Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 12
Evaluation of the safety and efficacy of a triple combination cream (hydroquinone, tretinoin, and fluocinolone) for treatment of melasma in Middle Eastern skin
Authors Ahmad Nasrollahi S , Sabet Nematzadeh M, Samadi A , Ayatollahi A , Yadangi S, Abels C, Firooz A
Received 20 January 2019
Accepted for publication 1 May 2019
Published 10 June 2019 Volume 2019:12 Pages 437—444
DOI https://doi.org/10.2147/CCID.S202285
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Saman Ahmad Nasrollahi,1,* Maede Sabet Nematzadeh,2,* Aniseh Samadi,1 Azin Ayatollahi,1 Somayeh Yadangi,1 Christoph Abels,3 Alireza Firooz1
1Center for Research & Training in Skin Diseases & Leprosy, Tehran University of Medical Sciences, Tehran, Iran; 2Pharmaceutical Sciences Branch, Islamic Azad University, Tehran, Iran; 3Dr. August Wolff GmbH & Co. KG Arzneimittel, Bielefeld, Germany
*These authors contributed equally to this work
Background: Melasma is the most common pigmentary skin disorder, especially in females and those with darker complexion. The current study evaluated the safety and efficacy of a triple combination cream containing hydroquinone 4%+tretinoin 0.05%+fluocinolone acetonide 0.01% (Januluma®, cream produced by Janus Pharmaceutical Co, Tehran, Iran) in the treatment of melasma.
Patients and methods: Twenty-two female volunteers (mean±standard deviation of age: 39.20±4.16 years) who fulfilled the eligibility criteria participated in this study after signing the informed consent. They were requested to apply the Januluma®,cream every night for 8 weeks. Modified melasma area and severity index (mMASI), skin lightness (L value), and severity of pigmentation (E value) by Visio Face, and skin biophysical parameters including pH, melanin index, erythema index, sebum, hydration, trans epidermal water loss, thickness and density of epidermis, and dermis (using 22 MHz ultrasonography) were measured before and 4 and 8 weeks after treatment. Also patients’ satisfaction was assessed 4 and 8 weeks after treatment using visual analog score.
Results: mMASI decreased significantly from 3.37 to 2.60 at week 4, and to 2.40 at week 8 (P-values=0.00 and 0.01, respectively). Also, E and L values improved significantly after 8 weeks of treatment (P=0.01 and 0.00, respectively). Skin melanin index decreased from 237.49 AU to 196.30 AU at week 8 (P=0.01). Also echo density of dermis increased significantly after 8 weeks of treatment (P=0.029). Almost all participants experienced some degrees of pruritus, scaling, and erythema, esspecially during the first month of application, which were generally mild and tolerable. The mean satisfaction of patients with the treatment was 6.77.
Conclusion: The triple combination formula was reasonably safe and effective for treatment of melasma in Middle Eastern patients.
Keywords: melasma, combination therapy, efficacy assessment, Middle Easterners
Introduction
Melasma is one of the most common diseases of skin pigmentation. The disease name is derived from the Greek word “melas”, which means black. It presents as symmetric, brown-to-black macules or patches with irregular and jagged borders).1 Melasma is frequently seen in women with darker complexions (skin type IV–VI). The most common locations are sun-exposed parts of the body, primarily on lower cheeks, forehead and upper lips, and, less commonly, on the forearms.2 Moreover, melasma is classified as epidermal, dermal, and a combination of the epidermal and dermal types according to the depth of melanin pigment in skin.3
Recently the histopathologic features of melasma investigated and considered that not only is melasma a melanocytes disease, but also because of conditions such as solar elastosis, altered basement membrane, increased vascularization, and increased mast cell count, it is classified as a photo-aging skin disorder.4
This chronic condition can be persistent and may cause depression, low self-esteem, anxiety, and social phobia in patients. The main risk factors are genetics, pregnancy, sun exposure, contraceptives, and cosmetics.3
Recently a triple combination formula which contains hydroquinone 4%, tretinoin 0.05%, and fluocinoloneacetonide 0.01% (Tri-Luma Cream, Galderma, USA) has been approved by the US FDA and considered as a gold standard for treatment of melasma.5
Hydroquinone (2–5%) is considered the primary and most effective topical agent for blocking melanin production through inhibition of tyrosinase enzyme in melanocytes.6 Hydroquinone shows low efficacy as monotherapy, and the disease usually recurs. Typically, 4–6 weeks are required for a visible response; however, complete treatment of melasma may take up to 6 months. Higher concentrations of hydroquinone might be more effective but with a higher potential for irritation (itching, burning, dermatitis). Therefore, in the combination formula, 4% were used as an optimum concentration with more effectiveness and less adverse reactions.7
Tretinoin, another component of the triple combination cream, works by motivating epidermal and dermal turnover, which may cause rapid loss of cell pigment.8 In addition, it inhibits tyrosinase enzyme, facilitates the penetration of hydroquinone, and neutralizes the stratum corneum thinning effects of the corticosteroid.9
Fluocinolone (a mid-potency class V steroid) can reduce the possible irritation or inflammation caused by tretinoin and hydroquinone, especially in sensitive skins.10
Numerous studies of treatment of melisma with triple combination have included patients from North and Latin America and Eastern Asia with dark skins.1,11,12 The aim of this study was to assess the efficacy and safety of a topical triple combination of hydroquinone, tretinoin, and fluocinolone (Januluma® cream produced by Janus Pharmaceutical Co, Tehran, Iran) in the treatment of melasma in Middle Eastern patients.
