Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Evaluation of the Efficacy and Safety of Blue Fenugreek Kale Extract on Skin Health and Aging: In-vitro and Clinical Evidences
Authors Kappler K , Grothe T, Srivastava S , Jagtap M
Received 13 May 2022
Accepted for publication 29 July 2022
Published 28 September 2022 Volume 2022:15 Pages 2051—2064
DOI https://doi.org/10.2147/CCID.S368576
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Katharina Kappler,1 Torsten Grothe,1 Shalini Srivastava,2 Manjiri Jagtap3
1Mibelle Group Biochemistry, Buchs, CH-5033, Switzerland; 2Vedic Lifesciences Private Limited, Mumbai, India; 3Skin Cure N Care, Mumbai, India
Correspondence: Torsten Grothe, Email [email protected]
Background: The skin is primarily affected by aging, especially when it is exposed to particulate matter present in the environment. It has been hypothesized that consumption of products with known antioxidant properties would help combat factors associated with both intrinsic and extrinsic aging factors.
Objective: The aim of the present study was to evaluate the effect of the formulation Blue Fenugreek Kale Extract (BFKE) on skin aging.
Methods: In this study, the effect of BFKE on protein oxidation was determined in human dermal fibroblasts by analysis of the level of protein carbonylation after cells were stressed with either H2O2 or urban pollution consisting of particulate matter and UV-A. Furthermore, a randomized, double-blind, placebo-controlled clinical study that evaluated the effect of BFKE consumption over a period of 56 days in 59 volunteers was performed. The major parameter studied was skin barrier dysfunction through the assessment of Transepidermal Water Loss (TEWL). Additional parameters analyzed clinically include skin moisture content, participant self-assessment of skin parameters, wrinkle severity, skin sagging and elasticity. Furthermore, low grade and allergic inflammatory biomarker levels were measured at the start and end of the treatment period, along with oxidative stress assessment using blood malondialdehyde levels.
Results: BFKE significantly reduced protein carbonylation in human dermal fibroblasts stressed with urban pollution. In the clinical study, the TEWL level reduced significantly and at the same time the skin moisture content levels increased by end of the treatment period. No significant changes were observed in wrinkle severity, skin sagging, elasticity, inflammatory and oxidative stress biomarker levels. Participant and investigator perception of treatment was significantly greater after product consumption, as was the improvement in skin parameters based on participant self-assessment.
Conclusion: BFKE reduces protein oxidation induced by H2O2 and restores skin barrier function and skin hydration, while also combating early signs of aging.
Keywords: blue fenugreek kale extract, transepidermal water loss, skin barrier function, skin aging, antioxidant, pollution
Introduction
The skin, being the largest and most visible organ of the human body, is primarily affected by aging.1 Aging most visibly affects the skin not only by worsening its physiological structure and function, but also by weakening the aesthetic appeal of the individual. The global anti-aging skincare product market accounted for 58.5 billion USD in 2020 and is expected to grow with a CAGR of 7.10% per annum to 88.30 billion USD by 2026.2 With aging, there is a decline in the functioning of the stratum corneum.3 Stratum corneum’s intercellular lipids are instrumental in providing barrier which has primary function of preventing Transepidermal water loss (TEWL). Prolonged exposure to the pollution also contributes to increased TEWL.4 The ambient particulate matter can induce skin aging through polycyclic aromatic hydrocarbons (PAHs), which are adsorbed on the surface of suspended particulates in air of urban areas and are able to activate the Aryl hydrocarbon receptor signaling, affecting the barrier function of the skin.5 TEWL serves as one of the most widely used objective measurement for assessing the skin barrier function in healthy individuals.6
Some of the common anti-aging measures include the use of topical retinoids,7 stem cell therapy8 and phytoceuticals.9 A body of data suggests that a polyphenol from pomegranate extract, ellagic acid, can decrease the skin pigmentation when taken orally.10 Many other phenolic compounds and carotenoids from fruits and vegetables have been studied successfully for their effects on skin aging.11
In recent times, there has been a manifold surge in consumer demand for clinically proven nutraceuticals for skin health. The phytochemicals from fenugreek are shown to modify the expression of the genes involved in the skin pigmentation.12 Extracts, phenolic compounds and saponins derived from various dietary plants, such as red ginseng, rosemary, olive leaves, green tea and pomegranate, have been discussed to counteract the cellular oxidative stress and inflammation induced by airborne particulate matter.13,14 Especially blue fenugreek (Trigonella caerulea), a dietary spice plant used in European cuisine seems to be a promising candidate attenuating the impacts of air pollution on skin tissues due to the presence of various phenolic compounds like glycosylated flavonoids with apigenin, luteolin and kaempferol as aglycons and furostene-type saponins.15,16 The current study was conducted to evaluate the effect of a novel proprietary formulation Blue Fenugreek Kale Extract (BFKE) on skin aging.
