Back to Journals » Biologics: Targets and Therapy » Volume 14
Evaluation of Therapeutic Drug Monitoring in the Clinical Management of Patients with Rheumatic Diseases: Data from a Retrospective Single-Center Cohort Study
Authors Pedersen L, Szecsi PB , Johansen PB, Bjerrum PJ
Received 12 May 2020
Accepted for publication 24 July 2020
Published 29 October 2020 Volume 2020:14 Pages 115—125
DOI https://doi.org/10.2147/BTT.S262511
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Doris Benbrook
Lise Pedersen, 1 Pal Bela Szecsi, 1 Per Birger Johansen, 2 Poul Jannik Bjerrum 1
1Department of Clinical Biochemistry, University of Copenhagen, Holbæk Hospital, Holbæk, Denmark; 2Department of Rheumatology, University of Copenhagen, Holbæk Hospital, Holbæk, Denmark
Correspondence: Lise Pedersen Department of Clinical Biochemistry
University of Copenhagen, Holbæk Hospital, Holbæk Hospital, Smedelundsgade 60, Holbæk 4300, Denmark
Tel +45 59484403
Email [email protected]
Purpose: Treatment of rheumatic diseases with tumor necrosis factor inhibitors leads to improved clinical outcomes. Therapeutic drug monitoring (TDM) may assist in guiding clinical decisions. This study investigates the impact of TDM on clinical outcome, decision-making and biologics cost expenditure.
Patients and Methods: In a retrospective observational study of 306 patients with rheumatic diseases treated with four different tumor necrosis factor inhibitors, drug levels and antidrug antibodies were measured over a period of one year. Primary outcomes were the clinicians’ response to each TDM result and the clinical outcome two years after TDM initiation. Outcomes were compared between the 111 TDM-guided patients and the 195 empirically guided patients.
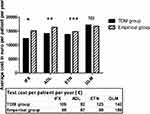
Results: Treatment change occurred in 55% of the patients in the TDM group, but in only 38% in the empirically guided group. In the TDM group, 89 (79.5%) patients were in remission or had low disease activity after two years follow-up compared to 128 (65.6%) patients in the empirical group. The average cost of biologics per patient per year was lower in the TDM group than in the empirical group for patients receiving infliximab, adalimumab or etanercept at baseline but not for golimumab.
Conclusion: TDM-guided decision-making is useful in rheumatic patients receiving TNFi and may optimize therapeutic decisions, leading to a better control of disease activity. Proactive TDM may support decisions on dose tapering, resulting in lower drug consumption and biologics cost expenditure.
Keywords: infliximab, adalimumab, etanercept, golimumab, anti-drug antibodies
Introduction
Rheumatic diseases are common in the population (prevalence ≈3%) and were previously highly debilitating. The development of biological disease-modifying anti-rheumatic drugs, including tumor necrosis factor inhibitors (TNFi), has greatly improved clinical outcomes. However, not all patients respond (primary non-response), and some patients lose effect over time (secondary loss of response), due to the development of anti-drug antibodies (ADA) and low serum concentrations of TNFi. In addition, some patients experience adverse events leading to discontinuing treatment. Adjustment of treatment is largely empirically based, but recently, therapeutic drug monitoring (TDM) guidance with measurement of drug concentration trough level and anti-drug antibody levels have emerged.1–4 TDM may be used both reactively and proactively. Reactive TDM is used in patients with loss of response to guide dose intensification or treatment change, and has shown to be cost-effective compared to empirical treatment.1–3 Proactive TDM is used in patients in remission to minimize future loss of response due to sub-therapeutic drug levels or to reduce overtreatment. Emerging evidence suggests that proactive TDM may improve the clinical outcome in inflammatory bowel disease. However, TDM is not recommended in recent guidelines.4–6 Here, we describe the everyday clinical use of both reactive and proactive TDM in a group of patients with rheumatic diseases, and compare those with results for a group of patients that were only treated empirically. Patient outcomes after two years and biologics expenditure are evaluated.
