Back to Journals » Clinical Ophthalmology » Volume 16
Evaluation of the EVO/EVO+ Sphere and Toric Visian ICL: Six Month Results from the United States Food and Drug Administration Clinical Trial
Authors Packer M
Received 5 April 2022
Accepted for publication 13 May 2022
Published 21 May 2022 Volume 2022:16 Pages 1541—1553
DOI https://doi.org/10.2147/OPTH.S369467
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mark Packer
Packer Research Associates, Boulder, CO, USA
Correspondence: Mark Packer, Packer Research Associates, 1400 Bluebell Ave., Boulder, CO, 80302, USA, Tel +1 (541) 915 – 0291, Email [email protected]
Purpose: To evaluate the safety and effectiveness of Collamer posterior chamber phakic refractive lenses with a central port design (EVO and EVO+ Sphere and Toric Visian ICLs) for correction of moderate-to-high myopia with or without astigmatism.
Patients and Methods: Six-month results of a multicenter clinical trial performed under United States FDA Investigational Device Exemption. Subjects 21 through 45 years of age with myopia ranging from − 3.00 D to − 20.00 D and astigmatism up to 4.00 D underwent implantation of EVO or EVO+ Sphere or Toric Visian ICLs. Uncorrected (UDVA) and corrected (CDVA) distance visual acuities, manifest refraction, intraocular pressure (IOP), endothelial cell density, and adverse events were evaluated over 6 months.
Results: This clinical trial enrolled 629 eyes of 327 subjects with mean age 35.6 ± 5.09 years. Mean preoperative spherical equivalent (SE) measured − 7.62 ± 2.75 D (range: − 3.00 to − 15.62 D). At 6 months, mean SE was − 0.079 ± 0.33 D, with 90.5% within ± 0.50 D of target and 98.9% within ± 1.00 D of target. Mean postoperative UDVA and CDVA were − 0.059 ± 0.10 logMAR and − 0.13 ± 0.08 logMAR, respectively. 52.3% of eyes gained lines of CDVA. Efficacy and safety indices were 1.06 and 1.24, respectively. No eye experienced pupillary block, required peripheral iridotomy or iridectomy, developed anterior subcapsular cataract or had elevated IOP due to angle narrowing or pigment dispersion. Mean endothelial cell density declined by 2.3%.
Conclusion: EVO ICL lenses demonstrated accuracy of refractive correction and achievement of high levels of UDVA. This clinical trial confirmed that the central port design of EVO and EVO+ Sphere and Toric Visian ICL lenses functions effectively to allow physiologic flow of aqueous humor, thus eliminating the requirement for preoperative peripheral iridotomies. The results of this clinical trial resulted in FDA approval on March 25, 2022.
Keywords: phakic refractive lens, myopia, astigmatism, Implantable Collamer Lens
Introduction
The benefits of the Visian Implantable Collamer Lens (ICL, STAAR Surgical, Monrovia, CA, USA) have been demonstrated by predictable, stable, refractive correction1 and a high efficacy index,2 as well as improvements in quality of vision3 and quality of life.4 Long-term studies of safety have demonstrated low rates of adverse events.5
 |
Figure 1 Scatter plot of attempted versus achieved correction of manifest refraction spherical equivalent, demonstrating the full range of refractive correction. |
In 2011, new ICL models were introduced: the EVO Visian Implantable Collamer Lens for Myopia and the EVO Visian Toric Implantable Collamer Lens for Myopia with Astigmatism. These lens models incorporate a central port to facilitate the flow of aqueous humor without the need for construction of peripheral iridotomies. In 2015, EVO+ lenses were introduced. These models include a slightly larger optic diameter than the EVO lens models to offer additional options for patients with larger pupil sizes.
