Back to Journals » Medical Devices: Evidence and Research » Volume 15
Evaluation of the Rheological Properties, Preclinical Safety, and Clinical Effectiveness of a New Dispersive Ophthalmic Viscoelastic Device for Cataract Surgery
Authors Palacio-Pastrana C , Muñoz-Villegas P , Dániel-Dorantes F, Sánchez-Ríos A, Olvera-Montaño O , Martínez-Montoya YI, Quintana-Hau JD, Baiza-Durán LM
Received 18 June 2022
Accepted for publication 12 August 2022
Published 24 August 2022 Volume 2022:15 Pages 293—305
DOI https://doi.org/10.2147/MDER.S379050
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Claudia Palacio-Pastrana,1 Patricia Muñoz-Villegas,2 Fernando Dániel-Dorantes,3 Alejandra Sánchez-Ríos,2 Oscar Olvera-Montaño,2 Yareni I Martínez-Montoya,1 Juan D Quintana-Hau,3 Leopoldo M Baiza-Durán2
1SalaUno Salud, SAPI de CV, México City, México; 2Regional Medical Affairs Department, Laboratorios Sophia SA de CV, Zapopan, Jalisco, México; 3Research and Development Department (CIS), Zapopan, Jalisco, México
Correspondence: Patricia Muñoz-Villegas, Laboratorios Sophia, SA de CV, Paseo del Norte 5255, Guadalajara Technology Park, Zapopan, Jalisco, 45010, México, Tel +52 3301 4200, Ext: 1018, Email [email protected]
Purpose: To evaluate the rheological properties of the ophthalmic viscoelastic device (OVD) PRO-149, its preclinical safety, and its effectiveness when used during cataract surgery in patients with age-related cataract.
Material and Methods: Control (HEC) and test (PRO-149) OVDs were compared through rheological measures, by two preclinical safety studies in rabbits, and under normal-use conditions during cataract removal and lens implantation in a parallel randomized clinical trial.
Results: Rheological properties were determined. Preclinical studies did not find any evidence of safety issues or toxicity. In the clinical trial, 36 subjects were included. After 29 days, there were no statistically significant differences in mean percentage of endothelial cell count change or in the postoperative intraocular pressure between groups. There were no significant differences between OVDs for any safety parameter studied. Finally, PRO-149 showed a statistically significant improvement in surgeon rating for ease of use during extraction (p < 0.05).
Conclusion: PRO-149 is a dispersive OVD. The rabbit models did not find evidence of clinical alterations or toxicity. The results of the clinical study support that the two studied OVDs were clinically similar in terms of safety and effectiveness for cataract surgery.
Trial Registration: The trial is registered at Clinical Trials.gov at NCT04702802 (21– 01-11).
Keywords: rheology, sodium hyaluronate, surgical procedure, viscoadaptative, viscosity
Introduction
Ophthalmic viscoelastic devices (OVDs) have been used in eye surgery for more than 50 years, initially as vitreous humor substitutes and later as a tool during cataract surgery.1,2
The study of the rheological properties of OVDs used during cataract surgery provides important information, allowing the ophthalmic surgeon to better understand and select the ideal OVD among the multiple currently available options.3 OVDs used in ophthalmic surgery can be classified as viscoadaptative, higher-viscosity cohesive, viscous-cohesive, viscous-dispersive, medium viscosity cohesive, viscous-cohesive, or lower viscosity-dispersive.3,4
The physical properties that are generally recognized as differentiators among OVDs are their viscosity, elasticity, stiffness, pseudoplasticity and cohesion. These characteristics are translated into tissue protection, space maintenance, and injection and aspiration ease.1
Viscosity is a parameter used on fluids. In pure substances, it varies significantly depending on the temperature and the pressure applied to them. The ease at which liquids flow is a way to evaluate their viscosity.5 The dynamic viscosity is the property of fluids characterized for their resistance to flow, due to the friction between their molecules. For some fluids, viscosity is constant, and it depends only on temperature and pressure. These can be grouped as Newtonian fluids. Fluids that do not follow this proportional ratio are denominated non-Newtonian. One of the properties of Newtonian fluids is how their viscosity depends on the shear-rate, property that can be evaluated and measured using the Carreau fluid model.6,7
The term plasticity refers to a material’s capacity to deform continuously, without breaking, when a sufficient force is applied and maintaining a new shape once the force has been interrupted. The more plasticity a material shows the force required to deform it decreases as the deformation velocity increases.1,5,8
The ability to become less viscous when the shear rate increases when there is a viscosity limited to zero shear is called pseudoplasticity. In this sense, an OVD with high viscosity at zero shear would be ideal to maintain formed spaces; however, pseudoplasticity would allow it to, when injected through a cannula (high shear rate), exhibit a decrease in viscosity, facilitating its application.1,5
Elasticity is defined as the tendency of a substance to recover its original shape after being deformed. Viscoelastics possess this characteristic, in various degrees. It is desirable for an OVD to be elastic to absorb the shocks produced by manipulation; however, viscosity should not be excessive since this would hinder fine surgical maneuvers.1,5
On the other hand, rigidity is also known as complex viscosity, and it refers to the sensation of perceived resistance when moving an object through a viscoelastic substance. The rigidity of an OVD depends on the shear-rate and vibration frequency, and it will be close to the viscosity or elasticity, whichever is larger during the operational conditions of the measurement. Therefore, both viscosity and elasticity will seem to predominate when influencing the tactile sensation of the surgeon when manipulating a certain OVD during a surgical procedure.1,5,7
Depending on their rheological properties, OVDs are classified into two large groups: cohesive or dispersive. Although their behavior is continuous, it is easier to study them if they are classified as such. Cohesion is the tendency of molecules constituting a certain material, to adhere to each other instead of dispersing. According to the type of hyaluronate, a low concentration of high molecular weight polymers in a solution will perform as a cohesive substance, while one with a high concentration of polymers will be more dispersive.1
The use of OVDs has had an enormous influence in cataract surgery technique developments since they provide protection for the corneal endothelium, reducing up to 70% the endothelial cell loss, as well as creation and maintenance of adequate spaces for manipulation during such procedures.1,9
Commercially available OVDs contain different concentrations of one or more of the following polymers: sodium hyaluronate (Na-HA), chondroitin sulfate, or hydroxypropyl methylcellulose. These products are composed mainly of water and share a very similar density (~1.000 kg/m3). The protective, retaining, cohesive and lubricant properties of OVDs are mostly granted by the polymeric structure, molecular weight, electric charge, purity and interchain interactions of their main ingredients.4,10,11
The development and eventual commercialization of OVDs require a thorough evaluation of biocompatibility, purity, and behavior within the ocular structures. The assessment of the rheological properties of this type of devices is crucial in determining its characteristics and behavior when it is used afterwards in a clinical setting. The evaluation of an OVD’s biocompatibility, that is the ability of a material to exist harmoniously with surrounding tissues and not produce an immune response from the host, must be part of the line of its development.