Method
Study Design
This retrospective, observational cohort study included patients with rheumatoid arthritis, ankylosing spondylitis or psoriatic arthritis treated with either infliximab (IFX); adalimumab (ADL); etanercept (ETN) or golimumab (GLM). TDM measurements were performed in the period of September 2017 to September 2018 every 3–4 months, depending on dose interval. Each measurement consisted of both drug level and ADA level and samples were taken immediately before drug administration (trough levels). The TDM measurements were supplemented with algorithms for reactive and proactive TDM (Figure 1). Treatment of some patients was guided by at least one TDM result and an algorithm (TDM group). Treatment of the remaining patients was not guided by TDM because some rheumatologist preferred an empirical approach without taking TDM into account (empirical group). Patients that had received less than two TNFi doses at baseline were excluded. TDM measurements not taken as trough levels were excluded. Clinical outcome was evaluated for all patients at TDM start (baseline) and after two years. Data were retrieved from patient records (diagnosis, gender, concomitant immune suppressor, clinical health assessment and drug amount/dose interval, as well as trough level TNFi and ADA). The disease activity was classified with DAS28 score or descriptive evaluations such as “in remission”, “good clinical response” or “rheumatologically doing well”. A DAS28 score <2.6 indicated remission while DAS28≤3.2 indicated low disease activity. Patients were categorized as “non-responders” in case of “no clinical effect” or “rheumatologically not well”. All adverse events, serious or non-serious; expected or unexpected; and study-related, possibly study-related, or not study-related were registered in the medical records. Loss of response was defined as primary non-response (absence of clinical improvement 12–20 weeks after initiation of treatment) or as secondary loss of response. Primary outcomes were treatment change in each group, clinical response at two years follow-up and biologics cost expenditure.
Cost Estimation
The total cost of the biologic treatment for each patient per year was summed (number of administrations per year x duration x dosage price). During a treatment pause, the dosage price was set to zero. The average cost for biologics and TDM per patient per year was calculated for each of the four TNFi. The dosage price is based on the manufacturers’ retail prices in Denmark as of September 2019 (Supplementary Table 1). The TDM test cost price (TNFi concentration and ADA) were 45 € for infliximab, adalimumab and etanercept and 60 € for golimumab. Cost estimations did not include utensils, human resources or immune suppressors for each treatment strategy.
Therapeutic Drug Monitoring and Algorithms
The trough level of drugs and ADA was measured using ELISA kits (Promonitor-ADL #5080230000; Promonitor-Anti-ADL #5090230000; Promonitor-IFX #5060230000; Promonitor-anti-IFX #5070230000; Promonitor-ETN #5110230000; Promonitor-anti-ETN#5120230000; Promonitor-GLM #5200230000 and Promonitor-anti-GLM #5210230000) (Progenika, Derio, Spain) on a Triturus 4-Plate platform (Grifols, Barcelona, Spain). Drug concentrations were expressed in mg/L, and cut-off values for ADA in arbitrary units according to the manufacture’s instruction, except for anti-IFX (>2 AU/mL). The cut-off value for anti-IFX were adjusted to the detection limit as the initial validation showed clearance of IFX at this level. Between patient variability was calculated based on the first TDM TNFi through level in all patients. The within patient variability were calculated in the subgroup of patients that had more than three consecutive TNFi trough level measurements and were not changed in treatment during the one year TDM measurement period. TDM algorithms for responders (proactive) and non-responders (reactive) are shown in Figure 1.
Statistics and Ethics
Data were analyzed using SPSS Version 21 (IBM, Armonk, NY, USA) or GraphPad Prism version 5.0, (GraphPad, La Jolla, CA, USA). Descriptive statistics were used generally, Mann–Whitney U-test for comparison of continuous variables. Receiver operating curves (ROC) and calculation of the area under curve were used to establish optimal discrimination trough level (cut-off values) for discriminate responders from non-responders and for predicting remission/low disease activity at follow-up. A post hoc power analysis of the cost estimation in the two treatment groups were performed (PS: Power and Sample Size Calculation version 3.1.6, October 2018).