The central port in the EVO/EVO+ sphere and toric ICL lens models allows the physiologic flow of aqueous humor through the lens, eliminating the need for peripheral iridotomies (PIs) prior to implantation. Where these lens models are available, patients require fewer office visits and procedures prior to surgery, without the introduction of new adverse events or increased occurrence of known adverse events as compared with the parent devices. Regarding the central port design, “Safety data suggest reduced rates of anterior subcapsular cataract and pupillary block compared with earlier models.”6
The clinical trial reported in this article was performed to gain United States FDA approval by evaluating the safety and collecting supportive data concerning the effectiveness of the EVO/EVO+ Sphere and Toric ICL lenses with the central port design. The FDA approved the EVO/EVO+ Sphere and Toric ICL lenses in March 2022.7
Materials and Methods
The EVO/EVO+ Visian Implantable Collamer Lens is a posterior chamber phakic refractive lens manufactured from Collamer, a proprietary hydroxyethyl methacrylate (HEMA)/porcine collagen containing biocompatible polymer material. The lens features a plate-haptic design with a central convex/concave optical zone and a 360 μ diameter central port; the lens incorporates a forward vault to minimize contact of the lens with the central anterior capsule of the crystalline lens. The EVO ICL lens optic diameter varies with the dioptric power: the smallest optic diameter is 4.9 mm and the largest is 5.8 mm, while the optic diameter of EVO+ lenses varies from 5.0 mm to 6.1 mm.
The Multicenter Clinical Evaluation of the EVO/EVO+ Visian Implantable Collamer Lens was designed as a prospective, 3-year, multicenter study to evaluate the safety and effectiveness of the EVO/EVO+ Visian Implantable Collamer Lens (EVO ICL). Safety outcomes included the incidence of pupillary block, adverse events, and endothelial cell loss. Effectiveness outcomes included refractive accuracy and uncorrected visual acuity. An interim analysis in support of FDA approval was performed after a minimum of 300 subjects completed the Month 6 Visit. The study was conducted with prior approval from Advarra Institutional Review Board (Columbia, MD), in accordance with HIPAA regulations, in compliance with the Declaration of Helsinki and under FDA Investigational Device Exemption (IDE) G191084. Registration information is available at https://clinicaltrials.gov/ct2/show/NCT04283149. Informed consent for the research was obtained from each subject prior to initiation of study-specific activities.
Subjects were eligible for enrollment if they were from 21 to 45 years of age, had moderate-to-high myopia ranging from −3.00 D to −20.00 D spherical equivalent (SE) in the spectacle plane with astigmatism up to 4.00 D, stable refractive history and minimum endothelial cell density (ECD) requirements for age and anterior chamber depth (ACD) consistent with FDA-approved labeling for the parent Visian Implantable Collamer Lens for Myopia (MICL) and Visian Toric Implantable Collamer Lens for Myopia (TICL). Potential subjects were excluded from enrollment if they had ACD <3.00 mm, anterior chamber angle (ACA) Grade < III, ocular hypertension or glaucoma, pseudoexfoliation, pigment dispersion, history or signs of uveitis, diabetes, previous ocular surgery, cataract, or progressive, sight-threatening disease.
Investigators used the STAAR Surgical Online Calculation and Ordering System (www.ocos.STAAR.com) to order study lenses. The lens power and size calculations were performed by investigators using OCOS: they were required to select a lens with the overall diameter (ie, 12.1, 12.6, 13.2 or 13.7 mm) recommended by the calculator based on ACD and white-to-white (WTW) measurements, which were obtained according to each investigator’s standard methods for ICL surgery. Following input of a subject’s preoperative assessment information, OCOS recommended a range of spherical powers along with the expected postoperative values (ie, residual SE) for the EVO Sphere lenses. In the case of EVO Toric lenses, OCOS provided a cylinder power and a range of spherical powers along with their expected postoperative values (ie, residual sphere, cylinder, axis and SE). Investigators were required to target postoperative emmetropia for all enrolled eyes with an acceptable variation of ± 0.50 D spherical equivalent. An Implantation Orientation Diagram was generated for each toric study lens identifying the amount and direction of rotation required after insertion of the lens in the eye to provide optimal astigmatic correction.
No peripheral iridotomies or iridectomies were performed prior to or during lens implantation. Study lenses were implanted through an incision of 3.5 mm or less following instillation of an ophthalmic viscosurgical device (OVD), hydroxypropylmethylcellulose (HPMC) 2%, in the anterior chamber. The use of prophylactic ocular hypotensive medication was not permitted. Postoperatively, an IOP check was performed 1 to 6 hours after surgery, prior to the release of the subject.