This paper describes the complete pipeline of an OVD, including preclinical in vitro, preclinical in vivo and clinical studies. The evaluated rheological parameters comprised the modulus crossover point (crossover point between loss modulus G” and storage modulus G’), zero-shear viscosity, and dynamic viscosity. Subsequently, a preclinical in vivo safety study including histopathological evaluations was performed to ensure the safety of the tested OVD after its application in the anterior chamber of New Zealand White (NZW) rabbits; and finally, a clinical study to evaluate the safety, efficacy and convenience of the new formulation is further detailed.
Materials and Methods
Rheological Measurements
Rheology is used to describe and assess the deformation and flow behavior of materials. The rheological properties of a Na-HA solution depend on the biopolymer’s structure and its polyelectrolyte characterization.11–13 The test OVD, PRO-149 (3% Na-HA, Laboratorios Sophia, SA de CV, Jalisco, Mexico) is a viscoelastic formulation with a comparable profile to other dispersive viscoelastic commercially available products, see Table S1. The present study used Healon EndoCoat® (HEC, 3% Na-HA, Johnson & Johnson Surgical Vision, Inc, CA, USA) as the control OVD because it is considered a similar device applied into the anterior chamber in order to safeguard the integrity of corneal endothelial cells throughout cataract surgery.14 Experimental data were obtained from 3 independent batches, and measurements were repeated two times per batch. Both OVDs were tested under controlled conditions following a validated methodology. Rheological parameters (modulus crossover point [G”/G’], zero-shear viscosity, and dynamic viscosity) of each OVD were analyzed with a rheometer (Discovery HR-2 Hybrid Rheometer, TA Instruments, NC, USA) equipped with a cone-plate (60 mm) and at an angle of 2 degrees (phase shift δ) at 25°C. The dynamic experiments took place within a frequency interval of 0.02–625 rad/s. Meanwhile, the shear rate (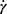 ) studies were conducted under a shear rate force of 0.001–2500 (1s−1), both experiments were executed under the linear viscoelastic zone (modulus G’ and G” are independent of deformation). Finally, the dynamic viscosity was analyzed with a viscometer (Brookfield DV2T Viscometer, AMETEK, Middleboro, MA, USA).
) studies were conducted under a shear rate force of 0.001–2500 (1s−1), both experiments were executed under the linear viscoelastic zone (modulus G’ and G” are independent of deformation). Finally, the dynamic viscosity was analyzed with a viscometer (Brookfield DV2T Viscometer, AMETEK, Middleboro, MA, USA).
Animals and Preclinical Experimental Design
All procedures adhered to the guidelines from the Association for Research in Vision and Ophthalmology (ARVO) resolution on the use of animals in ophthalmic and vision research, and the preclinical protocol approval was obtained from the Institutional Animal Care and Use Committee of Laboratorios Sophia, SA de CV (CICUALLS, identifier: HIVSHISTOCT17V2 [17–11-24]). A total of 20 experimental animals were included in this study. To calculate the sample size, a source equation method was implemented.15,16 Inclusion criteria comprehended: healthy male NZW rabbits, 2–3 months of age and weighing 2 to 3 kg, with no history of participation in any previous study. An ophthalmic eligibility screening including slit lamp (Luxvision®, Class I Type B, Doral FL, USA) examination and fluorescein staining (Bio GLOTM, HUB Pharmaceuticals, UK) was performed to ensure there were no exclusion criteria present. These included ocular abnormalities (secretion, conjunctival hyperemia, corneal or conjunctival lacerations, lens opacity, alteration in aqueous humor transparency, or corneal degeneration), or any pathological findings in the indirect fundoscopy performed with a 78D lens (Ocular Instruments, Bellevue, WA, USA), such as retinal detachment, or neovascularization. Any serious adverse event (AE) that required the administration of a complementary ophthalmic or systemic treatment, including any situation that entailed any compromise to the animal’s well-being, was an elimination criterion.
