Back to Journals » Clinical Ophthalmology » Volume 13
Evaluation Of The Timing Of Intravitreal Bevacizumab Injection As Adjuvant Therapy To Panretinal Photocoagulation In Patients With Diabetic Macular Edema Secondary To Diabetic Retinopathy
Authors Kartasasmita A , Harley O
Received 23 May 2019
Accepted for publication 6 September 2019
Published 26 September 2019 Volume 2019:13 Pages 1921—1926
DOI https://doi.org/10.2147/OPTH.S216790
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Arief Kartasasmita,1 Ohisa Harley2
1Departement of Ophthalmology, Universitas Padjadjaran/Cicendo Eye Hospital, Bandung, Indonesia; 2Netra Eye Hospital, Bandung, Indonesia
Correspondence: Arief Kartasasmita
Department of Ophthalmology, Universitas Padjadjaran, Jl. Cicendo No 4, Bandung 40141, Indonesia
Tel +62 22 4231280
Email [email protected]
Objective: To evaluate the difference in intravitreal bevacizumab (IVB) injection timing as adjuvant therapy to panretinal photocoagulation in patients with diabetic retinopathy combined with diabetic macular edema.
Methods: This was a retrospective nonrandomized study. Forty eyes with severe non-proliferative diabetic retinopathy (NPDR) or proliferative diabetic retinopathy (PDR) were divided into two groups; the IVB injection prior to, or after, panretinal photocoagulation. Changes in central macular thickness between the two groups were measured.
Results: There was no significant difference in change in central macular thickness between two groups after treatment (p=0.66), neither in eyes with severe NPDR groups (p=0.48) nor eyes with PDR (p=0.82).
Conclusion: IVB injection after panretinal photocoagulation gives insignificant difference in changes in central macular thickness with injection prior to laser treatment in patients with diabetic retinopathy combined with diabetic macular edema.
Keywords: diabetic retinopathy, diabetic macular edema, bevacizumab, panretinal photocoagulation
Introduction
Diabetic retinopathy is the most common microvascular complication of diabetes mellitus and occurs secondary to metabolic aberrations and retinal ischemia characteristic of diabetes mellitus.1 Proliferative diabetic retinopathy (PDR) and diabetic macular edema (DME) are the primary pathologies responsible for vision loss in diabetic eye disease. Panretinal photocoagulation (PRP) is the standard of care for prevention of vision loss in PDR.2 This treatment ablates neurons in the peripheral retina to decrease metabolic demand in the peripheral retina and facilitate oxygen and nutrient supply to the inner retina, alleviating the ischemia that drives neovascularization in PDR. However, PRP may induce macular edema (ME) due to retinal inflammation and increased vascular permeability.3 This PRP-induced ME may cause temporary or permanent vision loss.2
In clinical practice, eyes with untreated PDR may also have DME. Many studies have suggested administering intravitreal anti-VEGF injection as an additive therapy prior to PRP to prevent the progression of DME.4 However, this protocol extends the treatment period for patients. PRP is generally performed 1 week after intravitreal bevacizumab (IVB) injection. Some patients, especially those who live in rural areas or far away from the eye center, have difficulties traveling to the eye center for all the treatments. Like other minimally invasive surgeries, IVB injection requires family consent and internist examination to rule out the possibility of interfering the systemic conditions prior to treatment. These referral patients may be required to spend more time and money to travel to the vitreoretinal center for more treatments. IVB injection directly after laser treatment may be desirable in these patients because both procedures can be performed on the same day. IVB injection is performed after laser photocoagulation because the laser is less invasive and performing IVB injection after laser photocoagulation does not risk contamination at the injection site. Therefore, this protocol has minimal risk of side effects. Administering anti-VEGF therapy on the same day as PRP may also suppress DME more effectively, but the optimal timing for the injection is unknown. The purpose of the present study is to identify the optimal timing of IVB administration as an additive therapy to PRP for the treatment of DME.