Patients and methods
Study design
This was a phase II, before and after clinical trial, which was conducted in the Center for Research & Training in Skin Diseases & Leprosy, Tehran University of Medical Sciences from June 2016 to September 2017.
The study was conducted according to the ethical principles of the Declaration of Helsinki and in compliance with the guidelines of Good Clinical Practice (GCP). The study protocol was approved by the Ethics committee of Islamic Azad University (medical branch) with the acceptance code: IR.IAU.REC.1395.13 in May 2016, and registered in the Iranian Registry of Clinical Trials (IRCT) with the registration number of 2016121031288N2.
Patients
Women aged 18–50 years, skin type III–IV and a clinical diagnosis of mild-to-severe melasma (Melasma Global Severity Score, MGSS, grade 1 and more) were enrolled, after signing a written informed consent form. The subjects also provided consent for publication of any pictures.
Pregnant women, patients using oral contraceptive pills or immunosuppressive drugs, those with a history of hypertrophic scars or keloids, or with a recent history of chronic systemic disorders, patients who had contraindications for corticosteroid treatment, or any history of any procedural or topical or oral treatment for melasma within 3 months prior to the study were excluded. Volunteers were also excluded if they were expected to have any severe exposure to the sun during the study.
Product
The test product was Januluma® cream, produced by Janus Pharmaceutical Co, Tehran, Iran, which contains hydroquinone 4%, tretinoin 0.05%, and fluocinoloneacetonide 0.01% in O/W basis.
Intervention
Participant fulfilling inclusion and exclusion criteria were instructed to wash their skin with mild cleansers and then apply Januluma® on melasma lesions every night, 30 minutes before bedtime, for 8 weeks. They were also instructed to apply moisturizer and broad spectrum sunscreen with SPF 30 or more and a critical wavelength >375 nm regularly all day.
Clinical assessments
- Modified MASI scoring: Before intervention and 4 and 8 weeks later, the intensity and extension of melasma were assessed based on the Modified Melasma Area and Severity Index (mMASI) scoring method.13
The mMASI score was calculated according to the following equation:
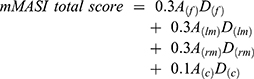
where A is the area of involvement, D is the darkness of melasma, f is the forehead region, lm is the left malar region, rm is the right malar region, and c is the chin region. The range of the total score is 0–24.
Area and darkness are scored as follows:
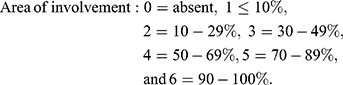
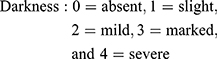
Standardized photographs were taken using a digital camera (Canon Power shot A495) at a fixed position and distance from the participant, and the mMASI score was measured by two independent dermatologists, and the mean was used for assessments.
- Severity of pigmentation (E value) and skin lightness (L value): In order to evaluate pigmentation and lightening of lesions, high-resolution frontal images of the face were taken using the VisioFace Quick system (Courage & Khazaka electronic GmbH, Cologne, Germany) and analyzed using the VisioFace CSI software before intervention, and 4 and 8 weeks after intervention.
- Biophysical parameters: Skin melanin index, erythema, pH, sebum, hydration, and TEWL were evaluated before intervention and 4 and 8 weeks after treatment in standardized conditions (temperature and humidity controlled room 20±1°C and 30–40%, respectively and after an acclimatization time of 20 minutes) according to in-house SOPs, using a non-invasive multi-probe adapter system MPA580 (Courage & Khazaka electronic GmbH, Cologne, Germany) respective probes: Mexameter, pH meter, Sebumeter, Corneometer, and TEWA meter.