Treated subjects were to be seen for 8 scheduled postoperative study visits per eye over the course of approximately 3 years after surgery (Day 1, Week 1, Month 1, Month 3, Month 6, Year 1, Year 2, and Year 3). In subjects who qualified to have both eyes included in the study, fellow eye implantation occurred between 7 days and 14 days after uneventful surgery in the first eye. Assessments included uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), manifest refraction, gonioscopy, slit-lamp examination, crystalline lens status per the Lens Opacities Classification System III (LOCS III),8 specular microscopy (with corneal endothelial cell density measurements performed by an independent reading center), intraocular pressure (IOP), dilated fundus examination, optical coherence tomography (OCT; lens vault), and reporting of adverse events.
Results
The study enrolled 629 eyes of 327 subjects at 14 clinical sites in the United States. The first enrolled subject underwent surgery in the primary eye in January 2020, and the last eye was implanted in December 2020. A total of 619 eyes (98.4%) of 321 subjects were available for analysis at the 6-month visit. Table 1 provides the preoperative characteristics of subjects and eyes enrolled.
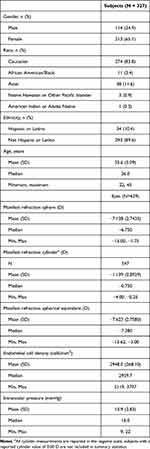 |
Table 1 Preoperative Characteristics |
Predictability
Figure 1 provides a scatterplot of the attempted versus achieved spherical equivalent (SE) correction (R2 = 98.6%). Six months after surgery, 90.5% of eyes were within ± 0.50 D of the targeted SE refraction, and 98.9% of eyes were within ± 1.00 D (Figure 2).
 |
Figure 2 Accuracy of spherical equivalent refractive correction at Month 6. 90.5% of eyes were within ± 0.50 D and 98.9% of eyes were within ± 1.00 D of emmetropia. |
Stability
Figure 3 provides the change in the mean SE and stability of refraction over time for the consistent cohort of 617 eyes with data at all time points. The mean SE was −7.62 ± 2.76 D preoperatively and −0.11 ± 0.30 D at 1 month, −0.03 ± 0.31 D at 3 months and −0.08 ± 0.34 D at 6 months.
 |
Figure 3 The stability of the spherical equivalent refraction from Month 1 to Month 6 is demonstrated in the consistent cohort of 617 eyes with data at all time points. |
Efficacy
Figure 4 provides the distribution of postoperative UDVA at 6 months. 87.6% of eyes achieved 20/20 or better and 99.7% of eyes achieved 20/40 or better postoperative UDVA. Only 2 of 619 eyes (0.3%) reported UDVA less than 20/40 at 6 months: 1 eye reported UDVA 20/50 due to a nuclear sclerotic cataract and 1 eye reported UDVA 20/50 due to under correction despite implantation with the highest available power EVO ICL lens (−16.0 D). The mean postoperative UDVA was −0.052 ± 0.10 logMAR, −0.066 ± 0.94 logMAR and −0.059 ± 0.10 logMAR at 1, 3 and 6 months, respectively. The efficacy index at 6 months was 1.06.
 |
Figure 4 Frequency distribution of uncorrected visual acuity at Month 6. All visual acuity testing was performed using an ETDRS chart and values were converted from logMAR to Snellen. |
Safety
Mean preoperative CDVA was −0.036 ± 0.07 logMAR; postoperative CDVA measured -0.12 ± 0.081 logMAR, −0.13 ± 0.079 logMAR and −0.13 ± 0.08 logMAR at 1, 3 and 6 months, respectively. Figure 5 provides the change in lines of CDVA for 619 eyes at 6 months. A total of 98.5% of eyes reported CDVA at 6 months that was equal to or better than preoperative CDVA. Overall, 52.3% of eyes gained lines of CDVA: 39.1% gained 1 line, 12.8% gained 2 lines, and 0.5% gained 3 or more lines. 46.2% of eyes experienced no change in CDVA, and 1.5% lost 1 line. No eye lost 2 or more lines of CDVA, and all eyes had CDVA 20/32 or better at 1, 3 and 6 months. The safety index at 6 months was 1.24.