Patients And Methods
This study is a nonrandomized retrospective study and was conducted in 2017. The study adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) at the University of Padjajaran. Patient informed consent was not required by the IRB because the data were collected retrospectively, and patient data were deidentified to protect patient privacy. Data were collected retrospectively from the electronic medical records at the Netra Eye Hospital in Bandung, Indonesia of patients that underwent IVB injection before laser treatment and patients treated with PRP before IVB injection. Inclusion criteria included patients >18 years of age with PDR or severe non-proliferative diabetic retinopathy (NPDR) who were treated with the above therapies and complied with testing and follow-up. The diagnostic criteria of NPDR are diabetic retinopathy with one or more of the following: hemorrhage in four quadrants, venous beading in two quadrants, and/or intraretinal microvascular abnormalities in one quadrant.5 PDR is defined as diabetic retinopathy with neovascularization.5 Exclusion criteria included patients with significant media opacities, neovascular glaucoma, and history of intraocular laser treatment or surgery within 3 months of treatment.
Ophthalmic examinations at baseline, before laser and injection treatment, and after treatment included ETDRS uncorrected visual acuity; measurement of IOP; slit lamp examination; indirect ophthalmoscopy; and ocular tomography (OCT) examination. Macular OCT was performed using a Heidelberg Spectralis Machine (Heidelberg Engineering GmbH, Heidelberg, Germany), and it was performed before treatment and 1 month after IVB injection to assess pre- and post-treatment (CMT). CMT or central subfield thickness, also known as foveal thickness, was defined as the average thickness of the macula in the central 1 mm ETDRS grid.6 OCT examination was conducted using the “fast macular volume” protocol, consisting of a 25-line horizontal raster scan covering 20°×20°, centered on the fovea. To ensure that the follow-up scan was in the same macular position, automatic scans in the “reference” and “follow-up” modes were used to obtain similar images to those in the pre-treatment scan. PRP was performed over three sessions at 1-week intervals using a solid-state 532 nm laser (LIGHTLas TruScan 532 Laser with built-in slit lamp biomicroscopy; Lightmed Corporation, San Clemente, CA, USA). An OMRA-PRP 165 (US Ophthalmic, Doral, FL, USA) ocular contact lens was used for PRP. The PRP technique was performed in each session aiming for a minimum of 1500 standard threshold fluence laser spots with a 200 μm spot size, 20 ms pulse duration, and 150–250 power resulting in typical grey-white lesions, evenly covering the entire retinal mid-periphery and periphery, as described previously.7–9 Patients were divided into two groups. Group A received IVB injection 1 week prior to the first session of PRP, while group B received IVB injection 1 week after the last session of PRP. The laser treatment was conducted using a low-duration laser with a 50 ms pulse and 300–500 μm spot with adjusted laser power to produce a grade 2 laser burn.
IVB injection was performed in an operating theatre in sterile conditions with topical anesthesia. After disinfection and draping, 0.05 mL of solution containing 1.25 mg bevacizumab (Avastin; Genetech, Inc., South San Francisco, CA, USA) was injected into the vitreous cavity using a 30-gauge needle on the superior pars plana. The optic nerve head and IOP were assessed after injection, and if IOP was elevated, paracentesis was performed. A topical antibiotic (Ciprofloxacin) was administered four times daily for 5 days post-injection, as described previously.3,4,10 An independent t-test was used to generate p-values, and a p-value <0.05 was considered significant. Statistical analysis was performed using the SPSS statistical software for Windows, version 16.0 (SPSS, Chicago, IL, USA).
Results
A total of 40 eyes of patients with severe NPDR or PDR were included. The patients' age ranged from 47 to 72 years of age (mean, 56.60±6.90) in group A (IVB injection before laser) and 46–76 years of age (mean, 60.70±10.64) in group B (IVB injection after laser). Patient demographics and baseline characteristics are summarized in Table 1. There was no significant difference in baseline data between the two groups. Visual acuity of the patients before and after treatment is shown in Table 1. There were significant differences between the two group on pre- and post-visual acuity.
 |
Table 1 Demographic And Baseline Characteristics Of The Patients |
After treatment, there was no significant difference in change in CMT between two groups (p=0.66), neither in eyes with severe NPDR groups (p=0.48) nor eyes with PDR (p=0.82) (Table 2).
 |
Table 2 Changes In Central Macular Thickness Between Two Groups |
The OCT photograph examples of the both groups are shown in Figure 1. The OCT shows the significant changes of edema on both groups after treatment.
