Back to Journals » Infection and Drug Resistance » Volume 16
Evaluation of Tigecycline Utilization and Trends in Antibacterial Resistance from 2018 to 2021 in a Comprehensive Teaching Hospital in China
Authors Zhou H , Sun X, Lyu S, Yu X, Li R, Wang H, An Z
Received 9 November 2022
Accepted for publication 3 February 2023
Published 14 February 2023 Volume 2023:16 Pages 879—889
DOI https://doi.org/10.2147/IDR.S395158
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Hong Zhou,1,* Xiangyu Sun,2,* Shaocheng Lyu,3 Xiaojia Yu,1 Ran Li,4 Huaguang Wang,1 Zhuoling An1
1Pharmacy Department of Beijing Chao-Yang Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Drug and Equipment Department, Beijing Chaoyang Emergency Medical Rescuing Center, Beijing, People’s Republic of China; 3Department of Hepatobiliary Surgery, Beijing Chao Yang Hospital, Capital Medical University, Beijing, People’s Republic of China; 4Department of Infectious Diseases and Clinical Microbiology, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Huaguang Wang; Zhuoling An, Pharmacy Department of Beijing Chao-Yang Hospital, Capital Medical University, Beijing, People’s Republic of China, Email [email protected]; [email protected]
Purpose: Tigecycline, the first glycylcycline antibiotic, which was widely used for off-label indications because of its broad-spectrum antibacterial activity. This study evaluated the indications for clinical use of tigecycline, clinical and microbiological effectiveness, factors associated with in hospital mortality, and bacterial resistance.
Methods: This retrospective study evaluated all inpatients who received tigecycline treatment for > 72 hours between January 2018 and December 2021 in a comprehensive teaching hospital in China. The evaluation included indications, administration regimen, etiology, efficacy and so on. Univariate and multivariate analyses were used to evaluate the risk factors for all-cause mortality.
Results: There were 203 patients treated with tigecycline. Tigecycline was commonly prescribed for off-label indications (83.25%, 169/203), and hospital-acquired pneumonia ranked first (79.29%, 134/169). The most common pathogen was Acinetobacter baumannii. Clinical and microbiological success was 57.14% (116/203) and 32.28% (41/127), respectively. Fifty-four patients died and all-cause mortality was 26.60%. Univariate and multivariate analyses showed no significant difference in age, gender, off-label indication, duration of treatment, combination with other drugs, multidrug-resistant or extensively drug-resistant infections and tigecycline application scoring with respect to mortality.
Conclusion: Although detection of A. baumannii has decreased in the past 4 years in our hospital, resistance to tigecycline has increased. For clinical application, physicians attach importance to detection of pathogenic microorganisms, but there is still empirical medication without bacterial culture reports. Therefore, an antibiotic stewardship program oriented toward tigecycline should be strengthened to curb bacterial resistance.
Keywords: off-label indication, antimicrobial resistance, rationality of medication, clinical prognosis
Introduction
With the emergence of multidrug-resistant and super-resistant bacteria, the problem of bacterial resistance has become a major challenge in global public health, and a concern for governments and society.1–3 In 2020, the National Health Commission of the People’s Republic of China published the theme on continuous management of the clinical application of antibiotics.4
Tigecycline, also known as GAR-936 or Tygacil, is a chemically modified minocycline (9-tbutylglycylamido derivative of minocycline).5 The main mechanism of action of tigecycline is causing protein synthesis inhibition by interaction with the bacterial 30S ribosome subunit.6 Tigecycline was approved by the US Food and Drug Administration (FDA) to treat complicated skin and soft tissue infections (cSSTIs), complicated intra-abdominal infections (cIAIs), and community-acquired pneumonia (CAP).7 The recommended standard dosing regimen for all indications is a 100 mg loading dose followed by 50 mg every 12 h.8 The recommended duration of treatment with tigecycline for cSSTI or cIAI and CAP is 5–14 and 7–14 days, respectively.9 Tigecycline has broad-spectrum antibacterial activity, especially against Gram-negative bacteria resistant to other antibiotics. Therefore, it has been widely used off-label for ventilator-associated pneumonia (VAP), hospital-acquired pneumonia (HAP), and bloodstream infections (BSIs) caused by multidrug-resistant (MDR) and extensively drug-resistant (XDR) pathogens, especially carbapenem-resistant bacteria.8 Consumption of tigecycline has increased as a result of its widespread off-label application in clinical practice and the clinical effectiveness varied between different indications.5 However, resistance to tigecycline has emerged since its approval.10 Related literature about the occurrence of tigecycline resistance to Klebsiella pneumonia and Stenotrophomonas maltophilia have been reported in China.11 Tigecycline was first introduced to the Chinese pharmaceutical market in 2012, but our hospital officially included it into the hospital antibacterial drug list in 2018. In the same year, rules for clinical application of tigecycline were published by the National Health Commission of the People’s Republic of China, which formulated the indications, administration regimen, etiology, efficacy evaluation, and consultation about and prescription of antibiotics for special use.12 According to the China Antimicrobial Surveillance Network (CHINET), which covered 1371 hospitals in China, the national average resistance rates of two commonly treated bacteria with tigecycline, A. baumannii and K. pneumoniae to carbapenems were 53.7% and 10.9%, respectively in 2020.13 Bacteriological monitoring reports in our hospital have shown an upward trend in drug resistance.The aim of this study was to evaluate the indications for clinical use of tigecycline, clinical and microbiological effectiveness, factors associated with in hospital mortality, and bacterial resistance in a comprehensive teaching hospital in China.