Materials and Methods
In-Vitro Analysis of Protein Carbonylation
Human dermal fibroblasts (Caucasian donor aged 39) were seeded in 96-well plates and grown in culture medium (Dulbecco's Modified Eagle Medium supplemented with 10% fetal bovine serum) at 37°C and 5% CO2. Cells were treated with either 2 mM N-acetylcysteine (reference compound) or BKFE (FenuKale™ Nu, Mibelle Biochemistry, Switzerland) at different concentrations (0.1, 0.05, and 0.01 mg/mL for H2O2 stress and 0.01, 0.005, and 0.001 mg/mL) for PM+UV-A stress for 24 hours. At the end of the treatment, the culture medium was replaced and cells were either stressed with 100 µM H2O2 for 30 minutes or with a combination of 0.1 µg/cm2 particulate matter (European Reference Material (ERM®) certified fine dust (PM10-like), containing Polycyclic Aromatic Hydrocarbons) and 1 J/cm2 UV-A. The cells were then fixed to the plate with ethanol/acetic acid (95%/5%) solution and carbonylated proteins were labeled with a specific fluorescent probe.17 After washing with Phosphate Buffered Saline, the fluorescence signal intensity was recorded using a microplate reader (Varioskan, Thermofisher, USA). Results are expressed in % of oxidation (carbonylation) as compared to the non-stressed cells (set to 100%). Statistical analysis was performed using multiple comparison ANOVA test.
Clinical Study Design and Ethics Statement
The study was approved by Aditya College of Engineering & Advanced Studies (ACEAS) – Independent Ethics Committee (IEC) (Ahmedabad, Gujarat, India) and was registered with the clinical trials registry of National Institutes of Health, “clinicaltrials.gov” bearing National Clinical Trial No: NCT04544982. The study was conducted in accordance with the revised Declaration of Helsinki. The clinical study was conducted in Mumbai, Maharashtra, India and recruited female participants aged between 30 and 55 years. The participants had fair to dark brown skin as indicated by Fitzpatrick skin type II to IV. The participants also had visible signs of aging and trans-epidermal water loss ≥15 g/m2/h in the forehead region. Written, signed and dated consent was obtained from all the study participants in the form of Informed Consent Forms . Females with a history of pathological dermatological conditions were excluded from the study. The study was a randomized, double-blinded, placebo-controlled study and was conducted under the supervision of a qualified dermatologist. Participants were randomized in a 1:1 ratio using block randomization at baseline visit. The first participant was enrolled in September 2020 and the last participant last visit was conducted in March 2021.
Study Product
The investigational product (IP) BFKE (FenuKale™ Nu, Mibelle Biochemistry, Switzerland) consisted of Blue Fenugreek (Trigonella caerulea) and Kale (Brassica oleracea var. acephala) Extract. The dried leaves of blue fenugreek and kale are extracted in a 4:1 ratio using 36% (w/w) food-grade ethanol as extraction solvent. The concentrated extract has been spray-dried using food-grade Gum Arabic as carrier resulting in a plant extract ratio of 2–4:1. The extract powder is standardized to ≥12 mg/g flavonoid glycosylates using authentic references (all provided by Analyticon Discovery GmbH, Potsdam, Germany) as standard. Each 700 mg capsule of BFKE capsule contained 175 mg of BFKE and 525 mg maltodextrin as filling material. The placebo capsule contained 700 mg of pharmaceutical grade maltodextrin. The research participants consumed “00” size one capsule before breakfast and one after lunch for 56 days. The capsules were matched for size, shape, color, texture and packaging to preserve the blinding for the clinical trial. The extract and capsules were manufactured in compliance with all the required food-manufacturing regulations like ISO22000 and hygiene practice certifications.