The study was conducted in accordance with the principles of the Declaration of Helsinki, but being part of current clinical practice, it was not considered a bioethics project according to the definition of the Danish Act on the Bioethics Committee System and the Processing of Bioethics Projects, and ethical approval and written informed consent were waived. Patient data were obtained from the medical records by the treating physicians in compliance with The Danish Data Protection Agency and local data protection and privacy regulations. Data were subsequently maintained with confidentiality in a secure manner.
Results
Of the 306 included patients, 111 patients' treatment was guided by TDM while the remaining 195 patients' treatment was guided empirically. The treatment response measurements were based on DAS28 scores in 175 patients and descriptive clinical evaluation in 131 patients. Two hundred seventy-six patients (90%) were categorized as in remission/having low disease activity at baseline, while 30 (10%) were categorized as non-responders. Demographic and clinical characteristics at baseline for the two groups appeared balanced (Table 1). The intra-patient variability (≥3 consecutive trough levels) was 23.6% (n=11) for IFX, 26.7% (n=12) for ADL, 23.2% (n=26) for ETN, and 45.7% (n=14) for GLM. Between-patient variability at first TDM was 90.5% (n=67) for IFX, 49.8% (n=60) for ADA, 63.3% for ETN (n=137) and 82.3% (n=39) for GLM. At first TDM, trough levels <1 mg/L for IFX were prevalent in non-responders n=3 (4%), but also in some patients in remission/having low disease activity n=9 (13%) of IFX patients (Table 2). Most (8/12) patients with low IFX trough levels at TDM start subsequently had treatment failure primarily due to the presence of ADA (data not shown). Interestingly, unmeasurable IFX trough levels were also seen in four patients without ADA, all with subsequent loss of response. Low ADL trough levels and ADA were found in two patients; one with loss of response resulting in a reactive change to another TNFi, and one with low disease activity that was proactively taken of treatment for seven months before being switched to another TNFi at signs of relapse (data not shown). A further two ADL patients were found to have unmeasurable levels of both TNFi through levels and ADA (double negative) that could not be explained by patient non-compliance. Both of these patients displayed loss of response. No ADA was detected in any ETN or GLM patients. However, there were four double negative ETN patients (unmeasurable levels of both TNFi and ADA). The median trough level in 39 GLM patients was 0.7 mg/L (interquartile range: 0.3–2.2 mg/L), with no difference between patients in remission/with low disease activity and non-responders at baseline or at follow-up. Remission/low disease activity at TDM start was observed in 19 patients (49%) with GLM trough levels <0.5 mg/L. Only a single patient with initial low GLM trough level below 0.5 mg/L subsequently experienced loss of response. Trough TNFi levels were lower in IFX patients receiving concomitant methotrexate compared to monotherapy, with median: 3.9 mg/L (2.5–5.0 mg/L; 95% confidence interval) vs 5.5 (4.4–7.5 mg/L; 95% confidence interval), P < 0.05. However, no differences in trough levels were observed in patients treated with ADL, ETN or GLM and receiving concomitant methotrexate compared to monotherapy. Serum trough levels for each TNFi in patients that are categorized as non-responders or in remission/having low disease activity at baseline as well as after two years follow-up are shown in Table 2. Receiver operating characteristic analysis and discrimination threshold for remission/low disease activity at follow-up indicated a cut-off level for IFX at 2.9 mg/L (P=0.001): however, the classification model’s performance for ADL, ETN and GLM was poor (Table 3). In the present study, ADA was found in 13% of IFX patients and in 3% of ADL patients. Patients with detectable ADA had higher baseline C-reactive protein values compared to patients without ADA (P=<0.0001) (data not shown), reflecting poor inflammatory suppression. Interestingly, only 5/11 (45%) patients with ADA displayed loss of response at the time of ADA detection and a single patient with ADA was in clinical remission resulting in a discontinuation of TNFi treatment.