Materials and Methods
Setting and Study Design
This single center retrospective observational study was conducted in Beijing Chaoyang Hospital affiliated to Capital Medical University. The hospital is a Level III A hospital integrating medical, teaching, scientific research and prevention under direct leadership of Beijing Municipal Administration of Hospitals, is the third clinical medical college of Capital Medical University, and is also the designated Category A medical institution for Beijing Municipal medical insurance. The study included inpatients who received tigecycline for >72 hours between January 1, 2018 and December 31, 2021, excluding those for whom detailed evaluation data were not available. The protocol was approved by the Drug and Therapeutics Committee and the Ethics Committee of Beijing Chao-Yang Hospital, Capital Medical University (Approval number: 2022-9-19-1).
Data Collection
The demographic information, diagnosis, medication details, biochemical and bacteriological test results of patients were collected from the electronic medical records system. Bacteriological surveillance data from 2018 to 2021 were provided by the Department of Infectious Diseases and Clinical Microbiology, and obtained through the Clinical Microbiology Laboratory Management System. The following data were collected: (1) gender and age; (2) diagnosis, including infection sites and treatment indications; (3) comorbidity, such as diabetes, heart disease, hypertension, hepatic dysfunction, and renal insufficiency; (4) medication details, such as dose, duration of treatment, and concomitant antibiotics; (5) biochemical test results, including alanine aminotransferase, aspartate aminotransferase, total bilirubin, direct bilirubin, indirect bilirubin, and creatinine; (6) microbiology laboratory results, such as specimen type, culture results, and drug sensitivity; and (7) indicators of infection, such as C-reactive protein, procalcitonin, white blood cell count, and erythrocyte sedimentation rate. A quality management plan for the research was developed before the study conducted. The data was collected by two-person and re-checked.
Tigecycline Application Scoring
The rationale for the use of tigecycline was evaluated based on the rules for clinical application of tigecycline, which was constructed and published by the National Health Commission of the People’s Republic of China (Table 1).14 Evaluation included indications, administration regimen, etiology, efficacy, prescription and consultation. There were 100 points in the evaluation form, and points were deducted if the evaluation criteria were not met.
 |
Table 1 Rules for Evaluation of Clinical Application of Tigecycline |
Evaluation of Clinical Outcomes
The “Technical Guidelines for Clinical Trials of Antibacterial Drugs” was used for clinical efficacy evaluation.15 According to the symptoms, signs, laboratory and etiological examinations, the therapeutic effect was divided into four grades: cured, effective, improved and ineffective. Cured referred to the disappearance or return to normal of clinical symptoms, signs, laboratory tests, and/or bacteriological eradication. Effective meant that the condition was significantly improved, but one of the above indicators had not completely returned to normal. Improved was defined as improvement of clinical symptoms and signs relative to the condition before treatment, but not significant relative to cured and effective. In clinical prognostic evaluation, cured, effective and improved were considered to be clinical success. Ineffective referred to unchanged or worsened symptoms and signs, and switching to other anti-infective treatment, which represented clinical failure.
