenetics as diagnostic and prognostic marker. Diabetes Metab Res Rev. 2019;35(5):e3155. doi:10.1002/dmrr.3155
35. Aslan O, Demir M, Koseoglu M. Kidney injury molecule levels in type 2 diabetes mellitus. J Clin Lab Anal. 2016;30(6):1031–1036. doi:10.1002/jcla.21976
36. Sueud T, Hadi NR, Abdulameer R, Jamil DA, Al-Aubaidy HA. Assessing urinary levels of IL-18, NGAL and albumin creatinine ratio in patients with diabetic nephropathy. Diabetes Metab Syndr. 2019;13(1):564–568. doi:10.1016/j.dsx.2018.11.022
37. Li L, Zhang X, Li Z, et al. Renal pathological implications in type 2 diabetes mellitus patients with renal involvement. J Diabetes Complications. 2017;31(1):114–121. doi:10.1016/j.jdiacomp.2016.10.024
38. Fernando B, Alli-Shaik A, Hemage RKD, et al. Pilot study of renal urinary biomarkers for diagnosis of CKD of uncertain etiology. Kidney Int Rep. 2019;4(10):1401–1411. doi:10.1016/j.ekir.2019.07.009
39. Zylka A, Dumnicka P, Kusnierz-Cabala B, et al. Markers of glomerular and tubular damage in the early stage of kidney disease in type 2 diabetic patients. Mediators Inflamm. 2018;2018:7659243. doi:10.1155/2018/7659243
40. Kapoula GV, Kontou PI, Bagos PG. Diagnostic accuracy of neutrophil gelatinase-associated lipocalin for predicting early diabetic nephropathy in patients with type 1 and type 2 diabetes mellitus: a systematic review and meta-analysis. J Appl Lab Med. 2019;4(1):78–94. doi:10.1373/jalm.2018.028530
41. de Carvalho JA, Tatsch E, Hausen BS, et al. Urinary kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin as indicators of tubular damage in normoalbuminuric patients with type 2 diabetes. Clin Biochem. 2016;49(3):232–236. doi:10.1016/j.clinbiochem.2015.10.016
42. Kim SS, Song SH, Kim IJ, et al. Clinical implication of urinary tubular markers in the early stage of nephropathy with type 2 diabetic patients. Diabetes Res Clin Pract. 2012;97(2):251–257. doi:10.1016/j.diabres.2012.02.019
43. Liu R, Zhu H, Yang JH, et al. Can urine albumin/creatinine ratio replace 24 hours urinary albumin?. Zhonghua Nei Ke Za Zhi. 2019;58(5):377–381. doi:10.3760/cma.j.issn.0578-1426.2019.05.009
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.