Effect of TDM Results on Clinical Management
In total, 686 TNFi through level measurements were performed with a range of 1–4 measurements in each patient per year. About half (54%, n=370) were in the proposed therapeutic ranges, 15% were below (n=106) and 31% (n=210) were above the proposed therapeutic ranges (Table 4). Most TDM results 77.8% (n=534) did not lead to any change in treatment, even in the TDM-guided group. TDM supported a change in treatment in 61 patients: 20 of the IFX patients, 21 of the ADL patients, 20 of the ETN patients and none of the GLM patients. Proactive changes were made in 40 patients in remission/with low disease activity, while reactive changes were made in 21 patients with loss of response. TDM guiding resulted in dose reduction (30.6%), switch to another TNFi (8%), switch to non-TNFi (9.8%), discontinued administration of biological drugs (3.6%), dose escalation (2.7%) or no change (45%). In some cases, ADA-positive patients were subjected to a proactive treatment pause of 3 to 7 months before switching to another TNFi/non-TNFi (2.7%). In the current study, 34 patients with high TNFi trough levels and low disease activity/in remission were reduced in dose guided by TDM. Thirty (88%) of patients undergoing TDM-guided dose reduction were in remission/had low disease activity at follow-up after two years. The remaining four patients had loss of response and needed rescue therapy. In the empirical group, the majority of treatment strategies involved no changes in treatment (62%) followed by reactive changes to another drug (32%). Flowcharts of the clinical management for each of the two groups are available in supplementary Figure 1. In the TDM group, 89 (80.2%) patients were in remission/had low disease activity at follow-up, compared to 128 (65.6%) in the empirical group. Clinical response and rate of adverse events at two years follow-up for both the TDM- and empirically guided groups are shown in supplementary Table 2. The average cost of biologics per patient per year was lower in the TDM group compared to the empirical group for patients receiving IFX, ADL or ETN at study start (Figure 2). The test cost for each drug was in average 90–159 € per patient per year (Figure 2). A post hoc powers calculation of the cost estimation in the two treatment groups using a common standard deviation within each drug group showed a power between 0.77 and 0.96, except for GLM of 0.11 (TDM resulted in no changes in the GLM group).
 |
Table 4 Changes in Clinical Management After TDM or Empirical Decision. All Values Represent Number (%) Unless Otherwise Indicated |
Discussion
Despite several studies on biological drugs, the clinical utilization of TDM with TNFi remains unclear.7–9 Recently, the American Gastroenterology Association recommended reactive TDM in patients with active disease and data on the clinical benefits of using proactive TDM have emerged.10,11 Routine clinical practice of TDM has also been proposed in treatment of psoriasis.12 To our knowledge, no published studies have reported on the routine clinical use of both proactive and reactive TDM in rheumatic patients. In this study, TDM was used to guide treatment in 111 patients (both proactive and reactive). We offered TDM in the clinic, but not all doctors utilized the possibility. This opened the opportunity for comparison of TDM with conventional treatment (empirical group). Only 22.2% of the TDM measurements made during one year resulted in treatment changes. This is in accordance with other studies regarding TDM and TNFi treatment. TDM resulted in treatment changes in 22% patients with Crohn’s disease, and in 37% of inflammatory bowel disease patients with secondary loss of response.13,14 Similar results have been seen in a pediatric inflammatory bowel disease cohort.15 Although dose reduction has been shown to be a feasible approach, such a proactive empirical approach in patients showing a good response has rarely been employed.16 An open-label randomized trial in rheumatoid arthritis patients with high trough levels concluded that TDM-guided dose reduction was equivalent to a conservative strategy.17 In addition, dose reduction in patients with inflammatory bowel disease and a high IFX trough levels did not increase remission rates, but reduced treatment costs.4 The lower cost expenditure observed in the TDM group may be due to a larger proportion of proactive dose reductions and proactive treatment pauses/discontinuations compared to a larger proportion of patients with no change in treatment in the empirical group. It is not possible to say whether it is proactive or reactive TDM that contributes the most to the lower cost in the TDM group. However, based on the higher proportion of proactive dose reductions