Evaluation of Microbiological Outcomes
Based on microbiological results and drug susceptibility testing, the microbiological efficacy was divided into eradication, no eradication and not available. Eradication was defined as the absence of the original pathogens in culture of specimens collected from the original site, which was identified with microbiological success. No eradication meant the persistence of original pathogens in follow-up cultures from the original infection site after treatment, which was identified with microbiological failure. Not available referred to specimen that was not available for estimation of eradication. In microbiological outcome evaluation, the not available cases were excluded.16
Mortality
The primary outcome of the study was in hospital mortality, which was defined as the status at the time of hospital discharge in survivors and non-survivors.17
Antimicrobial Susceptibility Test
Bacterial identification was performed according to standard microbiological procedures. Antibiotic susceptibility was performed using the VITEK-2 Compact and the disc diffusion method. All microbiological methods were acted in accordance with the Clinical and Laboratory Standards Institute (CLSI) guidelines of the corresponding year and the CLSI breakpoints of the corresponding year was used to identify the antimicrobial susceptibility. A 15-µg disc of tigecycline (Oxoid Ltd, Cambridge, UK) was used to determine susceptibility, and the breakpoints suggested by the FDA for Enterobacteriaceae (susceptible ≥19 mm; intermediate 15–18 mm, and resistant ≤14 mm), and gram-positive microorganisms (susceptible ≥19 mm) were used.18 Tigecycline clinical breakpoints using the disc-diffusion method against A. baumannii have not been established by both the CLSI and the European Committee on Antimicrobial Susceptibility Testing (EUCAST). Our laboratory applied the clinical breakpoints recommended by the expert consensus of operating procedures for tigecycline in vitro sensitivity test published in 2013 (susceptible ≥16 mm, intermediate 13–15 mm, and resistant ≤12 mm).19
Statistical Analysis
The data were analyzed using SPSS version 26.0 (SPSS, Inc, Chicago, IL, USA). The numerical variables were represented by mean ± standard deviation. The categorical variables were expressed by number and percentage. Univariate analysis was used to evaluate the effects of gender, age, course of treatment, off-label drug use, and combined drug use on mortality. Chi-square tests were used for univariate analysis of categorical variables. P<0.05 was considered to indicate statistical significance. Multivariate logistic regression was used to assess all-cause mortality. Results were presented as odds ratio (OR) and 95% confidence interval (CI).
Results
Between January 1, 2018 and December 31, 2021, 274 hospitalized patients were treated with tigecycline. We excluded 56 patients whose treatment course was ≤3 days, and 15 for whom basic information was missing, which left 203 patients for inclusion in the study.
Demographics and Epidemiology
The utilization rate of antibiotics in inpatients from 2018 to 2021 was 33.77%, 31.16%, 41.03% and 35.51%, respectively. At the same time, the utilization rate of tigecycline was 1.394‰, 2.196‰, 2.912‰ and 2.745‰. There were 203 patients, 154 male and 49 female, aged 17–97 years (median 62±14.65 years). All demographic and clinical characteristics are shown in Table 2. Over half the patients (105/203, 51.72%) were older than 65 years. Heart diseases were the main underlying conditions (102/203, 50.25%). Followed by liver-related diseases (93/203, 45.81%). According to Child-Pugh Score (CPS) classification, there were seven cases with CPSc, five with CPSB, and nine with CPSA.20 Pulmonary diseases (80/203, 39.41%) ranked the third. The rate of admission to intensive care unit was 73.89% (150/203).
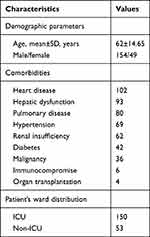 |
Table 2 Demographics and Epidemiology of Patients Treated with Tigecycline |
Diagnosis and Strategy of Treatment
Most infections were pulmonary or abdominal, and most cases were complicated with multiple infections (As shown in Table 3). Patients with indications for FDA-approved use of tigecycline included 18 with CAP, 14 with cIAIs, and two with cSSTIs. Off-label indications included 134 patients with HAP, 24 with BSI, nine with sepsis, and one each with VAP or urinary tract infection (UTI). We found that only 34 (16.75%) patients were treated with tigecycline for an indication approved by the FDA or European Medicines Agency. Conversely, the number of off-label indications was at a significantly high rate of 83.25% (169 patients). Tigecycline was combined with other antibiotics in 186 (91.62%) cases. Tigecycline was most frequently combined with cefoperazone–sulbactam (52/186, 27.96%) followed by carbapenems (29/186, 15.59%) and amikacin (11/186, 5.91%). The duration of treatment with tigecycline was 3–41 days; 52 patients were treated within 7 days, 52 for >14 days, and 99 within 7–14 days. The median length of hospital stay was 41±33.07 days, and the mean length of tigecycline treatment was 11±5.86 days.