 |
Figure 5 Lines of corrected distance visual acuity gained or lost. 52.3% of eyes gained lines of CDVA and 98.5% of eyes demonstrated CDVA at 6 months equal to or better than preoperative CDVA. |
Intraocular Pressure, Endothelial Cell Density and Vault
Figure 6 provides the mean IOP over time. Transient increase in IOP due to retained OVD (HPMC 2%) was reported in 19.9% (125/629) of eyes at the 1–6-hour postoperative visit and was treated, if indicated, by release of aqueous fluid from a previously constructed incision and/or ocular hypotensive medication. Of note, no prophylactic systemic or topical ocular hypotensive medications (eg, acetazolamide) were permitted during the study. Events of increased IOP attributed to a response to the topical steroid, which was prescribed as part of the standard perioperative medical regimen, occurred in 2.4% (15/629) of eyes from 6 to 31 days postoperatively. There were no instances of increased IOP related to blockage of aqueous flow through the central port, anterior chamber angle narrowing, pigment dispersion or intraocular inflammation.
 |
Figure 6 Mean postoperative intraocular pressure remained stable from Day 1 through Month 6. |
Mean endothelial cell density declined 2.3 ± 4.0% from preoperative to 6 months.
Figure 7 provides the mean vault of the EVO ICL lens over time. Mean vault measured 518 ± 232 μ, 510 ± 232 μ, and 492 ± 227 μ at 1, 3 and 6 months, respectively. There were no events of increased IOP related to narrowing of the anterior chamber angle associated with higher levels of vault.
 |
Figure 7 Vault measurements were consistent with values previously reported in the published literature. |
Adverse Events and Secondary Surgeries
No pupillary block, angle closure glaucoma, pigment dispersion or anterior subcapsular cataract have occurred in this clinical trial.
Two eyes (2/629, 0.3%) of 2 subjects underwent ICL repositioning and subsequent exchange because of narrowing of the anterior chamber angle in the absence of elevated IOP. Following lens exchange, vaults of 1380 μ and 1430 μ were reduced to vaults of 700 μ, and 340 μ, respectively. Neither eye experienced increased IOP, and the angle narrowing resolved following exchange for the next smaller overall diameter lens. Both eyes reported UDVA 20/16 following lens exchange.
One eye underwent toric ICL repositioning due to “blurry” UDVA of 20/32 secondary to residual astigmatism noted on the first postoperative day. After repositioning, UDVA improved to 20/16. One eye underwent ICL explantation due to a subjective report of halo and glare beginning on the first postoperative day. The fellow eye was not implanted, and symptoms resolved following explantation. CDVA in the primary eye returned to the preoperative level.
A nuclear sclerotic cataract developed in 1 eye (1/629, 0.02%) of a 36-year-old subject, resulting in myopic shift and reduced visual acuity. ICL explantation and uncomplicated cataract surgery were performed following the 6-month study visit. Three eyes (3/629, 0.5%) of 2 subjects underwent surgery for retinal detachment. One of these subjects reported UDVA 20/40 and CDVA 20/16 at 6 months in an eye which experienced a myopic shift following scleral buckle; the fellow eye reported UDVA 20/12.5. The other subject reported UDVA 20/12.5 in an eye which underwent laser retinopexy and UDVA 20/16 in an eye which underwent pars plana vitrectomy.
Discussion
The safety and effectiveness of the EVO/EVO+ Sphere and Toric lenses for the correction of myopia and myopia with astigmatism have been demonstrated in this prospective, multicenter clinical trial, and have confirmed results previously reported in the published literature. A recent review article considered a total of 27 peer-reviewed papers providing effectiveness data for the EVO ICL from prospective or retrospective case series and included data from a total of 1905 eyes with average weighted follow-up of 12.5 months.6 The review reported a mean efficacy index of 1.04 compared with 1.06 in this study, mean UDVA of −0.02 logMAR compared with −0.059 logMAR in this study, and 90.8% SE within ± 0.50 D and 98.7% SE within ± 1.00 D compared with 90.5% and 98.9%, respectively, in this study.