One eye of each animal was used to test an OVD, while the other was used as control (sham eye). The animals were anesthetized with xylazine (PROCIN®, Pisa, Hidalgo, Mexico) 10mg/kg and ketamine hydrochloride (ANESKET® VET, Pisa, Hidalgo, Mexico) 30mg/Kg intramuscularly. Tetracaine hydrochloride eyedrops were used for local anesthesia (Ponti Ofteno®, Laboratorios Sophia, SA de CV, Jalisco, Mexico). The surgical procedures were performed under a surgical microscope (Zeiss OPMI® Pico, Carl Zeiss Meditec, Inc, Dublin, CA, USA). A total of 10 NZW rabbits were assigned to aqueous humor exchange (AHE) model (0.05 mL of aqueous humor was exchanged with 0.05 mL of each OVD, by paracentesis pathway). In a separate experiment in 10 animals, the aqueous humor was exchanged with balanced salt solution in one eye through irrigation/aspiration, and a sham operation with injection and posterior extraction of the OVD was performed in the other eye (anterior chamber washout model [ACW]). In each experiment, five animals received the test OVD, PRO-149, and five animals received the control OVD, HEC. At the end of each intervention, a prophylactic broad-spectrum antibiotic eyedrop was applied (0.3% ciprofloxacin, Sophixin® Ofteno, Laboratorios Sophia, SA de CV, Jalisco, Mexico).
Slit lamp and indirect fundoscopy examinations were performed on all eyes before any intervention (basal examination), and it was repeated four times after surgery on days 1 (4 hours after OVD uses), 2, 3 and 5 (final safety visit). The animals were euthanatized by CO2 administration after the last evaluation. Eyes were enucleated and fixed for 24–48 hours in 4% paraformaldehyde for subsequent preparation by paraffin embedding, section preparation and staining with hematoxylin and eosin for examination with a light microscope.
Preclinical Safety and Toxicity Evaluations
The safety endpoints were corneal opacity, changes in intraocular pressure (IOP), anterior chamber cellularity and flare, iris congestion, conjunctival hyperemia, and incidence of AEs. Histological changes in the following structures were evaluated for toxicity endpoints: cornea, conjunctiva, ciliary body, retina, and optic nerve.
Clinical Trial Study Design
This controlled, parallel group, open, randomized clinical trial was conducted at one center in Mexico. The study was registered in the local MOH (COFEPRIS) as 203301410C0039/2021 before recruiting the first participant (21–08-09), and in ClinicalTrials.gov at NCT04702802 (21–01-11). An ethics committee reviewed and approved the study’s protocol and informed consent (see Ethical Approval section). This study was conducted in compliance with the Declaration of Helsinki and in accordance with Good Clinical Practices Standards. Informed consent was obtained from all participants included in the study. The patients were recruited between September 2021 (FPFV: 21–09-07) and January 2022 (LPLV: 22–01-13).
Patients
Inclusion criteria comprehended men and women (aged ≥ 49 years), with age-related cataract diagnosis (LOCS III cataract classification; NO and NC < 5),17 requiring phacoemulsification and intraocular lens (IOL) implantation, with an anterior chamber ≥2.8 mm deep (Zeiss IOL Master® 700, Carl Zeiss Meditec, Inc, Dublin, CA, USA) and a pre-surgical cardiologic evaluation that validated the patient’s eligibility to undergo the surgical procedure (including supporting studies). Exclusion criteria were as follows: history of Diabetes Mellitus with an A1C result ≥6.5% or glucose levels ≥126 mg/dL, poorly controlled systemic hypertension, history of previous ocular diseases which may have limited the best corrected visual acuity (BCVA) or which may have reactivated or worsened due to the surgical procedure, IOP ≥ 21 mmHg in the eye to withstand surgery, or previous history of IOP > 21 mmHg after topical steroid use, active ocular infection, pseudo exfoliation syndrome in the eye to withstand surgery, or any other such as zonular compromise. Patients having a previous history of any ophthalmological surgical procedure, within the last three months prior to the informed consent signing date or patients with a single functional eye were also excluded. For more information about inclusion and exclusion criteria see Supporting information (Table S2).
Treatment and Evaluations
Participants were assessed for eligibility during a screening visit scheduled two weeks before surgery. If the participant fulfilled all the inclusion criteria and presented none of the exclusion criteria, he/she was enrolled. Sample size calculation was performed to test the percentage of change in endothelial cell count (ECC) after cataract surgery at the end of 29-day study period, with an expected change of 7% for both OVDs.18 A sample size of 14 cases (eyes) was determined, with an alpha of 0.05 and an 80% power.19 Therefore, 18 eyes were considered per group, allowing as much 20% of excluded cases in the event of major protocol deviations. A total of 36 participants were randomized through computer software randomization numbers (SAS Institute, Inc, Cary, NC, USA) in a 1:1 ratio to receive either HEC (n = 18) or PRO-149 (n = 18). Both OVDs’ presentation was a pre-filled syringe for application in the ocular anterior chamber during phacoemulsification surgery. Starting 24 hours after surgery, patients of both groups initiated a prophylactic scheme with a drop of a fixed combination of 0.3% ciprofloxacin/0.1% dexamethasone (Sophixin DX®, Laboratorios Sophia, SA de CV, Jalisco, Mexico) drug topically in the inferior conjunctival sac of the operated eye four times a day for 10 days. Each patient was evaluated on five visits after enrollment: pre-op checkup (randomization), surgery, and safety visits on days 1, 8 and 29 ± 1 after surgery, see Figure S1.