Exploratory Outcome Measures
The exploratory outcomes for the study included the assessment of skin barrier by TEWL. At the same time, effect of IP on parameters of skin aging such as skin moisture content, wrinkle severity, skin sagging, elasticity, radiance, luminosity, smoothness, texture, firmness, and skin hydration were also assessed. Blood samples were collected during the baseline and end of study visits (Day 56) to assess chronic low grade inflammatory biomarkers associated with aging (IL-1β, IL-6, IL-8 and TNF-α), to evaluate allergic inflammation through Eosinophil to basophil (E/B) and Neutrophil to lymphocyte (N/L) ratios, and for the evaluation of oxidative stress using blood malondialdehyde (MDA) levels. The participants’ self-assessment for the effect of IP on various skin aging parameters were also assessed, along with the global assessments of treatment response.
TEWL was evaluated using a Tewameter (Tewameter® TM 300, Köln, Germany) on the baseline, Day 28 and Day 56 visits. Skin moisture content was analyzed using a moisture analyzer [Kostech, Mumbai, India]. Wrinkle severity was assessed using the Modified Fitzpatrick Wrinkle Severity Scale (MFWS),18 whereas the skin sagging was evaluated using Ezure sagging scale.19 Skin elasticity through pinch recoil time measurements and the self-assessment questionnaire containing numeric rating scales (NRS) were used to evaluate the participant’s perception of previously measured skin parameters. Furthermore, during the end of the study visit, the participant as well as the investigators rated the perceived treatment efficacy through the global assessment scales with scores ranging from 0 to 4. Safety of the IP was assessed by measuring vitals such as blood pressure and heart rate. Any adverse event/serious adverse event occurring during the study duration was to be reported and analyzed.
Statistical Analysis Plan
Based on data from similar studies for products containing polyphenols,20 a sample size of 30 participants/arm, was considered sufficient to evaluate the robust and reliable estimates for the efficacy and safety parameters.
The type I error probability associated with the null hypothesis test was set to 0.05. The ITT population was chosen for analysis. The efficacy and safety parameters were compiled using paired t-test/Wilcoxon test (within group analysis) and the two-sample t-test/Mann Whitney U-test (inter-group analysis). In case of in-equal number of observations in the treatment group, as per the convention, participants who do not had both baseline and post-baseline assessment were excluded from the analysis and only the p-value was derived for available paired observation.
Results
Result of Protein Carbonylation
Urban pollution and H2O2 are major stressors for cells and lead to increased oxidative damage, which can be measured by the level of protein carbonylation. In this study, human dermal fibroblasts were stressed with either 100 µM H2O2 or with a combination of 0.1 µg/cm2 particulate matter and 1 J/cm2 UV-A to simulate urban pollution. These stressors significantly increased protein carbonylation in dermal fibroblasts. Preventive treatment with BKFE significantly decreased H2O2-induced protein oxidation in a dose-dependent manner. The level of protein carbonylation in cells treated with the lowest dose of 0.001 mg/mL BFKE was comparable to the levels of cells treated with the reference compound N-acetylcysteine. Cells treated with the highest dose (0.1 mg/mL) did not show any difference to non-stressed control cells in the extent of protein carbonylation. Protein carbonylation induced by urban pollution (UV-A + PM) was also significantly reduced by treatment with BFKE. All concentrations tested led to a similar reduction in the level of protein carbonylation, which was comparable to the level of cells treated with the positive control N-acetylcysteine (Figure 1 and 2).
 |
Figure 1 BFKE decreased protein oxidation induced by urban pollution. |
 |
Figure 2 BFKE decreased protein oxidation induced by H2O2. |
Clinical Trial - Participant Demographics and Baseline Characteristics
A total of 64 participants were recruited for the clinical trial, with 4 (6.25%) participants being excluded from the study due to screening failure. Figure 3 presents participant disposition during the study. The mean age of the participants in the BFKE and placebo arms was 39.77 ± 5.64 and 39.87 ± 5.51 years, respectively. In case of BMI, the BFKE group participants recorded a mean BMI of 26.27 ± 2.40 kg/m2, while the placebo group had an average BMI of 25.34 ± 3.09 kg/m2. As per the TEWL eligibility criteria, all the participants included in the study had deranged TEWL values, which indicated a damaged skin barrier. A summarized description of the demographic and baseline characteristics has been presented in Table 1.