 |
Table 3 Indications, Duration and Treatment Strategy in Patients Receiving Tigecycline |
Bacterial Isolates and Drug Susceptibility
All the 203 patients were sent for etiology test, and the positive proportion of pathogen culture results was 97.54% (198/203). The main sources of bacteria were sputum, bronchoalveolar lavage fluid, ascites, and venous whole blood; among which, sputum specimens accounted for 43.60% (194/445). A total of 55 bacteria were isolated. The top 10 were A. baumannii (n=110), Klebsiella pneumoniae (n=86), Pseudomonas aeruginosa (n=35), Enterococcus faecium (n=27), Stenotrophomonas maltophilia (n=21), Staphylococcus epidermidis (n=18), Escherichia coli (n=18), Enterobacter cloacae (n=14), Staphylococcus aureus (n=11), and Staphylococcus hominis subspecies hominis (n=9). We detected 236 drug-resistant bacteria among the above-mentioned strains (detected as different secretions from patients). Among them, MDR A. baumannii was the most commonly detected strain, followed by MDR K. pneumoniae (Table 4).
 |
Table 4 Distribution of Top 10 Detected Bacteria and Drug Sensitivity |
Trends in Microbiology and Antibacterial Resistance
In the past 4 years, the top five most frequently isolated Gram-negative bacilli in our hospital were E. coli, P. aeruginosa, K. pneumoniae, A. baumannii and E. cloacae. E. coli was always listed as the most common isolate from 2018 to 2021. The percentage of K. pneumoniae isolates showed a decreasing trend from 17.98% in 2018 to 17.18% in 2020, and then increasing to 20.09% in 2021. A. baumannii decreased from 12.52% in 2018 to 7.24% in 2021 (Figure 1). These two types of bacteria were also the most common bacteria among patients treated with tigecycline.
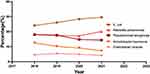 |
Figure 1 Top five Gram-negative bacilli isolated from 2018 to 2021. |
A. baumannii had a high rate of resistance against six commonly used antibiotics. The resistance rates of A. baumannii to cefoperazone sulbactam, piperacillin-tazobactam, imipenem, meropenem, amikacin and tigecycline was 60.60%, 64.54%, 63.56%, 63.12%, 62.54% and 32.71%, respectively. The resistance rates of K. pneumoniae against those six commonly used antibiotics was 25.62%, 19.32%, 15.05%, 14.36%, 11.00% and 24.04%, respectively. The year by year trends were shown in Figures 2–3.
 |
Figure 2 Resistance profile of Acinetobacter baumannii species for six commonly used antimicrobials from 2018 to 2021. |
 |
Figure 3 Resistance profile of Klebsiella pneumoniae species for six commonly used antimicrobials from 2018 to 2021. |
Rationale for Tigecycline Use and Outcome
The rationale for tigecycline prescription was judged according to its evaluation for clinical application. The result was shown in Table 5. Among the 203 cases, one hundred and sixty-three (80.30%) scored 100 points, which fully met the evaluation criteria of four aspects in Table 1. Eight cases (3.94%) were 90 points, and the deduction reason was lack of specialist consultation records. Thirty-two (15.76%) cases were 0 points because of without indications listed in Table 1, such as UTI.
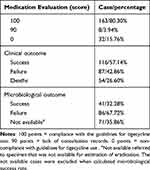 |
Table 5 Rationale for Tigecycline Use and Outcome |
Microbiological evaluation was based on the 198 patients whose pathogen culture was positive. The microbiological success rate was 32.28% (41/127). According to the efficacy evaluation in “Technical Guidelines for Antimicrobial Clinical Trials”, among the 203 patients who received tigecycline, 116 (57.14%) achieved clinical success and 87 (42.86%) were judged as clinical failure. This included 54 patients who died, which resulted in an all-cause mortality rate of 26.60%. Univariate and multivariate analyses showed that there was no significant difference in age, gender, off-label indication, duration of treatment, combination with other drugs, MDR or XDR infections and tigecycline application scoring. With respect to comorbidities, patients with cardiovascular disease had a higher mortality rate than those without cardiovascular disease by univariate analysis (P=0.012) and multivariate analysis (OR=2.111, 95% CI 1.006–4.179, P=0.032) (Table 6). The univariate analysis (P=0.001) and multivariate analysis (OR=4.554, 95% CI 1.667–12.441, P=0.003) showed a significant difference in the mortality in the patients admission to ICU.