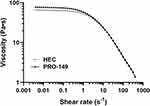 |
Figure 1 Shear rate (s−1) versus average viscosity (Pascal second) for test OVD (PRO-149) compared to control OVD (HEC) in logarithmic scale. Data from 3 batches. |
Clinical Study Endpoints
The primary endpoints were as follows: ECC measured preoperatively and 29 days postoperatively by specular microscopy and changes in IOP measured through Goldmann tonometer after topical anesthetic (0.5% tetracaine, Ponti Ofteno®, Laboratorios Sophia, SA de CV, Jalisco, Mexico), and fluorescein application. Any IOP measurement of 30 mmHg or higher was considered an IOP “spike” and recorded as an AE. Secondary outcomes were changes in central corneal thickness (by OCT, Carl Zeiss Meditec, Inc, Dublin, CA, USA), changes in anterior chamber cellularity and flare (measured during evaluation with a slit lamp), changes in BCVA (evaluated by Snellen chart), and the incidence of AEs. The degree of cellularity and flare were determined according to the standardized uveitis nomenclature.20 Lastly, to compare the clinical performance of the OVDs during the phacoemulsification and IOL implantation, the surgeon completed a post-surgery OVD clinical performance questionnaire (Table S3) at the end of each surgical case.21
Statistical Analyses
The zero-shear viscosity was performed with basis on the Carreau Fluid Model under the following equation:6,7
Where η (viscosity), η∞ (viscosity at zero shear rate), λ (relaxation time), and p (power index) are material coefficients. Each time point was analyzed two times per batch, without assuming a consistent standard deviation (SD). A total of 52 timepoints were used for the shear rate, and the data were obtained from 3 independent batches.
Statistical analyses were carried out using the R statistical software package (The R Foundation for Statistical Computing; http://www.R-project.org). All data are expressed as mean ± SD unless indicated otherwise. For the preclinical study, statistical significance was determined using a Mann–Whitney test for continuous data and Chi-square test or Fisher’s exact test for categorical data. In the clinical trial, statistical evaluations for differences were performed with Student’s t-test for continuous data, and the ordinal variables were analyzed using p x q contingency tables and the differences were calculated with Pearson Chi-square test. A p-value less than 0.05 was considered significant.
Results
Rheological Evaluation
PRO-149 is a transparent, non-toxic, nonpyrogenic, sterile OVD, which does not provoke any reaction when used intraocularly (Table S1).
The rheological properties of both test and reference OVDs are shown in Table 1. The apparent viscosity (OVD’s resistance to flow, calculated by the cone-plate model) of PRO-149 exhibits a dispersive profile, similar to the control OVD (HEC). The dynamic viscosity of HEC was also like PRO-149ʹs (53,950 Pa vs 53,913 Pa). Likewise, the structural viscosity (rheometer) between these OVDs was non-different (65.61 Pa·s vs.76.91 Pa·s). An ideal viscoelastic should maintain space for accurate instrumentation during the surgery, meanwhile its viscoelasticity allows it to absorb traumatic forces like shocks during phacoemulsification and the unfolding of IOL during implantation.22 Figure 1 is a graphic depiction of the rheological profile of test OVD formulation (PRO-149) compared to that of the control OVD (HEC). The zero-shear viscosity (η0, at 25°C) for HEC was 64.98 ± 1.08 Pa·s [1.66% CV] vs 76.27 ± 0.79 Pa·s [1.04% CV] for PRO-149. Finally, one of the key parameters when developing biomedical substances is osmolality because of the limited range of the body’s tolerance to affecting endogenous or exogenous liquids in this regard.12 The osmolality should be found within the range from 200 to 400 mOsm/Kg. Both OVDs laid within this interval (310 mOsm/Kg vs 320 mOsm/Kg, respectively).
 |
Table 1 Rheologic Characterization |
Preclinical Safety and Toxicity
Before AHE experiment and 4 hours after surgery, both groups showed 100% of clear cornea frequency. As shown Table 2, operated eyes revealed a similar appearance of corneal opacity (slight to moderate) in 1 of 5 (HEC) and 2 of 5 (PRO-149) eyes in each OVD in the following evaluations (p-values >0.05). Likewise, in the ACW model, operated eyes showed slight to mild corneal opacity without differences between OVDs in 3 of 5 eyes which received HEC, and 2 of 5 eyes which received PRO-149.
 |
Table 2 Clinical Ophthalmic Observations for NZW Rabbits at 4 Hour and 5 Days Post Procedure in Eyes Injected with Control OVD (HEC) and Test OVD (PRO-149) |
The IOP before the AHE experiment was similar between groups (9.0 ± 1.0 mmHg vs 9.0 ± 0.7 mmHg, p = 1.000). The OVDs did not cause postoperative IOP increase (Figure 2A). In all cases, the IOP did not show significant differences between groups after 4 (p = 0.913), 24 (p = 0.369), 72 (p = 0.736) hours or 5 days (p = 0.488). Likewise, the ACW experiment did not have any incidence of postoperative IOP increase in both groups (Figure 2B). Ocular tonometry showed no significant differences in basal values (8.8 ± 0.8 mmHg vs 9.2 ± 0.5 mmHg, p = 0.343) nor changes in IOP between HEC and PRO-149 eyes at 4 (p = 0.663), 24 (p = 0.339), 72 (p = 0.419) hours, or after 5 days (p = 0.729). This did not correlate to previously reported results for IOP evaluation after AHE experiment since other authors found peaks of IOP increase after the procedure. However, what did coincide with other publications is the presence of considerable variation within individuals for this variable.23
As shown in Table 2, ophthalmic examination revealed mild cellularity in the anterior chamber at 4 hours post procedure in 3 of 5 eyes from the HEC group and 2 of 5 eyes in the PRO-149 group. No anterior chamber cells were observed in the subsequent evaluations. No anterior chamber flare was observed at any time for any group during the experiment. Similarly, in the ACW experiment, 4 hours after surgery, 1 of 5 eyes that received PRO-149 showed mild cellularity in the anterior chamber (p = 1.000). No anterior chamber cells or flare was observed in the subsequent evaluations for any OVD group.