The preservation of CDVA observed in this clinical trial, in which 98.5% of eyes reported CDVA at 6 months that was equal to or better than their preoperative CDVA, is at least equivalent to that observed with the MICL and TICL parent lenses.9 In the MICL PMA study, 19 eyes (4%, 19/478) were reported to have a decrease of 1 line of CDVA, and 2 eyes (0.4%, 2/478) were reported to have a decrease of 2 lines of CDVA at 6 months. In the TICL PMA study, 3 eyes (1.4%, 3/210) were reported to have a loss of ≥2 lines of CDVA between the preoperative and 12-month visits. Comparison of these data confirms that the addition of the central port to the ICL platform has no impact on corrected distance visual acuity.
The mean ECD loss of 2.3% for all eyes in this clinical trial is similar to the rate reported in the literature for EVO ICL lenses (ie, 2.6%, with weighted average follow-up of 14.7 months).6 The rate is also similar to that reported in the MICL PMA, which was 3.19% at 12 months.9 Comparisons of the rates of cell loss in these clinical trials and the published literature are valid despite the differences in length of follow-up because cell loss measured in these time periods is presumed to be primarily related to the surgical procedure. The use of an independent reading center to evaluate specular microscopy in this clinical trial supports the scientific validity of these results. Nevertheless, a limitation of this report regarding corneal endothelial health is the length of follow-up. The full 3-year study results will allow additional insight regarding long-term changes in endothelial cell density.
The most frequently reported adverse event in this clinical trial was a transient increase in IOP 1 to 6 hours postoperatively due to retained OVD (19.9%, 125/629 of all eyes). These events were due to incomplete removal of OVD and unrelated to the EVO ICL; all resolved without secondary surgical intervention and without sequelae by the first postoperative day. The potential for elevation of IOP following the use of an OVD with ICL implantation is referenced as a Precaution in the MICL/TICL Directions for Use: “Inadequate flushing of the viscoelastic from the eye may lead to IOP spikes.”9 There were no reports of increased IOP in this trial related to blockage of aqueous flow through the central port, anterior chamber angle narrowing, pigment dispersion or intraocular inflammation.
Similar results to those observed in this clinical trial have been reported in a prospective, randomized, masked, fellow-eye comparative study demonstrating that 20% of eyes in which HPMC 2% was used without prophylactic IOP-lowering medications experienced IOP > 30 mmHg (range, 33–45 mmHg) at the 2-hour postoperative time point.10 All eyes in which HPMC 2% was used had at least a transient increase in IOP > 22 mmHg within the first 24 hours. As with the published study, no prophylactic systemic or topical IOP-lowering medications (eg, acetazolamide) were allowed during this clinical trial. The rate of increased IOP in the immediate postoperative period reported in the published study (20%) is remarkably similar to the rate reported in this clinical trial (19.9%). As noted in a recent publication, “A large number of studies have shown that early postoperative IOP elevation is a common complication due to residual OVDs following ICL implantation … ”11
The development of a nuclear sclerotic cataract in 1 eye (1/629, 0.2%) fits the description provided by Kaufman and Sugar of young patients with axial myopia who develop “discrete, opalescent” nuclear cataract.12 The mean age of the patients in these authors’ series was 44 years (range, 34–54 years). The mean best corrected visual acuity of the patients was 20/50, and the ocular history of the patients included a progressive decrease in vision. The authors note that “Our series suggests that an opalescent cataract can accompany axial myopia with or without notable induced myopic shift.”12 Given that ICL implantation has never been associated with the development of nuclear sclerotic cataract and that this subject’s course corresponds with a known clinical entity of opalescent cataract in young myopic patients without any history of ocular surgery, the nuclear cataract in this case is considered not related to either the EVO ICL or the implantation procedure.