- Ultra-sonography: High-frequency ultrasound digital apparatus (DUB-USB skin scanner, tpm Co. Luneburg, Germany) with probe 22 MHz was used to measure the thickness and echo-density of the epidermal and dermal layers.
Visual analog scale (VAS)
The participants’ satisfaction was measured by VAS (0=no satisfaction and 10=highest satisfaction) in both follow-up visits.
Safety assessment
To evaluate the tolerability of the tested cream, the subjects were asked to record any dryness, itching, stinging, redness, desquamation, or ocular intolerance during the treatment. In case of any severe adverse effects, the area was photographed and, if the participant could not continue the study, the use of cream was stopped and the measurements were done on the same or next day.
Statistical analysis
Statistical analysis was performed using SPSS Software version 20.0 (IBM, USA), and results were presented as the mean±standard deviation for quantitative variables. Skin parameters at weeks 4 and 8 were compared with baseline values using paired sample t-test, and the statistical significance was set at P-values less than 0.05.
Results
Twenty-two female patients with mild-to-severe melasma were enrolled, and the background characteristics and demographics are shown in Table 1; 60% of patients had experience of prior treatments for melasma with no satisfactory clearance. Of these cases, 82% were on topical depigmentation creams (cosmetic, pharmaceutical, or compounding preparations).
 | Table 1 Demographic data of all included patients (n=22) |
Seventeen patients (mean age 39.20±4.16 years) completed the study and five discontinued the study in week 4. Two decided to have a baby and three of them said the treatment was not effective.
Figure 1 demonstrates the mean mMASI score, which improved significantly during the study. It decreased from 3.61±0.81 at baseline to 2.81±1.41 at week 4 (P=0.001), and to 2.45±0.81 at week 8 (P<0.001).
 | Figure 1 Significant reduction in mean mMASI from baseline to end of the study. |
As Figure 2 shows, 4 and 8 weeks after applying Januluma, skin lightening factor (L value) increased, and this enhancement was significant in week 8 (P=0.01).
 | Figure 2 The improvementwas seen in skin lightness (L value) and skin pigmentation (E value) during the study. |
The average E Value, which reflects the skin pigmentation severity, decreased significantly from baseline (5.34±2.32) after 4 weeks (3.40±2.23), and 8 weeks (3.23±1.89) (P-value=0.001).
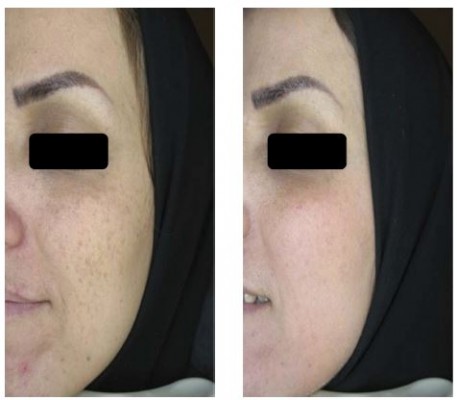
The melanin index was monitored by Mexameter after intervention at weeks 4 and 8. The average level of melanin index reduced in both follow-up visits (Figure 3), and this reduction was statistically significant at week 8 (P=0.001). The reduction of skin pigmentation and melanin in one of the patients 8 weeks after treatment with Januluma is shown in Figure 4.
 | Figure 3 A significant decrease in skin melanin index 8 weeks after treatment with Januluma (P<0.05). |
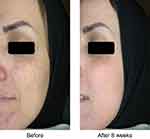 | Figure 4 Patient treated with Januluma cream at: (A) baseline and (B) week 8. |
TEWL showed a significant increase 8 weeks after treatment with Januluma, while there were no significant changes in other skin biophysical parameters, including hydration, erythema, sebum, and pH (Table 2).
 | Table 2 The mean±standard deviation of skin biophysical parameters at baseline, 4, and 8 weeks after treatment with Januluma |
High frequency ultrasonography evaluation of epidermis and dermis thickness and echo-density did not show any significant changes, except for a significant increase in echo density of the dermis 8 weeks after treatment (Table 3 and Figure 5).
 | Table 3 The mean±standard deviation of thickness and echo-density of epidermis and dermis at baseline, 4, and 8 weeks after treatment with Januluma |
The mean scores of subject satisfaction rate based on VAS 4 and 8 weeks after treatment were 6.32±1.41 and 6.77±0.96, respectively.