 |
Table 1 Summary of Randomized Participants’ Demographic and Baseline Characteristics |
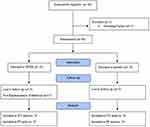 |
Figure 3 Disposition of participants. |
Efficacy Outcomes
TEWL
The baseline TEWL values of the BFKE and placebo groups were 22.54 ± 5.33 and 19.74 ± 4.24 g/m2/h respectively, with BFKE group having significantly higher TEWL (p-value = 0.0163). The values showed a declining trend in both the groups at day 28. By the end of the treatment period, the reduction in the TEWL in the BFKE group was significantly higher as compared to the placebo group (p = 0.0222), as is seen in Table 2. The absolute change in the mean TEWL is presented in Figure 4.
 |
Table 2 Summary and Absolute Change - Transepidermal Water Loss (g/m2/h) |
 |
Figure 4 Absolute change in average TEWL. |
Skin Moisture Content
The skin moisture content levels at the forehead region of the participants were comparable between both the treatment arms at baseline. On day 28, the BFKE group skin moisture content increased by 2.13% and the placebo moisture content decreased by 1.24%. The trend continued till the end of the treatment period, with a significant 2.24% increase and a 2.15% decrease in the BFKE and placebo arms, respectively (p = 0.0357) as seen in Table 3.
 |
Table 3 Summary and Absolute Change - Skin Moisture Content (Forehead) |
Participants-Assessment of Skin Aging
The NRS scores for each of the skin parameters (Skin radiance, freshness, luminescence, homogeneity, smoothness, texture, firmness and hydration (Table 4)) were comparable between the treatment groups (p > 0.05). After 28 days of product consumption, all the aforementioned parameters showed a significant improvement in scores for the BFKE group. At the same time, all the parameters improved in placebo group with the exception of skin homogeneity. Significantly better results were obtained at Day 56 in BFKE group.
 |
Table 4 Change in Participants’ Assessment - Skin Quality |
Investigator and Participant Global Assessment of Treatment Response
It was found that the investigator as well as the participants global assessment rating for the treatment efficacy was greater than the placebo at the end of the treatment period. The investigator’s scores were 3.07 ± 0.75 for BFKE as compared to 2.60 ± 077 in placebo, while the participant scores were 3.10 ± 0.56 and 2.80 ± 0.66, respectively. The investigators and participant assessment demonstrated that overall skin health was better improved in the BFKE participants. The details are presented in Tables 5 and 6. No significant change was observed in wrinkle severity, skin sagging, elasticity, inflammatory and oxidative stress biomarker assessment.
 |
Table 5 Investigator’s Global Assessment of Treatment Response |
 |
Table 6 Participant’s Global Assessment of Treatment Response |
Safety Analysis
After 28 and 56 days from the baseline visit, no clinically significant changes were visible for systolic and diastolic blood pressure as well as pulse rate, demonstrating an absence of any safety concerns related to the study product. No adverse event was reported in any of the treatment groups throughout the duration of the study.
Discussion
Flavonoids from Trigonella caerulea and Brassica oleracea have been studied for their antioxidant properties and phytochemical composition,21,22 as well as their anti-carcinogenic, neuro-protective, antimicrobial, cardio-protective, and anti-diabetic properties.23,24 Supplementation with BFKE was thus hypothesized to fortify the skin against oxidative stress and inflammatory responses. Recent study has suggested that botanical supplements can be effective in reducing the ill effects of environmental pollution exposure.25
The study results confirmed the protective effect of BFKE in human epidermal fibroblasts, which were stressed with increased protein oxidation.
At the same time, the current clinical study was conducted in Mumbai, a major city in India which is considered to be highly polluted. The study published in 2021 by Hu et al26 evaluated the air quality of Mumbai between the years 2018 to 2021. It stated that the PM2.5 levels in Mumbai were usually high, i.e. in the range of 71.7–149.2. Furthermore, the Air Quality Index in Mumbai around the end of the study period was stated to be 126, which is 4 times higher than the WHO exposure recommendation.27 The increased exposure to particulates leads to extrinsic skin aging and leads to skin barrier dysfunction. Therefore, the main objective of this study was to evaluate the effect of BFKE on skin barrier as assessed by TEWL. Furthermore, the formulation’s effect on skin elasticity, skin firmness, sagging, radiance, wrinkles, pigmentation, oxidative stress and systemic inflammation were also studied. Thus, formulation’s effect on both intrinsic and extrinsic aging was studied.