 |
Table 6 Analysis of Risk Factors Associated with Mortality |
Discussion
Tigecycline was first approved by the FDA in 2005 for treatment of cIAIs and cSSTIs in adults.21 In 2009, the FDA added CAP in adults to the list of approved indications. Tigecycline has demonstrated promising in vitro activity against extended spectrum beta-lactamase-producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and extensively drug-resistant A. baumannii.22–25 Because of the lack of other treatment options, tigecycline was prescribed for off-label indications such as HAP, VAP, UTI, sepsis, bacteremia, and febrile neutropenia.26,27 In an Chinese consensus statement, tigecycline was recommended for the treatment of XDR-Enterobacteriaceae and XDR-A. baumannii.28 Among the 203 cases included in this study, there were more off-label indications (83.25% 169/203) than FDA-approved indications (16.75%, 34/203). It was accordance with previous studies.27 HAP (79.29%, 134/169) was the most common indication for off-label use. However, the application of off-label indications remains controversial. The 2016 guidelines for the diagnosis and treatment of HAP jointly formulated by the Infectious Disease Society of America and American Thoracic Society clearly oppose the use of tigecycline for treatment of HAP caused by A. baumannii. These recommendations place a higher value on avoiding potential adverse effects of combination therapy with rifampicin and colistin, than on achieving increased microbial eradication rate, because eradication rate was not associated with improved clinical outcome.29 Although strongly recommended, the quality of the evidence is low. The etiology and bacterial resistance in China differ from those in the USA; therefore, more clinicians agree that the existing evidence is far from denying the value of tigecycline for treatment of A. baumannii-induced HAP. At present, tigecycline-based combination therapy is an indispensable and important option for HAP caused by A. baumannii in China.30
According to the regulation of antibiotics grading management in China, tigecycline belongs to the special use class of antibiotics. This means that it can be used only after consultation with a clinician or clinical pharmacist specialized in infection. In special cases, it can be used for 24 hours and recorded in the medical records. In our study, eight patients (3.94%) lacked consultation records; therefore, the rationale evaluation was 90 points. In the rules for evaluation in Table 1, the indications for tigecycline have been extended from CAP, cIAI and cSSTI to MRAB and CRE infections (central nervous system infections and UTIs not included). We had 32 patients (15.76%) which did not meet the above indications; one of whom received tigecycline for treatment of UTI. Tigecycline has good tissue penetration and distribution in bone, liver, spleen and kidney, but only 33% of the total dose is excreted unchanged in urine.31 A meta-analysis of 19 studies showed that clinical cure was 77.4% and microbiological eradication accounted for 65.2% of these cases.32 However, only 31 patients were included in that study, and considering the pharmacokinetic characteristics of tigecycline, it is still not recommended for UTI treatment.
A. baumannii is a cause of serious healthcare-associated infections worldwide.33 Mortality associated with invasive A. baumannii infection is high, especially for carbapenem-resistant cases. Crude mortality for carbapenem-resistant A. baumannii infections ranges from 16% to 76%, as opposed to 5–53% for carbapenem-susceptible infections.34 In our study, the total mortality was 26.6%. Univariate and multivariate analyses showed that patient with cardiovascular disease and patients admitted to ICU had significant effect on all-cause mortality. In September 2013, the FDA approved a new box warning about an increased risk of death with tigecycline, and updated the warnings and precautions and adverse reactions sections. Therefore, tigecycline should be reserved for use in situations in which alternative treatments are not suitable.35
This study had some limitations. First, it was a single center retrospective analysis and the sample size was limited. There was potential for inclusion bias because some basic information was missing. The observed microbiological success rate was subject to surveillance bias because some cases did not have follow-up cultures. Nevertheless, we believe this was a meaningful comparative clinical study in which we analyzed the rationale for tigecycline use, clinical efficacy, and risk factors for all-cause mortality. In the future, a prospective multicenter study should be conducted to determine the efficacy and safety of tigecycline.
Conclusion
We found that tigecycline is commonly used in off-label indications, among which, HAP ranked first. From the bacteriological perspective, tigecycline is mostly used for treatment of A. baumannii infection. Although the detection rate of A. baumannii has decreased in the past 4 years, resistance to tigecycline has increased in our hospital. In clinical applications, physicians attach importance to detection of pathogenic microorganisms, but there is still empirical medication without bacterial culture reports. Therefore, antibiotic stewardship programs oriented toward tigecycline should be strengthened to curb bacterial resistance.