Adverse events including erythema, scaling, and skin dryness were reported in 8, 10, and 14 patients at week 4 and in 7, 8, and 11 patients at week 8, respectively. Most events were mild in intensity and none was severe. The frequency of adverse events reduced from week 4 to week 8. These adverse effects were drug-related and, after finishing the treatment, all side-effects disappeared. No patients discontinued the treatment due to the side-effects.
Discussion
Melasma (also mentioned as chloasma or the pregnancy mask) is a common and benign disorder of facial pigmentation in darker skin prototypes living in areas of intense ultraviolet (UV) radiation, but people of all ages, races, and skin colors may be affected. Presently, melasma treatment remains a challenge, and the search for safe and effective therapy continues.7,14
Despite various pharmaceutical and cosmeceutical ingredients and procedures, combination treatment with once daily application of a mixture of hydroquinone, tretinoin, and fluocinolone acetonide is the only commercial and effective treatment approved by the FDA.5,15
This triple combination has been studied widely in East Asians, Mediterranean Africans, Latino, and Indians ethnics and, proven to be effective in reducing of pigmentation and improving the quality-of-life (QoL) in patients with melasma.10,16–21
As there are no studies in Middle East patients, we assessed a local combination formula (Januluma®) on 22 Iranian females with mild-to-severe treatment resistant melasma for 8 weeks.
Triple therapy over 8 weeks reduced MASI scores significantly (Figure 1). This reduction was observed as early as 4 weeks after treatment, and is similar to the previous reports.11,22,23 In all of these studies patients used combination therapy for 8 weeks and showed significant reductions (P<0.001) from baseline in MASI at weeks 4 and 8.
Melasma improvement was also accompanied with a significant decrease in skin pigmentation (E value), as shown in in Figure 2. On the other hand, we observed a non-significant increase in skin lightness (L value) and a decrease in melanin index 4 weeks after treatment. These changes might become significant if we had a larger sample size.
This rapid decrease in melanin after 4 weeks is due to hydroquinone, as a tyrosinase inhibitor,24 and also penetration enhancement effect of tretinoin.25 Hydroquinone is one of the key ingredients for bleaching, and its effect is usually started after 2–3 weeks.26 Tretinoin can also reduce melanin synthesis by inhibition of tyrosinase transcription after 8 weeks of use,27 and this mechanism adds to hydroquinone action.
Another interesting finding was the significant increase in the echo-density of dermis (Table 3 and Figure 5). This improvement during treatment is related to the effect of tretinoin, which induces dermal proteins production. This is the first study using high frequency ultrasonography for evaluation of a triple combination formula, and confirmed previous reports showing that tretinoin might improve the skin density due to the remodeling effect on collagen and elastin fibers, as well as deposition of glycosaminoglycans.9,28 It also shows redistributing effects on melanin content, which leads to improvement of the hyperpigmentation.29
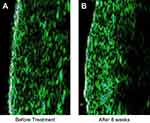 | Figure 5 High-frequency ultrasound pictures of treated area at (A) baseline and (B) 8 weeks after treatment. |
Generally, fluorinated steroids (used in tested formulation) are used to decrease the irritation potential of hydroquinone and tretinoin. They also have bleaching effects.30 The only concern with topical fluorinated corticosteroids is the potential of skin atrophy. However, according to ultrasound results, topical application of Januluma®, not only did not cause skin atrophy, but also lead to a relative increase in dermal thickness and density, which is probably due to tretinoin (Table 3). Our non-invasive ultrasonic result is consistent with Bhawan et al's31histological analysis in 2009. They evaluated the skin biopsies in patients treated with a mixture of hydroquinone, tretinoin, and fluocinolone acetonide, and no epidermal or dermal atrophy was seen.
After 8 weeks of treatment, TEWL increased significantly, which might be due to the peeling effect of tretinoin. Skin desquamation and xerosis are common side-effects of tretinoin, which correlates with increased TEWL due to damage to the skin barrier function.10,11
No significant changes were found in skin hydration, erythema, sebum, and pH. This correlates with our observation that Januluma® was well tolerated. The majority of adverse events were transient and mild, and none of the patients discontinued treatment due to side-effects. The most common related adverse events were skin irritation, scaling, and discomfort, which were all expected from active ingredients.10,32
The fixed triple combination as the first-line treatment for all melasma types was recommended by many academies of dermatologists, and other combinations (such as hydroquinone+peeling ingredients, 20% azelaic acid+tretinoin and kojic acid+hydroquinone+peeling ingredients) or single agents were applied only for patients who could not tolerate the adverse effects.27
During treatment with the topical triple combination, patients must be instructed to avoid sun exposure, apply a broad-spectrum sunscreen with a sun-protection factor (SPF) of >30, reapply sunscreen every 2 hours, use moisturizers during the day, and wash their face with mild cleansers.33 This skincare regime can help to reduce all side-effects.