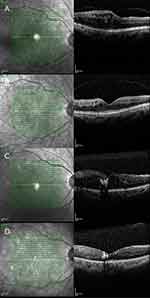 |
Figure 1 The examples of patient ocular tomography. Notes: A) is group A pre-treatment; B) is group A post treatment; C) is group B pre-treatment; D) is group B post treatment. |
Discussion
PDR and DME are the two manifestations of diabetic eye disease that are responsible for the majority of vision loss. Hypoxia, metabolic disturbances, and chronic retinal inflammation due to poorly controlled diabetes may cause alterations of the blood-retinal barrier.11 This alteration, which is characterized by endothelial cell junction breakdown and pericyte loss, may increase vascular permeability, which can lead to DME.12
PRP has been the standard of care for PDR for several decades.13 Photocoagulation causes destruction of photoreceptors in the peripheral retina, decreasing oxygen consumption and metabolic demand. PRP will subsequently increase the oxygen flow from the choroid to the inner retina.14 This retinal restoration may decrease PDR and DME. Many studies reported the improvement of visual acuity, although this may not occur until 3 months after treatment.10,15 However, in eyes with PDR combined with DME, PRP may cause more inflammation.16 This could induce increased ME, temporarily or permanently decreasing vision quality.
IVB injection may prevent or lessen PRP-induced ME. Bevacizumab is a complete full-length humanized antibody designed to directly bind VEGF-A, inhibiting binding of the VEGF molecule to its receptor on the surface of endothelial cells.17 Reduction in VEGF activity is key for the treatment of DME.18 Haritoglou et al reported a significant reduction in macular thickness 2 weeks after IVB injection lasting 3 months post-injection.19 Sameen et al reported the benefit of IVB as an additive treatment to PRP in reducing DME and ME secondary to PRP.20 In the present study, eyes in group A received IVB injection 1 week prior to the first session of PRP, while eyes in group B received IVB injection 1 week after the last session of PRP. CMT was evaluated before and 1 month after injection. CMT reduction of eyes in group A was consistent with previous studies.21 Bevacizumab prevented ME secondary to PRP in these patients. However, eyes in group B also had decreased CMT. There was no significant difference in the change of macular thickness between groups. Reduction of VEGF activity was still able to catch up to PRP, stabilizing secondary ME. The visual acuity pre- and post-treatment between the two groups were significantly significant; however, these findings were suggested due to the different staging and severity of the DR.
This study was unable to evaluate the efficacy of a single session of PRP and IVB injection in reducing the number of patient visits. However, the study demonstrated that IVB injection is effective within this range of time, regardless of administration before or after PRP. Further studies are needed with larger sample sizes and longer follow-up times.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008;88(11):1322–1335. doi:10.2522/ptj.20080008
2. Kartasasmita As Ho, Irfani I, Iskandar E. Evaluation of the inner-retinal cells survival after low duration laser treatment measured by electroretinogram in patient with diabetic retinopathy. J Eye Ophthalmol. 2017;4(2)1–4.