Three eyes (3/629, 0.5%) of 2 subjects underwent surgical intervention for retinal detachment. The annual risk of retinal detachment among the US population with myopia greater than −3.1 D has been reported to be 117 in 100,000, with a lifetime risk of 9.3%.13,14 The increased risk of retinal detachment in myopia is attributed to anatomic and physiologic characteristics of myopic eyes:
In addition to axial length greater than or equal to 26 mm, the high myopic eye exhibits a thin and fragile sclera, choroidal hemodynamic changes, early and strong vitreous liquefaction followed by earlier posterior vitreous detachment and complex vitreous–retinal interface, pathologic vitreoretinal attachments with higher peripheral retinal break occurrence, irregularity of the posterior border of the vitreous base, and weak retinal adhesion.15
Regarding retinal detachment following posterior chamber phakic IOL implantation, “data suggest that these retinal detachments are part of the natural history of retinal detachment in high-degree myopia.”16 The authors have concluded that “we cannot demonstrate that the correction of high myopia by the implantation of phakic lenses plays a role in the eventual occurrence of retinal detachment.”17 The American Academy of Ophthalmology Preferred Practice Pattern further states that
Phakic intraocular lenses have not been associated with increased risk of retinal detachment compared with other intraocular interventions in highly myopic patients.18
Therefore, the retinal events reported for 2 subjects in this clinical trial are considered unrelated to both the EVO ICL and the implantation procedure.
One eye (1/629, 0.02%) underwent ICL explantation due to a subjective report of halo and glare. Unwanted optical side effects such as halo and glare have been previously reported following ICL implantation,19 including effects due to the presence of a peripheral iridotomy. For example, as noted during the FDA Advisory Committee Meeting for the TICL,
There was one patient who observed a photopsia immediately following the peripheral iridotomy and prior to the placement of the ICL. This symptom remained after the ICL was implanted, and it persisted after the ICL was removed at the patient’s request. It was felt that the photopsia was caused by the iridotomy and not specifically by the ICL.20
Two eyes of 2 subjects (0.3%, 2/629) in this clinical trial experienced anterior chamber angle narrowing in the absence of elevated IOP, which was related to excessive vault and resulted in secondary surgical intervention. Neither of these eyes developed increased IOP or other findings related to angle closure, eg, no peripheral anterior synechiae, no loss of pupil reactivity and no iris atrophy were observed. These findings reinforce the conclusion that “extremes of vault must be viewed as risk factors for adverse events, not as adverse events in and of themselves.”5 The absence of increased IOP, pigment dispersion or anterior subcapsular cataract, which have previously been associated with extremes of vault, demonstrate that a wide range of vault is well tolerated because the addition of the central port design facilitates physiologic aqueous flow.
For comparison of rates of surgical intervention related to vault, in the MICL Study 3 (0.6%, 3/526), eyes underwent ICL replacement due to the lens being “too long” through 3 months postoperative.9 In the TICL Study, one (0.5%, 1/210) eye underwent ICL replacement due to the lens being “too long” through 1 year.9 The reported rate of EVO replacement for excessive vault in the published literature is 0.03% (1/2970).6 In addition, the mean and standard deviation of vault observed in this clinical trial are similar to those observed in the published literature for both MICL and TICL lenses without the central port as well as for EVO and EVO+ lenses with the central port, irrespective of the sizing methodology employed.5 We conclude that the addition of the central port does not impact the physical vaulting behavior of the lens, although it does appear to mitigate events such as anterior subcapsular cataract and increased IOP related to angle narrowing which have previously been associated with extremes of vault.
The zero incidence of pupillary block reported in this clinical trial demonstrates the safety of the central port design, which maintains physiologic aqueous flow without the requirement for peripheral iridotomies. The rate of pupillary block observed with EVO is less than the rate reported in earlier clinical trials of lenses which required peripheral iridotomies, including the TICL PMA study (0.5% [1/210] incidence of pupillary block) and the MICL PMA study (3.2% [17/526] incidence of pupillary block).9 In this clinical trial of the EVO/EVO+ Sphere and Toric ICL lenses, no events of pupillary block occurred and no peripheral iridotomies were performed.