The recurring nature of melasma needs maintaining the efficacy achieved after triple combination treatment. A study conducted by Arellano et al23 demonstrated the twice-weekly application of triple combination cream for 6 months was more effective with a lower relapse in severe melasma, while the tapering regimen (three times weekly for the first month, twice weekly for the second month, and once weekly for the fourth month) was more appropriate for those with moderate melasma. In both groups, the median time for relapsing was around 4 months. This study also confirmed that applying triple combination intermittently over a long time period is tolerable, safe, and improves patient’s quality-of-life.
The main limitation of this study is the lack of a control group. So a randomized, controlled study with a larger sample size is necessary to confirm its results. However, a justification for the lack of placebo group would be referral to the available previous data of the approved product. Also it is recommended to assess the patient quality-of-life in addition to objective and subjective measures of improvement.
Conclusion
In conclusion, this study showed that Januluma® cream is an effective and well tolerated treatment to improve melasma.
Data sharing statement
The authors do not intend to share individual deidentified participant data separately.
Acknowledgments
This study has been supported by a research grant from the Center for Research and Training in Skin Diseases and Leprosy, Tehran University of Medical Sciences grant number 94-03-34-27706. The authors appreciate Janus Pharmaceutical Co for providing Januluma® creams in the present study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cestari T, Arellano I, Hexsel D, Ortonne J, Academy LAPD. Melasma in Latin America: options for therapy and treatment algorithm. J Eur Acad Dermatol Venereol. 2009;23(7):760–772. doi:10.1111/j.1468-3083.2009.03251.x
2. Rajaratnam R, Halpern J, Salim A, Emmett C. Interventions for melasma. Cochrane Database Syst Rev. 2010;7(7).CD003583
3. Shankar K, Godse K, Aurangabadkar S, et al. Evidence-based treatment for melasma: expert opinion and a review. Dermatol Ther (Heidelb). 2014;4(2):165–186. doi:10.1007/s13555-014-0064-z
4. Kwon SH, Hwang YJ, Lee SK, Park KC. Heterogeneous pathology of melasma and its clinical implications. Int J Mol Sci. 2016;17(6). doi:10.3390/ijms17060824
5. Taylor SC, Torok H, Jones T, et al. Efficacy and safety of a new triple-combination agent for the treatment of facial melasma. Cutis. 2003;72(1):67–73.
6. Jimbow K, Obata H, Pathak MA, Fitzpatrick TB. Mechanism of depigmentation by hydroquinone. J Invest Dermatol. 1974;62(4). doi:10.1111/1523-1747.ep12701679
7. Gupta AK, Gover MD, Nouri K, Taylor S. The treatment of melasma: a review of clinical trials. J Am Acad Dermatol. 2006;55(6):1048–1065. doi:10.1016/j.jaad.2006.02.009
8. Kimbrough-Green CK, Griffiths CE, Finkel LJ, et al. Topical retinoic acid (tretinoin) for melasma in black patients: a vehicle-controlled clinical trial. Arch Dermatol. 1994;130(6):727–733.
9. Rendon M. Utilizing combination therapy to optimize melasma outcomes. J Drugs Dermatol. 2004;3(5 Suppl):S27–S34.
10. Torok HM, Jones T, Rich P, Smith S, Tschen E. Hydroquinone 4%, tretinoin 0.05%, fluocinolone acetonide 0.01%: a safe and efficacious 12-month treatment for melasma. Cutis. 2005;75(1):57–62.
11. Chan R, Park K, Lee M, et al. A randomized controlled trial of the efficacy and safety of a fixed triple combination (fluocinolone acetonide 0·01%, hydroquinone 4%, tretinoin 0· 05%) compared with hydroquinone 4% cream in Asian patients with moderate to severe melasma. Br J Dermatol. 2008;159(3):697–703. doi:10.1111/j.1365-2133.2008.08717.x
12. Torok HM. A comprehensive review of the long-term and short-term treatment of melasma with a triple combination cream. Am J Clin Dermatol. 2006;7(4):223–230.