Inflammation seen after surgery followed the same course in both experiments. No significant differences were observed regarding iris congestion or conjunctival hyperemia, between HEC and PRO-149 in all safety visits (p > 0.05, Table 2). The incidence of AEs was similar between groups. Air bubble formation and white mucus were observed in 2 of 5 eyes which received HEC and 3 of 5 eyes which received PRO-149 in the AHE experiment. No discernible fibrin presence was observed for any group. Meanwhile, in the ACW experiment, AEs were observed in 3 of 5 eyes, which received HEC and 4 of 5 eyes, which received PRO-149. The events included one or more of the following signs: white mucus, lack of integrity of anterior capsule, lens fragments in the anterior chamber, lens opacity, neo vessels and presence of fibrin in the anterior chamber.
Lastly, light microscopy was performed in all eyes (operated and sham). No cornea, conjunctiva, retina, or optic nerve toxicity was found in any eyes. For the ciliary body, the reported changes were all considered within normal limits or as incidental findings (2 and 3 eyes which received PRO-149 shown minimal and occasional presence of the changes in both the AHE and ACW experiments, respectively). The histology of both OVD of injected eyes (HEC and PRO-149) after AHE and ACW experiments did not show signs of toxicity or structural damage, see Figure S2.
None of the sham operation eyes nor AHE or ACW experiments had any effect on postoperative IOP or any safety and toxicology evaluation (see Table S4).
Clinical Effectiveness and Safety
Thirty-six patients were enrolled and completed the entire protocol without deviations up to day 29 (per protocol, PP population). Demographic and baseline characteristics were similar between the OVD groups without significant differences. Mean age was 69.8 ± 10.1 years (range 49–88); 66.7% of the patients were female and 80.6% had comorbidities, see Table 3.
 |
Table 3 Initial Characteristics of Each Group in the Clinical Trial (N = 18 Patients per Group) |
Baseline ECC was similar between HEC and PRO-149 OVD (2558.3 ± 363.4 cells/mm2 vs 2584.4 ± 385.5 cells/mm2, p = 0.836). By the final visit (day 29), as expected, no difference was observed between the two groups regarding the percentage of change in ECC (t(34)=0.073, p = 0.942).
On day 1, one patient in the PRO-149 group showed a postoperative IOP increase of 18 mmHg (17 to 35 mmHg, reported as an AE); the patient showed a normal IOP by day 29. For the HEC group, the initial IOP was 15.8 ± 2.2 mmHg, with a postoperative increase of 0.72 mmHg after surgery. On day 29, the IOP decreased to 14.9 ± 2.7 mmHg, 0.83 mmHg lower than that of eligibility visit. Meanwhile, for the patients that received PRO-149, the initial IOP was 15.8 ± 2.4 mmHg, with a postoperative increase of 3.4 mmHg, which decreased to 15.2 ± 3.0 mmHg, 0.61 mmHg lower than that of initial value on day 29. No differences were observed between the groups at any of the safety visits (p-values; 0.211, 0.892, and 0.826 respectively), as represented in Table 4 and showed in Figure 3A.
 |
Table 4 Clinical Effectiveness and Safety (N = 18 Patients per Group) |
At day 29, there was a slight increase in corneal thickness (by OCT) compared to 2-weeks before surgery in both HEC and PRO-149 groups (t(34)=1.006, p = 0.322). At the final visit, the corneal thickness increased 2.8 ± 20.2 µm for the HEC group vs 9.9 ± 22.2 µm in PRO-149 group, see Table 4.
The percentage of patients with grade 0 (no cells) 24 hours after surgery was similar between HEC and PRO-149 groups (0% vs 5.6%, p = 0.560). By the final visit, there was a significant improvement in anterior chamber cell grade in both groups, compared with day 1 (p = 0.001). All the patients in both groups showed 100% of grade 0 anterior chamber cellularity by day 29, see Table 4. Similar findings were observed in the analysis of anterior chamber flare; the percentage of patients with grade 0 flare was not different between HEC and PROP-149 groups (33.3% vs 61.1%, p = 0.230). As shown Table 4, by day 29, all patients in both groups presented grade 0 flare.
There was a statistically significant difference in both OVDs in terms of the postoperative mean BCVA at day 29 (22.5 ± 3.5 vs 22.2 ± 4.9) compared to preoperative values (69.4 ± 84.4 vs 106.1 ± 114.2, p < 0.0001), see Figure 3B. However, no significant differences were found between groups regarding the mean BCVA at any safety visit (p-values: 0.981, 0.834, and 0.847, respectively), see Table 4.
Surgical complications were rare, with only three patients reporting AEs (2 in the HEC and 1 in the PRO-149 groups). The AEs reported for HEC were conjunctival hyperemia and Descemet’s membrane striae, both classified as mild. The patient in the PRO-149 group showed posterior capsule rupture during the surgical procedure and ocular hypertension, both classified as moderate. No surgical procedures outside the protocol were performed.