and treatment pauses in the TDM group, it seems that that TDM helps in identifying patients with a good clinical response that are overdosed or receive a drug while also having ADA. It is speculative whether any treatment failure in the empirical group might have been avoided if TDM had been used to adjust dose to a target concentration, or if treatment had changed in the acknowledgement of ADA. Our results suggest that TDM-guided treatment results in a larger proportion of patients in remission/with low disease activity after two years compared to the empirically treated group. There are still challenges with regard to identifying candidates for dose reduction, however. One reason for this is the lack of internationally recommended trough levels for dose reduction. The TDM algorithms and cut-off values used in this study were based on data from other studies, as well as our experience.17–21 Trough level cut-off may vary depending on several factors, such as disease nature, phenotype, gender and serum albumin, as well as the measurement method/assay, etc., making it difficult to establish universal ranges.22 Establishing “true” therapeutic ranges and cut-off levels requires large-scale studies relating concentration to effect, with unbiased measurement of the outcomes.
Infliximab, Adalimumab and Etanercept
We identified an IFX trough level threshold around 2.8 mg/L for discriminating between future treatment failure and remission/low disease activity, in line with results for inflammatory bowel disease patients.4,14,23 Accordingly, we adjusted our therapeutic ranges for IFX to 3–7 mg/L. Pouw et al found a correlation between disease activity and ADL trough levels, identifying a therapeutic window of 3–12 mg/L, while Keystone et al recommend a narrower interval, 5–8 mg/L.21,24 In accordance with other studies,17,20 we found that proactive TDM could be used to prolong the dose interval of ADL in patients in remission/with low disease activity having serum trough levels >8 mg/L. This indicates that >8 mg/L may be used as an upper cut-off value for successful dose reduction of adalimumab. Like others, we found a low frequency of ADA in ETN patients.9,28,29 For etanercept patients there was a significant difference between trough levels in patients in remission/with low disease activity and in non-responders at baseline as well as follow-up (Table 3); however, a clear discriminatory threshold as found in previous studies was not seen.30
Golimumab
Only limited data on GLM trough levels are available from other studies, but they compare well with our results. The PURSUIT study found trough levels at 0.69–0.83 mg/L in patients with ulcerative colitis with an estimated threshold of 1.4 mg/L.31 In a prospective, small, observational cohort of rheumatoid arthritis patients median trough levels were 0.55 mg/L (interquartile range: 0.27–1.48), with a significantly higher trough level in responders.32 In the present study, proactive TDM of GLM was of limited use, mainly because no patients were identified above the proposed cut-off values for dose reduction. The retrospective evaluation of data do not support any clear threshold level.
As in some other studies, we found low trough levels of all for TNFis in a number of patients with low disease activity.25–27 The clinical course of rheumatic diseases is marked by exacerbations and remissions, which may occur spontaneously or in response to treatment changes and it is possible that these patients may have attained spontaneous remission and therefore no longer required the presence of drug to achieve a good response.
Anti-Drug Antibodies
Several studies have shown that ADA formation may increase the risk of loss of response.1,33,34 ADA may also be present in patients in clinical remission, as TNF-alpha may fade with time in a subset of patients due to spontaneous remission.34 It is speculative whether more patients in remission and with ADA may be discontinued or be subjected to proactive treatment pauses instead of being immediately switched to another drug. In all our cases, presence of ADA was accompanied by unmeasurably low drug levels, suggesting rapid clearance of drug. Our findings indicate that measurement of ADA is most relevant in cases where IFX and ADL trough levels are <1 mg/L, as found in a mixed cohort of gastroenterology and rheumatology patients on maintenance IFX therapy.35 A proportion of patients had unmeasurable levels of both TNFi and ADA. The actual mechanism for this remains unclear, although BMI, gender, disease activity and albumin levels as well as non-immune clearance may play a role.7,36 Another explanation may also be false-negative results as a result of the bridging ELISA’s inability to detect functionally monovalent IgG4 ADA.