13. Pandya AG, Hynan LS, Bhore R, et al. Reliability assessment and validation of the Melasma Area and Severity Index (MASI) and a new modified MASI scoring method. J Am Acad Dermatol. 2011;64(1):78–83. e2. doi:10.1016/j.jaad.2009.10.051
14. Prignano F, Ortonne J-P, Buggiani G, Lotti T. Therapeutical approaches in melasma. Dermatol Clin. 2007;25(3):337–342. doi:10.1016/j.det.2007.04.006
15. Malakooti S, Nasrollahi SA, Kashani MN, et al. Formulation of triple cream for treatment of melasma. Jundishapur J Nat Pharm Prod. 2019;14(2).
16. Balkrishnan R, Kelly A, McMichael A, Torok H. Improved quality of life with effective treatment of facial melasma: the pigment trial. J Drugs Dermatol. 2004;3(4):377–381.
17. Cestari TF, Hexsel D, Viegas M, et al. Validation of a melasma quality of life questionnaire for Brazilian Portuguese language: the MelasQoL‐BP study and improvement of QoL of melasma patients after triple combination therapy. Br J Dermatol. 2006;156:13–20. doi:10.1111/j.1365-2133.2006.07591.x
18. Pasricha J, Khaitan BK, Dash S. Pigmentary disorders in India. Dermatol Clin. 2007;25(3):343–352. doi:10.1016/j.det.2007.05.004
19. Halder R, Grimes P, McLaurin C, Kress M, Kenney JJ. Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32(4):388–390.
20. Ferreira Cestari T, Hassun K, Sittart A. de Lourdes Viegas M. A comparison of triple combination cream and hydroquinone 4% cream for the treatment of moderate to severe facial melasma. J Cosmet Dermatol. 2007;6(1):36–39. doi:10.1111/j.1473-2165.2007.00288.x
21. Sivayathorn A. Melasma in orientals. Clin Drug Investig. 1995;10(2):34–40. doi:10.2165/00044011-199500102-00006
22. Grimes P, Kelly AP, Torok H, Willis I. Community-based trial of a triple-combination agent for the treatment of facial melasma. Cutis. 2006;77(3):177–184.
23. Arellano I, Cestari T, Ocampo‐Candiani J, et al. Preventing melasma recurrence: prescribing a maintenance regimen with an effective triple combination cream based on long‐standing clinical severity. J Eur Acad Dermatol Venereol. 2012;26(5):611–618.
24. Wolverton SE. Comprehensive Dermatologic Drug Therapy. Oxford (UK). Elsevier Health Sciences; 2012.
25. Khunger N, Sarkar R, Jain R. Tretinoin peels versus glycolic acid peels in the treatment of melasma in dark‐skinned patients. Dermatol Surg. 2004;30(5):756–760. doi:10.1111/j.1524-4725.2004.30212.x
26. Khanna N, Rasool S. Facial melanoses: indian perspective. Indian J Dermatol Venereol Leprol. 2011;77(5):552. doi:10.4103/0378-6323.84046
27. Rendon M, Berneburg M, Arellano I, Picardo M. Treatment of melasma. J Am Acad Dermatol. 2006;54(5):S272–S81. doi:10.1016/j.jaad.2005.12.039
28. Kong R, Cui Y, Fisher GJ, et al. A comparative study of the effects of retinol and retinoic acid on histological, molecular, and clinical properties of human skin. J Cosmet Dermatol. 2016;15(1):49–57. doi:10.1111/jocd.12193
29. Kang HY, Valerio L, Bahadoran P, Ortonne J-P. The role of topical retinoids in the treatment of pigmentary disorders. Am J Clin Dermatol. 2009;10(4):251–260.
30. Kanwar A, Dhar S, Kaur S. Treatment of melasma with potent topical corticosteroids. Dermatology. 1994;188(2):170. doi:10.1159/000247129
31. Bhawan J, Grimes P, Pandya AG, et al. A histological examination for skin atrophy after 6 months of treatment with fluocinolone acetonide 0.01%, hydroquinone 4%, and tretinoin 0.05% cream. Am J Dermatopathol. 2009;31(8):794–798. doi:10.1097/DAD.0b013e3181a9070d
32. Guevara IL, Werlinger KD, Pandya AG. Tolerability and efficacy of a novel formulation in the treatment of melasma. J Drugs Dermatol. 2010;9(3):215–218.
33. Seth V, Pandya A. Melasma: a comprehensive update part II. J Am Acad Dermatol. 2011;65:699–711. doi:10.1016/j.jaad.2011.06.001
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