Finally, the rate of user acceptance for the OVD was measured on a 5-point scale (0 = very bad, 1 = bad, 2 = acceptable, 3 = good and, 5 = very good). The rates of user acceptance (“good to very good”) amounted to 97.2% for each OVD (see Figure 4). The overall rate of acceptance for both OVDs was similar (p = 0.713). No significant differences were observed for the following variables: capability to maintain a formed anterior chamber during continuous circular capsulorhexis and lens implantation (p = 0.154), retention during surgery (p = 0.641), user-friendliness (p = 0.684), and ergonomics (p = 0.189). The transparency/visibility of the OVD during the surgery, was “very good” in all cases for both OVDs. Ease of use during extraction/removal was rated “good” in 83.3% in HEC vs 94.4% of the ratings for PRO-149, favoring PRO-149 (p = 0.044).
Discussion
PRO-149 is a viscoelastic solution composed of sodium hyaluronate 3% developed for use as an OVD in cataract surgery.
Dispersive OVDs’ molecules are less likely to adhere to each other (less cohesion) as well as to the corneal endothelial cells.24 Usually, this type of viscoelastics have a low molecular weight, such as PRO-149 and HEC. The interpretation of the rheological profile is not trivial considering that the Na-HA used has a low molecular weight (800Da ~ 1.0 MDa) and is made of short chains. The initial structure adopted in both viscoelastic solutions is comprised of many junction points or intramolecular bonds that give it a certain degree of flexibility and allow it to achieve different configurations and structural orders within the solution (area comprised from 0.001 to 1 seg−1).13 When the OVDs experiment a certain degree of deformation due to applied force (area comprised from 1 to 1000 seg−1) this shear force transmits the stress applied to all the molecules in the solution, generating a rearrangement or alignment of the molecules in the direction of the applied velocity gradients, causing a decrease in the resistance to it. This deformation causes the temporary formation of a different structure during the experiments. Thus, the rheological characteristics of the Na-HA present in viscoelastic solutions, even when these are intended for the same indication, allow for a range of different options and OVD behavior in ophthalmological surgical procedures.10,13
In general, OVDs aid the ophthalmic surgeon by dividing tissue, resolving adhesions, acting as a wetting agent or even as an instrument. Particularly for cataract surgery, OVDs protect ocular structures by preventing mechanical trauma by creating the required space that allows a safe manipulation of the tissues and shields the corneal endothelium. Depending on the characteristics of a particular OVD, it can be classified according to its cohesion and viscosity, and therefore considered more useful for a certain procedure. For example, either dispersive OVDs or a combination of agents may be more adequate for assuring tissue protection.25,26
However, as much as OVDs are indispensable for the obtention of successful surgical results, some studies have reported that both cohesive and dispersive OVDs have a similar capacity to protect the endothelium.27,28 Even for hard cataracts grade 3/4, no evidence of significant difference in endothelial cell count has been reported between groups treated with OVDs of different rheological characteristics.29 Nevertheless, it must be considered that most published trials evaluating OVD performance generally include a relatively small number of patients, with a high basal endothelial cell count, and do not take into account particularly challenging cases. Currently, there is no single OVD considered clearly superior to others for various ophthalmologic surgical techniques, therefore it is important that OVDs continue to be developed and their safety and efficacy proven.8
PRO-149 has undergone a thorough development process including rheological studies which demonstrated that its dynamic and structural arrangements adopted were similar to the reference product’s, showing a similar profile concerning the zero-shear viscosity and physicochemical osmolality of both substances. The synergic interaction of these characteristics makes PRO-149 a solution fit to use during cataract surgery, showing a dispersive profile useful for maintaining spaces that permit surgical manipulation while protecting the corneal endothelium and other intraocular structures.1
Safety and toxicity for PRO-149 were also evaluated in an in vivo model. Based on previously published similar studies, the NZW rabbit model was chosen since it has proven to be a reliable model for this purpose.23,30 Corneal opacity, IOP, cellularity in the anterior chamber, flare, iris congestion, conjunctival hyperemia, secretion, conjunctival edema, and adverse events were some of the studied variables. Post-surgery inflammation followed the same general time course in both experiments, and it is relevant to point out that no anti-inflammatory drugs were administered during the post-surgical period. No significant differences were observed for iris congestion or conjunctival hyperemia, between HEC and PRO-149 OVD in all safety visits. The incidence of adverse events was also similar between groups. Furthermore, a histopathological evaluation took place in order to confirm the safety and biocompatibility of PRO-149 when in contact and interaction with live tissues, and no signs of toxicity or structural damage were observed.
Regarding the clinical study in which PRO-149 was evaluated during cataract surgery, thirty-six patients were subjected to phacoemulsification and insertion of an IOL and later evaluated through endothelial cell count, and postoperative intraocular pressure.
By the final visit (day 29), as expected, no difference was observed between the two groups regarding the percentage of change for ECC. This aligns with previously described data in multiple studies regarding OVD’s effectiveness in protecting corneal endothelial cells.1,9,18,31
There was a mild IOP increase in early postoperative visits, as it has been described by other authors and in accordance with the known transient adverse event of hypertension after the use of any OVD.26,32 There was only one case in which the IOP raised up to 18 mmHg on day 1, and it is worth mentioning that all patients presented a normal IOP by the last visit and that there were no statistically significant differences between groups at any of the visits. This IOP increment is consistent with the reported results shown to be transient and mild in this type of procedure, having no clinical impact on patients’ final results and long-term ocular integrity.25,28,33
As demonstrated in other publications, complications related to OVDs during cataract surgery are rare and generally mild and transient.25,33 A total of 4 adverse events were reported by 3 subjects. The HEC group presented a case of mild conjunctival hyperemia and another case of mild Descemet membrane striae, both of which were satisfactorily resolved. Meanwhile, PRO-149 group presented 2 successfully resolved adverse events: one moderate rise in IOP and a posterior capsule rupture.