Conclusion
EVO/EVO+ Sphere and Toric ICL lenses have enjoyed growing popularity around the world, with over 1,000,000 implants in 75 countries.21 This prospective, multicenter clinical trial, conducted under an Investigational Device Exemption and Good Clinical Practice has rigorously confirmed the results of numerous studies published in the peer-reviewed literature by demonstrating excellent safety and effectiveness, including safety and efficacy indices of 1.24 and 1.06, respectively; uncorrected distance visual acuity of 20/20 or better in 87.6% of eyes; spherical equivalent within ± 0.50 D of target in 90.5% of eyes; minimal loss of endothelial cell density; no events of increased IOP secondary to pupillary block, angle narrowing, pigment dispersion or intraocular inflammation; no events of anterior subcapsular cataract; and no new or unanticipated adverse events. The continuation of follow-up through 3 years of study will provide additional, long-term data. The results of this clinical trial have definitively demonstrated the safety and effectiveness of EVO/EVO+ Sphere and Toric ICL lenses for the correction of myopia and myopia with astigmatism.
Data Sharing Statement
No further data beyond those provided will be shared.
Ethics Approval and Informed Consent
The study was conducted with prior approval from Advarra Institutional Review Board (Columbia, MD), and in accordance with HIPAA regulations under FDA Investigational Device Exemption (IDE) G191084. Registration information is available at https://clinicaltrials.gov/ct2/show/NCT04283149. Informed consent for the research was obtained from each subject prior to initiation of study-specific activities.
Acknowledgments
Clinical investigators who collected data and provided care for study patients include Michael Aronsky, MD, Kremer Eye Center, King of Prussia, PA; Jason Brinton, MD, Brinton Vision, St. Louis, MO; Alan Faulkner, MD, Aloha Laser Vision, Honolulu, HI; Lance Kugler, MD, Kugler Vision, Omaha, NE; Majid Moshirfar, MD, Hoopes Vision, Draper, UT; Greg Parkhurst, MD, Parkhurst NuVision, San Antonio, TX; Scott Perkins, MD, Barnett Dulaney Perkins, Phoenix, AZ; Frank Price, MD, Price Vision Group, Indianapolis, IN; Jonathan Solomon, MD, Bowie Vision Institute, Bowie, MD; Jason Stahl, MD, Durrie Vision, Overland Park, KS; Vance Thompson, MD, Vance Thompson Vision, Sioux Falls, SD, Jeffrey Whitman, MD, Key-Whitman Eye Center, Dallas, TX; William Wiley, MD, Cleveland Eye Clinic, Brecksville, OH; Zachary Zavodni, MD, The Eye Institute of Utah, Salt Lake City, UT.
Funding
This study was supported by STAAR Surgical Company.
Disclosure
The author reports personal fees from (Advanced Vision Science [Santen], Alcon, Amaros Medical, Aquea Health, Bausch + Lomb, Beaver-Visitec International, Cassini Technologies, ClearSight, International Biomedical Devices, Keranova, LENSAR, LensGen, PhysIOL, MedTrials, Precision for Medicine, Presbia USA, Promedica International, Rayner, Refocus Group, STAAR Surgical, Stroma Medical, Tarsus Pharmaceuticals, Visant Medical); equity owner (Aerie Pharmaceuticals, Amaros Medical, Aquea Health, Cassini Technologies, ClearSight, Digital Surgery Systems, International Biomedical Devices, Ira, Keranova, LENSAR, LensGen, Refocus Group, STAAR Surgical, Tarsus Pharmaceuticals, TrueVision, Visant Medical). The author reports no other conflicts of interest in this work.
References
1. Sanders DR, Doney K, Poco M; ICL in Treatment of Myopia Study Group. United States Food and Drug Administration clinical trial of the Implantable Collamer Lens (ICL) for moderate to high myopia: three-year follow-up. Ophthalmology. 2004;111(9):1683–1692.