Limitations of Study
Firstly, this study is a small-scale single-center study that is not powered to assessing all branches of the proposed algorithms. Secondly, this study is based on a heterogeneous group of different rheumatic diseases, which may limit the interpretation. Thirdly, the retrospective design and the non-randomization into TDM and empirical groups may result in a potential selection bias and residual confounding. Furthermore, as this is a retrospective study, collected data on clinical outcome rely on record keeping and may be subject to interpretation bias. The study may also be limited by the use of subjective clinical evaluations of treatment response instead of DAS28 scores in 43% of the cases. Lastly, the use of the TDM algorithms was not as strict as would be expected in a clinical trial. For this reason, it is not possible to say whether TDM was the primary driver for treatment changes in all cases in the TDM group. A technical limitation to this study is the inability to measure IgG4 ADA in the presence of circulating TNFi, which may result in underreporting of ADA.
Conclusion
The combined use of both proactive and reactive TDM in the clinical care of patients with rheumatic diseases treated with IFX, ADL or ETN may be associated with improved clinical outcome and lower biologics expenditure compared to empirical treatment. For GLM, there is insufficient data to support the clinical use of proactive TDM, although reactive TDM for clear indications may be of use.
Trial Registration
Not relevant.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Garcês S, Demengeot J, Benito-Garcia E. The immunogenicity of anti-TNF therapy in immune-mediated inflammatory diseases: a systematic review of the literature with a meta-analysis. Ann Rheum Dis. 2013;72(12):1947–1955. doi:10.1136/annrheumdis-2012-202220
2. Steenholdt C. Proactive and reactive therapeutic drug monitoring of biologic therapies in inflammatory bowel disease are complementary, not mutually exclusive. Clin Gastroenterol Hepatol. 2018;16(4):597–598. doi:10.1016/j.cgh.2017.11.033
3. Velayos FS, Kahn JG, Sandborn WJ, Feagan BG. A test-based strategy is more cost effective than empiric dose escalation for patients with Crohn’s disease who lose responsiveness to infliximab. Clin Gastroenterol Hepatol. 2013;11(6):654–666. doi:10.1016/j.cgh.2012.12.035
4. Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148(7):1320–1329. doi:10.1053/j.gastro.2015.02.031
5. Papamichael K, Juncadella A, Wong D, et al. Proactive therapeutic drug monitoring of adalimumab is associated with better long-term outcomes compared with standard of care in patients with inflammatory bowel disease. J Crohns Colitis. 2019;13(8):976–981. doi:10.1093/ecco-jcc/jjz018
6. Vaughn BP, Martinez-Vazquez M, Patwardhan VR, Moss AC, Sandborn WJ, Cheifetz AS. Proactive therapeutic concentration monitoring of infliximab may improve outcomes for patients with inflammatory bowel disease: results from a pilot observational study. Inflamm Bowel Dis. 2014;20(11):1996–2003. doi:10.1097/MIB.0000000000000156
7. Jani M, Chinoy H, Warren RB, et al. Clinical utility of random anti-tumor necrosis factor drug-level testing and measurement of antidrug antibodies on the long-term treatment response in rheumatoid arthritis. Arthritis Rheumatol. 2015;67(8):2011–2019. doi:10.1002/art.39169
8. Mazilu D, Opriş D, Gainaru C, et al. Monitoring drug and antidrug levels: a rational approach in rheumatoid arthritis patients treated with biologic agents who experience inadequate response while being on a stable biologic treatment. Biomed Res Int. 2014;2014:702701. doi:10.1155/2014/702701
9. Chen D-Y, Chen Y-M, Tsai W-C, et al. Significant associations of antidrug antibody levels with serum drug trough levels and therapeutic response of adalimumab and etanercept treatment in rheumatoid arthritis. Ann Rheum Dis. 2015;74(3):e16–e16. doi:10.1136/annrheumdis-2013-203893
10. Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S. American gastroenterological association institute clinical guidelines committee. American gastroenterological association institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology. 2017;153(3):827–834. doi:10.1053/j.gastro.2017.07.032
11. Papamichael K, Vogelzang EH, Lambert J, Wolbink G, Cheifetz AS. Therapeutic drug monitoring with biologic agents in immune mediated inflammatory diseases. Expert Rev Clin Immunol. 2019;15(8):837–848. doi:10.1080/1744666X.2019.1630273