Other secondary variables such as central corneal thickness, and best corrected visual acuity did not show any statistically significant difference when comparing both groups, demonstrating a similar safety profile. Both groups showed a significant improvement in post-surgery vision, compared to preoperative values, as expected for the characteristics of the treated pathology.
Flare and cellularity in the anterior chamber after phacoemulsification presented a similar tendency in both groups, observing no difference in the percentage of patients with grade 0 (no cells) 24 hours after surgery. On the final visit both groups presented no incidence of either flare or cellularity. Other previous studies have described similar results for these variables, presenting a significant yet mild incidence of flare and cellularity during the early postoperative period with a complete resolution by the 1-month evaluation.32
Furthermore, the clinical performance of both HEC and PRO-149 was evaluated with a survey based in the general performance key aspects during a cataract surgery, as other authors have done previously.21 The acceptance rate for the OVDs was evaluated and 97.2% for each group were rated as “good to very good”. Parameters such as capability to maintain a formed anterior chamber during continuous circular capsulorhexis and lens implantation, retention during surgery, user-friendliness, and ergonomics presented no statistically significant differences between groups. Ease of use during extraction/removal was rated “good” in 83.3% in HEC vs 94.4% of the ratings for PRO-149, favoring PRO-149. This characteristic is clinically relevant both during surgical procedures and afterwards in the postoperative period since it portrays how easy it is for surgeons to remove the OVD from the anterior chamber, assuring no product remains in this space which could potentially cause a rise in IOP after the procedure.8,21,25
It has been demonstrated in previous studies the similarity between HEC and Viscoat®, another broadly marketed OVD, regarding endothelial cell decrease 3 months after phacoemulsification. In this study, safety was also evaluated through the study of rise in IOP to ≥30 mmHg. Since its safety and efficacy have been well established and because the rheological properties of currently marketed HEC are like PRO-149ʹs, HEC was chosen as the reference product for the development process of PRO-149.34
Finally, this study presents a few limitations such as follow-up time after phacoemulsification. However, previously published studies have reported a very similar percentage of ECC loss at both, the 30-day and 90-day time frames.31,32
Another limitation was the fact that in the preclinical in vivo study the evaluation of OVDs was not undertaken through a fully realistic surgical technique which would have included the use of a phacoemulsification ultrasound hand piece as well as the implantation of an intraocular lens. Nevertheless, similar studies have been reported in which only AHE experiment was performed, whereas our study included both AHE and ACW models.23,30
Conclusion
After a comprehensive and exhaustive assessment of PRO-149ʹs properties, following a complete pipeline including the rheological, preclinical in vivo, and clinical studies detailed in this paper, it has been established that it is a safe, effective and convenient OVD in phacoemulsification and IOL implantation surgery.
Abbreviations
ACW, anterior chamber washout; AE, adverse event; AHE, aqueous humor exchange; BCVA; best corrected visual acuity; CCT, central corneal thickness; ECC, endothelial cells count; HEC, control OVD; IOP; intraocular pressure; Na-HA; sodium hyaluronate; OVD, ophthalmic viscoelastic device; PP, per-protocol population; PRO-149, test OVD.
Data Sharing Statement
The additional data that supports the findings of this study are available from the corresponding author [PMV], upon reasonable request.
Ethical Approval
This study was conducted in compliance with the Declaration of Helsinki and in accordance with Good Clinical Practices Standards. Informed consent was obtained from all participants included in the study. The location of the study was SalaUno Salud, SAPI de CV, Mexico City (Mexico). The study protocol was approved by their respective Institutional Review Board, Centro de Investigación Clínica Acelerada, SC. The study was registered in local MOH (COFEPRIS) before recruiting the first participant as 203301410C0039/2021 (21-08-09), and in ClinicalTrials.gov at NCT04702802 (21-01-11).
Acknowledgments
The authors thank Dr Ricardo Jauregui Franco, for the medical support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was endorsed by Laboratorios Sophia, SA de CV (Zapopan, Jalisco, Mexico).
Disclosure
The funder provided support in the form of salaries for authors [PMV, ASR, OOM, and LBD], but this commercial affiliation did not have any additional role in the data collection. The rest of the authors declared that they had no personal, financial, commercial, or academic interest.
References
1. Arshinoff S. Ophthalmic viscosurgical devices. In: Kohnen T, Koch D, editors. Cataract and Refractive Surgery Essentials in Ophthalmology. Berlin, Heidelberg: Springer; 2005:37–62.
2. Miller D, Stegmann R. Healon (Sodium Hyaluronate). A Guide to Its Use in Ophthalmic Surgery. New York: John Wiley & Sons; 1983:5–28.
3. Arshinoff S, Hofmann I, Nae H. Role of rheology in tears and artificial tears. J Cataract Refract Surg. 2021;47(5):655–661.
4. Arshinoff SA, Jafari M. New classification of ophthalmic viscosurgical devices–2005. J Cataract Refract Surg. 2005;31(11):2167–2171.
5. Lubrication Maintenance Management. Encyclopedia of Lubricants and Lubrication. Springer, Berlin: Heidelberg; 2014.
6. Tian Z, Duan L, Wu L, Shen L, Li G. Rheological properties of glutaraldehyde-crosslinked collagen solutions analyzed quantitatively using mechanical models. Mater Sci Eng C Mater Biol Appl. 2016;63:10–17.