Ethics Approval and Consent to Participate
The study protocol was approved by the Ethics Committee of Beijing Chaoyang Hospital, Capital Medical University and was conducted in accordance with the principles of the Declaration of Helsinki. Patient information collected in the case system did not contain name, address or other personal information, so the patient’s written informed consent was exempt.
Acknowledgments
We thank Cathel Kerr, BSc, PhD, from Liwen Bianji (Edanz) for editing the English text of a draft of this manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Huemer M, Mairpady Shambat S, Brugger SD, Zinkernagel AS. Antibiotic resistance and persistence-Implications for human health and treatment perspectives. EMBO Rep. 2020;21(12):e51034. doi:10.15252/embr.202051034
2. Köck R, Becker K, Idelevich EA, et al. Prevention and control of multidrug-resistant bacteria in the Netherlands and Germany-the impact of healthcare structures. Int J Environ Res Public Health. 2020;17(7):2337. doi:10.3390/ijerph17072337
3. Blake KS, Choi J, Dantas G. Approaches for characterizing and tracking hospital-associated multidrug-resistant bacteria. Cell Mol Life Sci. 2021;78(6):2585–2606. doi:10.1007/s00018-020-03717-2
4. National Health Commission. Notice on continuing to do a good job in clinical application management of antibacterial drugs. National Health Office Medical Letter [2020, No. 8]; 2020. Available from: http://www.gov.cn/zhengce/zhengceku/2020-07/24/content_5529693.htm.
5. Yaghoubi S, Zekiy AO, Krutova M, et al. Tigecycline antibacterial activity, clinical effectiveness, and mechanisms and epidemiology of resistance: narrative review. Eur J Clin Microbiol Infect Dis. 2022;41(7):1003–1022. doi:10.1007/s10096-020-04121-1
6. Kaewpoowat Q, Ostrosky-Zeichner L. Tigecycline: a critical safety review. Expert Opin Drug Saf. 2015;14(2):335–342. doi:10.1517/14740338.2015.997206
7. Brink AJ, Bizos D, Boffard KD, et al. Guideline: appropriate use of tigecycline. S Afr Med J. 2010;100(6 Pt 2):388–394. doi:10.7196/samj.4109
8. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
9. LiverTox. Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
10. Hoban DJ, Bouchillon SK, Johnson BM, Johnson JL, Dowzicky MJ. In vitro activity of tigecycline against 6792 gram-negative and gram positive clinical isolates from the global tigecycline evaluation and surveillance trial (test program, 2004). Diagn Microbiol Infect Dis. 2005;52:215–227. doi:10.1016/j.diagmicrobio.2005.06.001
11. Zhao J, Liu Y, Liu Y, et al. Frequency and genetic determinants of tigecycline resistance in clinically isolated stenotrophomonas maltophilia in Beijing, China. Front Microbiol. 2018;9:549. doi:10.3389/fmicb.2018.00549
12. National Health Commission. Notice on Printing and Distributing 3 Technical Documents including Expert Consensus on Clinical Application of Carbapenems Antibacterial Drugs. National Health Office Medical Letter [2018, No. 822]; 2018. Available from: http://www.nhc.gov.cn/yzygj/s7659/201809/95f65ca473b44746b24590e94468b8ff.shtml.
13. China Antimicrobial Resistance Surveillance System. National antimicrobial resistance surveillance report. CARSS; 2020. Available from: http://www.carss.Cn.
14. National Health and Family Planning Commission. National action plan for containing bacterial resistance. Available from: http://www.nhc.gov.cn/yzygj/s7659/201809/95f65ca473b44746b24590e94468b8ff.shtml.
15. Guidance for clinical trials of anti-bacterial drugs; 2015. Available from: https://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20150403120001449.html.