3. Kartasasmita AS, Takarai S, Switania A, Enus S. Efficacy of single bevacizumab injection as adjuvant therapy to laser photocoagulation in macular edema secondary to branch retinal vein occlusion. Clin Ophthalmol. 2016;10:2135–2140. doi:10.2147/OPTH.S116745
4. Li X, Zarbin MA, Bhagat N. Anti-vascular endothelial growth factor injections: the new standard of care in proliferative diabetic retinopathy? Dev Ophthalmol. 2017;60:131–142. doi:10.1159/000459699
5. Wu L, Fernandez-Loaiza P, Sauma J, Hernandez-Bogantes E, Masis M. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes. 2013;4(6):290–294. doi:10.4239/wjd.v4.i6.290
6. Pokharel A, Shrestha GS, Shrestha JB. Macular thickness and macular volume measurements using spectral domain optical coherence tomography in normal Nepalese eyes. Clin Ophthalmol. 2016;10:511–519. doi:10.2147/OPTH.S95956
7. Figueira J, Fletcher E, Massin P, et al. Ranibizumab plus panretinal photocoagulation versus panretinal photocoagulation alone for high-risk proliferative diabetic retinopathy (PROTEUS study). Ophthalmology. 2018. doi:10.1016/j.ophtha.2017.12.008
8. Reddy SV, Husain D. Panretinal photocoagulation: a review of complications. Semin Ophthalmol. 2018;33(1):83–88. doi:10.1080/08820538.2017.1353820
9. Mitsch C, Pemp B, Kriechbaum K, Bolz M, Scholda C, Schmidt-Erfurth U. Retinal morphometry changes measured with spectral domain-optical coherence tomography after pan-retinal photocoagulation in patients with proliferative diabetic retinopathy. Retina. 2016;36(6):1162–1169. doi:10.1097/IAE.0000000000000855
10. Solaiman KA, Diab MM, Dabour SA. Repeated intravitreal bevacizumab injection with and without macular grid photocoagulation for treatment of diffuse diabetic macular edema. Retina. 2013;33(8):1623–1629. doi:10.1097/IAE.0b013e318285c99d
11. Zhang W, Liu H, Al-Shabrawey M, Caldwell RW, Caldwell RB. Inflammation and diabetic retinal microvascular complications. J Cardiovasc Dis Res. 2011;2(2):96–103. doi:10.4103/0975-3583.83035
12. Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med. 2012;2012:918267.
13. Jampol LM, Odia I, Glassman AR, et al. Panretinal photocoagulation versus ranibizumab for proliferative diabetic retinopathy: comparison of peripapillary retinal nerve fiber layer thickness in a randomized clinical trial. Retina. 2017;39(1):69–78.
14. Desapriya E, Khoshpouri P, Al-Isa A. Panretinal photocoagulation versus ranibizumab for proliferative diabetic retinopathy: patient-centered outcomes from a randomized clinical trial. Am J Ophthalmol. 2017;177:232–233. doi:10.1016/j.ajo.2017.01.035
15. Kumar A, Sinha S. Intravitreal bevacizumab (Avastin) treatment of diffuse diabetic macular edema in an Indian population. Indian J Ophthalmol. 2007;55(6):451–455. doi:10.4103/0301-4738.36481
16. Shimura M, Yasuda K, Nakazawa T, et al. Panretinal photocoagulation induces pro-inflammatory cytokines and macular thickening in high-risk proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2009;247(12):1617–1624. doi:10.1007/s00417-009-1147-x
17. Hsieh YT, Yang CM, Chang SH. Bevacizumab and Panretinal photocoagulation protect against ocular hypertension after posterior subtenon injection of triamcinolone acetonide for diabetic macular edema. J Formos Med Assoc. 2017;116(8):599–605. doi:10.1016/j.jfma.2016.09.014
18. Bressler SB, Beaulieu WT, Glassman AR, et al. Factors associated with worsening proliferative diabetic retinopathy in eyes treated with panretinal photocoagulation or ranibizumab. Ophthalmology. 2017;124(4):431–439. doi:10.1016/j.ophtha.2016.12.005
19. Haritoglou C, Kook D, Neubauer A, et al. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006;26(9):999–1005. doi:10.1097/01.iae.0000247165.38655.bf
20. Sameen M, Khan MS, Mukhtar A, Yaqub MA, Ishaq M. Efficacy of intravitreal bevacizumab combined with pan retinal photocoagulation versus panretinal photocoagulation alone in treatment of proliferative diabetic retinopathy. Pak J Med Sci. 2017;33(1):142–145. doi:10.12669/pjms.331.11497
21. Preti RC, Mutti A, Ferraz DA, et al. The effect of laser pan-retinal photocoagulation with or without intravitreal bevacizumab injections on the OCT-measured macular choroidal thickness of eyes with proliferative diabetic retinopathy. Clinics. 2017;72:81–86. doi:10.6061/clinics/2017(02)03
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