12. Liau MM, Oon HH. Therapeutic drug monitoring of biologics in psoriasis. Biologics. 2019;13:127–132.
13. Kamperidis N, Middleton P, Tyrrell T, Stasinos I, Arebi N. Impact of therapeutic drug level monitoring on outcomes of patients with Crohn’s disease treated with Infliximab: real world data from a retrospective single centre cohort study. Frontline Gastroenterol. 2019;10(4):330–336. doi:10.1136/flgastro-2018-101024
14. Mitchell RA, Shuster C, Shahidi N, et al. The utility of infliximab therapeutic drug monitoring among patients with inflammatory bowel disease and concerns for loss of response: a retrospective analysis of a real-world experience. Can J Gastroenterol Hepatol. 2016;2016:5203898. doi:10.1155/2016/5203898
15. Deora V, Kozak J, El-Kalla M, Huynh HQ, El-Matary W. Therapeutic drug monitoring was helpful in guiding the decision-making process for children receiving infliximab for inflammatory bowel disease. Acta Paediatr. 2017;106(11):1863–1867. doi:10.1111/apa.14008
16. Den Broeder AA, Creemers MCW, van Gestel AM, van Riel PLCM. Dose titration using the Disease Activity Score (DAS28) in rheumatoid arthritis patients treated with anti-TNF-alpha. Rheumatology. 2002;41(6):638–642. doi:10.1093/rheumatology/41.6.638
17. l’Ami MJ, Krieckaert CL, Nurmohamed MT, et al. Successful reduction of overexposure in patients with rheumatoid arthritis with high serum adalimumab concentrations: an open-label, non-inferiority, randomised clinical trial. Ann Rheum Dis. 2018;77(4):484–487. doi:10.1136/annrheumdis-2017-211781
18. Verhoef LM, van den Bemt BJ, van der Maas A, et al. Down-titration and discontinuation strategies of tumour necrosis factor-blocking agents for rheumatoid arthritis in patients with low disease activity. Cochrane Database Syst Rev. 2019;5:CD010455.
19. van Vollenhoven RF, Østergaard M, Leirisalo-Repo M, et al. Full dose, reduced dose or discontinuation of etanercept in rheumatoid arthritis. Ann Rheum Dis. 2016;75(1):52–58. doi:10.1136/annrheumdis-2014-205726
20. Bouman C, van Herwaarden N, van den Hoogen F, van der Maas A, van den Bemt B, den Broeder AA. Prediction of successful dose reduction or discontinuation of adalimumab, etanercept, or infliximab in rheumatoid arthritis patients using serum drug levels and antidrug antibody measurement. Expert Opin Drug Metab Toxicol. 2017;13(6):597–604. doi:10.1080/17425255.2017.1320390
21. Pouw MF, Krieckaert CL, Nurmohamed MT, et al. Key findings towards optimising adalimumab treatment: the concentration-effect curve. Ann Rheum Dis. 2015;74(3):513–518.