2. Lisa C, Alfonso JF, Alfonso-Bartolozzi B, Fernández-Vega L, Pérez-Vives C, Montés-Micó R. Collagen copolymer posterior chamber phakic intraocular lens supported by the ciliary sulcus to treat myopia: one-year follow-up. J Cataract Refract Surg. 2015;41(1):98–104. doi:10.1016/j.jcrs.2014.05.036
3. Sanders DR, Vukich JA. Comparison of implantable contact lens and laser assisted in situ keratomileusis for moderate to high myopia. Cornea. 2003;22(4):324–331. doi:10.1097/00003226-200305000-00009
4. Ieong A, Hau SC, Rubin GS, Allan BD. Quality of life in high myopia before and after implantable Collamer lens implantation. Ophthalmology. 2010;117(12):2295–2300. doi:10.1016/j.ophtha.2010.03.055
5. Packer M. Meta-analysis and review: effectiveness, safety, and central port design of the intraocular collamer lens. Clin Ophthalmol. 2016;10:1059–1077. doi:10.2147/OPTH.S111620
6. Packer M. The implantable collamer lens with a central port: review of the literature. Clin Ophthalmol. 2018;12:2427–2436. doi:10.2147/OPTH.S188785
7. Approval for the EVO/EVO+ Visian implantable collamer lens. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P030016S035.
8. Chylack LT Jr, Wolfe JK, Singer DM, et al. The lens opacities classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol. 1993;111(6):831–836. doi:10.1001/archopht.1993.01090060119035
9. Visian® TORIC ICL (Implantable Collamer® Lens) for myopia directions for use. https://www.accessdata.fda.gov/cdrh_docs?pdf3/P030016S001d.pdf (Accessed May 19, 2022)
10. Ganesh S, Brar S. Comparison of surgical time and IOP spikes with two ophthalmic viscosurgical devices following Visian STAAR (ICL, V4c model) insertion in the immediate postoperative period. Clin Ophthalmol. 2016;10:207–211. doi:10.2147/OPTH.S89487
11. Qin Q, Bao L, He Z, et al. Pure ICL implantation: a novel ophthalmic viscosurgical device-free method. J Ophthalmol. 2021;2021:1–11. doi:10.1155/2021/7363267
12. Kaufman BJ, Sugar J. Discrete nuclear sclerosis in young patients with myopia. Arch Ophthalmol. 1996;114(10):1178–1180. doi:10.1001/archopht.1996.01100140378001
13. Haarman AEG, Enthoven CA, Tideman JWL, Tedja MS, Verhoeven VJM, Klaver CCW. The complications of myopia: a review and meta-analysis. Invest Ophthalmol Vis Sci. 2020;61(4):49. doi:10.1167/iovs.61.4.49
14. Burton TC. The influence of refractive error and lattice degeneration on the incidence of retinal detachment. Trans Am Ophthalmol Soc. 1989;87:
15. Bernheim D, Rouberol F, Palombi K, Albrieux M, Romanet JP, Chiquet C. Comparative prospective study of rhegmatogenous retinal detachments in phakic or pseudophakic patients with high myopia. Retina. 2013;33(10):2039–2048. doi:10.1097/IAE.0b013e31828992ac
16. Martínez-Castillo V, Boixadera A, Verdugo A, Elíes D, Coret A, García-Arumí J. Rhegmatogenous retinal detachment in phakic eyes after posterior chamber phakic intraocular lens implantation for severe myopia. Ophthalmology. 2005;112(4):580–585. doi:10.1016/j.ophtha.2004.09.025
17. Ruiz-Moreno JM, Montero JA, de la Vega C, Alió JL, Zapater P. Retinal detachment in myopic eyes after phakic intraocular lens implantation. J Refract Surg. 2006;22(3):247–252. doi:10.3928/1081-597X-20060301-09
18. Flaxel CJ, Adelman RA, Bailey ST, et al. Posterior vitreous detachment, retinal breaks, and lattice degeneration preferred practice pattern®. Ophthalmology. 2020;127(1):P146–P181. doi:10.1016/j.ophtha.2019.09.027
19. Eom Y, Kim DW, Ryu D, et al. Ring-shaped dysphotopsia associated with posterior chamber phakic implantable collamer lenses with a central hole. Acta Ophthalmol. 2017;95(3):e170–e178. doi:10.1111/aos.13248
20. Transcript, ophthalmic devices panel of US FDA. Free State Reporting, Inc; 2014.
21. STAAR surgical. Available from: https://investors.staar.com/press-releases/press-release-details/2022/STAAR-Surgical-Announces-U.S.-FDA-Approval-of-EVO-Visian-Implantable-Collamer-Lenses/default.aspx.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