16. Montravers P, Dupont H, Bedos JP, Bret P; The Tigecycline Group. Tigecycline use in critically ill patients: a multicentre prospective observational study in the intensive care setting. Intensive Care Med. 2014;40:988–997. doi:10.1007/s00134-014-3323-7
17. Wang X, Wang Q, Cao B, et al. Retrospective observational study from a Chinese network of the impact of combination therapy versus monotherapy on mortality from carbapenem-resistant Enterobacteriaceae bacteremia. Antimicrob Agents Chemother. 2019;63(1):e01511. doi:10.1128/AAC.01511-18
18. Wang H, Yu YS, Wang MG, et al. Expert consensus on the operating procedures of tigecycline in vitro drug sensitivity test. Chin J Lab Med. 2013;36(7):584–587. doi:10.3760/cma.j.issn.1009-9158.2013.07.004
19. Babaei S, Haeili M. Evaluating the performance characteristics of different antimicrobial susceptibility testing methodologies for testing susceptibility of gram-negative bacteria to tigecycline. BMC Infect Dis. 2021;21(1):709. doi:10.1186/s12879-021-06338-7
20. Amann LF, Alraish R, Broeker A, Kaffarnik M, Wicha SG. Tigecycline dosing strategies in critically ill liver-impaired patients. Antibiotics. 2022;11(4):479. doi:10.3390/antibiotics11040479
21. Levonorgestrel I. Drugs and Lactation Database (Lactmed) [Internet]. Bethesda (MD): National Library of Medicine (US); 2006.
22. McKeage K, Keating GM. Tigecycline: in community-acquired pneumonia. Drugs. 2008;68(18):2633–2644. doi:10.2165/0003495-200868180-00008
23. Xu L, Wang YL, Du S, Chen L, Long LH, Wu Y. Efficacy and safety of tigecycline for patients with hospital-acquired pneumonia. Chemotherapy. 2016;61(6):323–330. doi:10.1159/000445425
24. Wang J, Pan Y, Shen J, Xu Y. The efficacy and safety of tigecycline for the treatment of bloodstream infections: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob. 2017;16(1):24. doi:10.1186/s12941-017-0199-8
25. Babinchak T, Ellis-Grosse E, Dartois N, et al. The efficacy and safety of tigecycline for the treatment of complicated intraabdominal infections: analysis of pooled clinical trial data. Clin Infect Dis. 2005;41:354–367. doi:10.1086/431676
26. Ellis-Grosse EJ, Babinchak T, Dartois N, et al. The efficacy and safety of tigecycline in the treatment of skin and skin structure infections: results of 2 double-blind Phase 3 comparison studies with vancomycin-aztreonam. Clin Infect Dis. 2005;41:341–353. doi:10.1086/431675
27. Moghnieh RA, Abdallah DI, Fawaz IA, et al. Prescription patterns for tigecycline in severely ill patients for Non-FDA approved indications in a developing country: a compromised outcome. Front Microbiol. 2017;8:497. doi:10.3389/fmicb.2017.00497
28. Guan X, He L, Hu B, et al.; Chinese XDR Consensus Working Group. Laboratory diagnosis, clinical management and infection control of the infections caused by extensively drug-resistant Gram-negative bacilli: a Chinese consensus statement. Clin Microbiol Infect. 2016;Suppl 1. S15–S25. doi:10.1016/j.cmi.2015.11.004
29. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated Pneumonia: 2016 clinical practice guidelines by the infectious disease Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):61–111. doi:10.1093/cid/ciw353
30. Liu YN. Whether tigecycline can be used to treat hospital-acquired pneumonia caused by Acinetobacter. Chin Tuber Respir Dis. 2016;39(10):753–754. doi:10.3389/fmed.2022.772372
31. Meagher AK, Ambrose PG, Grasela TH, et al. The pharmacokinetic and pharmacodynamic profile of tigecycline. Clin Infect Dis. 2005;5:333–340. doi:10.1086/431674
32. Liu YX, Le KJ, Shi HY, et al. Efficacy and safety of tigecycline for complicated urinary tract infection: a systematic review. Transl Androl Urol. 2021;10(1):292–299. doi:10.1086/431674
33. Viehman JA, Nguyen MH, Doi Y. Treatment options for carbapenem resistant and extensively drug-resistant Acineto bacter baumannii infections. Drugs. 2014;74:1315–1333. doi:10.1007/s40265-014-0267-8
34. Lemos EV, de la Hoz FP, Einarson TR, et al. Carbapenem resistance and mortality in patients with Acineto bacter baumannii infection: systematic review and meta-analysis. Clin Microbiol Infect. 2014;20(5):416–423. doi:10.1111/1469-0691.12363
35. US FDA. FDA Drug Safety Communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new Boxed Warning; 2013. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-increased-risk-death-iv-antibacterial-tygacil-tigecycline.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