22. Daïen CI, Morel J. Predictive factors of response to biological disease modifying antirheumatic drugs: towards personalized medicine. Mediators Inflamm. 2014;2014:386148. doi:10.1155/2014/386148
23. Adedokun OJ, Sandborn WJ, Feagan BG, et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology. 2014;147(6):1296–1307. doi:10.1053/j.gastro.2014.08.035
24. Keystone EC, Kavanaugh A, Weinblatt ME, Patra K, Pangan AL. Clinical consequences of delayed addition of adalimumab to methotrexate therapy over 5 years in patients with rheumatoid arthritis. J Rheumatol. 2011;38(5):855–862. doi:10.3899/jrheum.100752
25. St Clair EW, Wagner CL, Fasanmade AA, et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46(6):1451–1459. doi:10.1002/art.10302
26. van der Maas A, van den Bemt BJF, Wolbink G, van den Hoogen FHJ, van Riel PLCM, den Broeder AA. Low infliximab serum trough levels and anti-infliximab antibodies are prevalent in rheumatoid arthritis patients treated with infliximab in daily clinical practice: results of an observational cohort study. BMC Musculoskelet Disord. 2012;13:184. doi:10.1186/1471-2474-13-184
27. Lamers-Karnebeek FBG, Jacobs JWG, Radstake TRDJ, van Riel PLCM, Jansen TL. Adalimumab drug and antidrug antibody levels do not predict flare risk after stopping adalimumab in RA patients with low disease activity. Rheumatology. 2019;58(3):427–431. doi:10.1093/rheumatology/key292
28. Sanmarti R, Inciarte-Mundo J, Estrada-Alarcon P, et al. Towards optimal cut-off trough levels of adalimumab and etanercept for a good therapeutic response in rheumatoid arthritis. Results of the INMUNOREMAR study. Ann Rheum Dis. 2015;74(8):e42.
29. Hoshino M, Yoshio T, Onishi S, Minota S. Influence of antibodies against infliximab and etanercept on the treatment effectiveness of these agents in Japanese patients with rheumatoid arthritis. Mod Rheumatol. 2012;22(4):532–540. doi:10.3109/s10165-011-0567-8
30. Daïen CI, Daïen V, Parussini E, Dupuy A-M, Combe B, Morel J. Etanercept concentration in patients with rheumatoid arthritis and its potential influence on treatment decisions: a pilot study. J Rheumatol. 2012;39(8):1533–1538. doi:10.3899/jrheum.111522
31. Adedokun OJ, Xu Z, Marano CW, et al. Pharmacokinetics and exposure-response relationship of golimumab in patients with moderately-to-severely active ulcerative colitis: results from Phase 2/3 PURSUIT induction and maintenance studies. J Crohns Colitis. 2017;11(1):35–46. doi:10.1093/ecco-jcc/jjw133
32. Kneepkens EL, Plasencia C, Krieckaert CL, et al. Golimumab trough levels, antidrug antibodies and clinical response in patients with rheumatoid arthritis treated in daily clinical practice. Ann Rheum Dis. 2014;73(12):2217–2219. doi:10.1136/annrheumdis-2014-205983
33. Ducourau E, Mulleman D, Paintaud G, et al. Antibodies toward infliximab are associated with low infliximab concentration at treatment initiation and poor infliximab maintenance in rheumatic diseases. Arthritis Res Ther. 2011;13(3):R105. doi:10.1186/ar3386
34. Eng GP, Bendtzen K, Bliddal H, et al. Antibodies to infliximab and adalimumab in patients with rheumatoid arthritis in clinical remission: a cross-sectional study. Arthritis. 2015;2015.
35. Barlow NL, Mohammed P, Berg JD. Serum trough infliximab and anti-infliximab antibodies in a cohort of gastroenterology and rheumatology patients’ infliximab therapeutic drug monitoring. Ann Clin Biochem. 2016;53(Pt 4):477–484. doi:10.1177/0004563215604866
36. Mahil SK, Arkir Z, Richards G, Lewis CM, Barker JN, Smith CH. Predicting treatment response in psoriasis using serum levels of adalimumab and etanercept: a single-centre, cohort study. Br J Dermatol. 2013;169(2):306–313. doi:10.1111/bjd.12341
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.