The results obtained from the study confirmed that ingestion of BFKE lead to the restoration of skin barrier function, with the TEWL significantly reducing at the end of the treatment period when compared to the placebo (−5.86 g/m2/h vs −2.32 g/m2/h; p < 0.05). The results were comparable to the results from a study conducted using collagen tripeptide (CT) and a combination of the same with Vitamin C (500 mg/day) in a randomized trial. After 12 weeks of 3 g/day CT supplementation, the maximum decrease was only stated to be −3.78 g/m2/h and −2.86 g/m2/h for each arm respectively. Even after 28 days of BFKE supplementation the reduction was seen to be −4.61 g/m2/h, this value being better than in both the CT and CT with Vitamin C arm of the mentioned study.28
In terms of skin hydration, the moisture content in the forehead region for the BFKE arm participants was greater at the end of the study period in comparison to the placebo, where it gradually declined throughout the study period. The TEWL and skin moisture content provide information about the epidermal barrier function of the skin under healthy, diseased or experimentally perturbed conditions.29 Throughout the study duration, the participants were instructed to consume a similar volume of water as noted during their screening visit. This criterion was included to homogenize water consumption for the participants, which would affect the water content within the skin. In addition to skin barrier function, the effect of BFKE extract on various physical properties of skin was also assessed which included wrinkles, sagging, elasticity, firmness, radiance, luminescence, pigmentation, etc. Superior skin hydration has been shown to be associated with lesser appearance of wrinkles.30
Assessment of skin lines and wrinkles through the MFWS questionnaire demonstrated a reduction in visible wrinkles, although the change was not statistically significant. Moreover, the wrinkle severity reduced gradually over the study duration, with the reduction being greater after 56 days as compared to day 28. In a clinical trial conducted by Foolad et al,31 the effect of almond consumption on TEWL, wrinkle severity and depth was evaluated for a duration of 16 weeks, the wrinkle severity assessment being done using high-resolution photographs. Twenty percent of the participant’s daily energy requirement had to be met through almond consumption or a nut-free caloric equivalent (control). The wrinkle severity and depth were reduced by 9% and 10%, respectively, at the end of 16 weeks (p < 0.02). The above study suggests that a longer treatment period may have produced more significant results in the current study.
The findings from the participant self-assessment questionnaire revealed that the participants believed their skin to be more radiant, fresh, and glowing in the BFKE group in comparison to the placebo, and the inter- and intragroup differences were statistically significant. Similar results were obtained for the other skin qualities like homogeneity, smoothness, texture, firmness, and hydration, where consumption of BFKE for 56 days resulted in significant improvement of the aforementioned qualities from baseline and in comparison to placebo. These improvements were observed even after 28 days of treatment, which progressively improved over a longer period of dosing. Similar improvement in the skin qualities mentioned earlier was observed even in the placebo group (except skin hydration). Despite such ambiguous results, global assessment scores revealed that after 56 days of study product consumption, the participants in the BFKE group reported better improvement in overall skin condition in comparison to the ones receiving placebo. Similar results were observed for the investigator’s assessment of treatment efficacy, where the participants in BFKE group were reported to have better skin appearance than the placebo group.
For skin sagging, a statistically significant reduction was observed after 28 and 56 days of BFKE consumption, but it was not statistically significant when compared to the placebo. Even for elasticity, a significant reduction in pinch recoil time was observed after 56 days, but again the results were not significant when compared to the placebo arm. Numerous other studies which demonstrated a clinical significance in these parameters, utilized a larger study population and a greater study duration, especially in studies using nutricosmetic products. For example, in a study assessing skin texture changes after the consumption of collagen bioactive peptides and antioxidants, it was observed that after a duration of 90 days, skin elasticity increased by 7.5%, the increase being statistically significant (p < 0.001). The study had 120 participants, with 60 in the treatment and 60 in placebo arms.32 As mentioned earlier, collagen peptides have been studied extensively for their anti-skin aging properties and thus a study with a longer treatment period and restricted inclusion criteria is suggested to obtain more robust and significant results. However, it must be noted that the formulation in the present study would not be as effective as other formulations that demonstrate a significant improvement within 8 weeks. These include rose hip powder, which showed a significant improvement in skin wrinkles, moisture and elasticity after 8 weeks of ingestion,33 and marine collagen peptides, which demonstrated a significant improvement in both skin elasticity and sebum production after a treatment period of two months.34
As stated earlier, the components of the BFKE extract have all been studied for their anti-oxidant and anti-inflammatory properties. An enormous body of evidence has strongly linked chronic inflammation and oxidative stress with skin aging.35,36 It has been reported that IL-6, TNF-α, IL-8, and IL-1β are dual markers for inflammation and aging.37,38 In addition to this, TNF-α inhibits collagen synthesis and enhances collagen degradation by increasing the production of matrix metallopeptidase-9 (MMP-9).39 Thus, the peripheral levels of several inflammatory (IL-1β, IL-6, IL-8, TNF-α) and oxidative stress (malondialdehyde) biomarkers were assessed to determine the anti-inflammatory and anti-oxidative potential of BFKE. After the consumption of BFKE for 56 days, a slight decrease in serum levels of pro-inflammatory cytokines IL-6 and IL-8 was observed while a statistically significant increase in TNF-α levels was observed from baseline. Furthermore, no change in the levels of IL-1β in the BFKE group was observed, whereas it decreased drastically in the placebo group, the intragroup difference being statistically insignificant. Similar enigmatic results were obtained for other biomarkers of systemic inflammation (E/B and N/L ratio).