7. Amangeldi M, Wang Y, Perveen A, Zhang D, Wei D. An Iterative Approach for the Parameter Estimation of Shear-Rate and Temperature-Dependent Rheological Models for Polymeric Liquids. Polymers. 2021;13(23):4185.
8. Higashide T, Sugiyama K. Use of viscoelastic substance in ophthalmic surgery - focus on sodium hyaluronate. Clin Ophthalmol. 2008;2(1):21–30.
9. American Academy of Opthalmology. Lens and Cataract. San Francisco: AAO; 2016.
10. Bissen-Miyajima H. Ophthalmic viscosurgical devices. Curr Opin Ophthalmol. 2008;19(1):50–54.
11. Gibbs DA, Merrill EW, Smith KA, Balazs EA. Rheology of hyaluronic acid. Biopolymers. 1968;6(6):777–791.
12. Snetkov P, Zakharova K, Morozkina S, Olekhnovich R, Uspenskaya M. Hyaluronic Acid: the Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. Polymers. 2020;12(8):1800.
13. Balazs EA. Viscoelastic Properties of Hyaluronan and Its Therapeutic Use. In Chemistry and Biology of Hyaluronan.
14. Johnson & Johnson Vision Inc. HEALON EndoCoat® Dispersive OVD [Package Insert]. Santa Ana, California: Johnson & Johnson Vision Inc; 2020.
15. Charan J, Kantharia ND. How to calculate sample size in animal studies? J Pharmacol Pharmacother. 2013;4(4):303–306.
16. Festing MF. Reduction of animal use: experimental design and quality of experiments. Lab Anim. 1994;28(3):212–221.
17. Chylack LT, Wolfe JK, Singer DM, et al. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol. 1993;111(6):831–836.
18. Storr-Paulsen A, Nørregaard JC, Farik G, Tårnhøj J. The influence of viscoelastic substances on the corneal endothelial cell population during cataract surgery: a prospective study of cohesive and dispersive viscoelastics. Acta Ophthalmol Scand. 2007;85(2):183–187.
19. Chow S, Shao J, Wang H. Chapter 3. Comparing Means. In: Sample Size Calculation in Clinical Research.
20. Foster C, Vitake A, eds. Diagnosis and Treatment of Uveitis. Philadelphia: WB Saunders Company; 2002.
21. Daas L, Larrosa JM, Gavin A, et al. Clinical Comparison of the Performance of Two Marketed Ophthalmic Viscoelastic Devices (OVDs): the Bacterially Derived Healon PRO OVD and Animal-Derived Healon OVD. J Ophthalmol. 2020;2020:8874850.
22. Kaur K, Gurnani B. Viscoelastics. Treasure Island, FL: StatPearls Publishing; 2022.
23. Törngren L, Lundgren B, Madsen K. Intraocular pressure development in the rabbit eye after aqueous exchange with ophthalmic viscosurgical devices. J Cataract Refract Surg. 2000;26(8):1247–1252.
24. Kretz FT, Limberger IJ, Auffarth GU. Corneal endothelial cell coating during phacoemulsification using a new dispersive hyaluronic acid ophthalmic viscosurgical device. J Cataract Refract Surg. 2014;40(11):1879–1884.
25. Borkenstein AF, Borkenstein EM, Malyugin B. Ophthalmic Viscosurgical Devices (OVDs) in Challenging Cases: a Review. Ophthalmol Ther. 2021;10(4):831–843.
26. Holzer MP, Tetz MR, Auffarth GU, Welt R, Völcker HE. Effect of Healon5 and 4 other viscoelastic substances on intraocular pressure and endothelium after cataract surgery. J Cataract Refract Surg. 2001;27(2):213–218.
27. Van den Bruel A, Gailly J, Devriese S, Welton NJ, Shortt AJ, Vrijens F. The protective effect of ophthalmic viscoelastic devices on endothelial cell loss during cataract surgery: a meta-analysis using mixed treatment comparisons. Br J Ophthalmol. 2011;95(1):5–10.
28. Auffarth GU, Auerbach FN, Rabsilber T, et al. Comparison of the performance and safety of 2 ophthalmic viscosurgical devices in cataract surgery. J Cataract Refract Surg. 2017;43(1):87–94.
29. Vajpayee RB, Verma K, Sinha R, Titiyal JS, Pandey RM, Sharma N. Comparative evaluation of efficacy and safety of ophthalmic viscosurgical devices in phacoemulsification [ISRCTN34957881]. BMC Ophthalmol. 2005;5:17.
30. Leang RS, Kloft LJ, Gray B, Gwon AE, Huang LC. Preclinical Safety Evaluation of Ophthalmic Viscosurgical Devices in Rabbits and a Novel Mini-Pig Model. Ophthalmol Ther. 2019;8(1):101–114.
31. Ravalico G, Tognetto D, Baccara F, Lovisato A. Corneal endothelial protection by different viscoelastics during phacoemulsification. J Cataract Refract Surg. 1997;23(3):433–439.
32. Chang DH, Christie WC, Loden JC, Smith PJ, Jackson BE. Clinical evaluation of a bacterially derived sodium hyaluronate 2.3% ophthalmic viscosurgical device. J Cataract Refract Surg. 2019;45(12):1789–1796.
33. Chan E, Mahroo OA, Spalton DJ. Complications of cataract surgery. Clin Exp Optom. 2010;93(6):379–389.
34. Maár N, Graebe A, Schild G, Stur M, Amon M. Influence of viscoelastic substances used in cataract surgery on corneal metabolism and endothelial morphology: comparison of Healon and Viscoat. J Cataract Refract Surg. 2001;27(11):1756–1761.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.