A potential explanation for such ambiguous results could be the increasing pro-inflammatory biomarker levels associated with age that modulate the levels of anti-inflammatory biomarkers, where the outcomes vary depending on pre-existing reserve, type of stimulation and the current immune state. This suggested that “Inflamm-aging” is not simply an increase in pro-inflammatory biomarkers due to age.40 In addition to this, a decrease in malondialdehyde levels was observed at the end of the study from baseline. However, the change was not statistically significant in comparison to baseline or placebo. A significant reduction in malondialdehyde level has been observed in studies assessing the antioxidant status of participants, with one of these studies being conducted over a period of 12 weeks assessing the skin health and anti-oxidant status in post-menopausal women after the consumption of a blend containing Glycine max, Cimicifuga racemosa, Vitex agnus-castus, and Oenothera biennis extracts.41 The results obtained suggest that the current study was slightly underpowered to draw a meaningful conclusion regarding BFKE and its effect on the inflammatory and oxidative stress cascade.
Comparable antioxidant results and reduced skin-ageing effects like TEWL and hydration had been reported for a polyphenol-enriched dietary supplement based on 4 standardized plant extracts in response to air pollution.25 A similar study design has been used as 100 Italian outdoor-workers participated for a 3-month study duration. Antioxidant and anti-inflammatory properties linked to its polyphenol content have been discussed as potential mechanism. Such benefits have been described several times for natural compounds like phenolics, flavonoids and saponins. The phenolic compound Eupafolin protects HaCaT keratinocytes from particulate matter-induced inflammation and oxidative stress by impacting the ROS/MAPK and COX2/PGE2 pathways.42 Protective effect of green tea catechin against urban fine dust particle-induced skin aging have been described by regulation of NFkappa-B, AP-1, and MAPKs signalling pathways.43 Remarkable are also the reports about the anti-pollution benefits of ginsenosides, saponins present in red ginseng. Inhibitory effects of a ginsenosides have been demonstrated in a particulate matter-induced pulmonary injury model.44 In a particulate matter induced skin damage model, ginsenosides showed excellent skin protection and could inhibit mitochondria and ER stress-dependent apoptosis.45 Most probably phenolic compounds like the flavonoid glycosylates and the saponins present in BFKE contributes to the activity of the extract.
Study Limitation
The current sample size seems to be insufficient to demonstrate the effect of the product on certain biomarkers. An adequately powered study with a longer treatment duration is warranted to determine effect of BFKE on systemic inflammation and oxidative stress to obtain more robust and significant results.
Conclusion
The in-vitro results demonstrated that BKFE significantly reduced protein carbonylation in pollution stressed human dermal fibroblasts, therefore establishing the skin protecting property of the extract. The clinical study further reconfirmed that BFKE significantly restores skin barrier function and hydrates the skin hence correcting the early signs of aging. However, the study could not show effect of the investigational product on inflammation or oxidative stress.
Trial Registration
The study was registered at National Clinical Trial (NCT) registry (NCT04544982).
Data Sharing Statement
All data collected for the study, including individual participant data and a data dictionary defining each field in the set, will be made available on request to the corresponding author.
Ethical Statement
The study was approved by an independent ethics committee (IEC, Aditya) registered with the Office for Human Research Protections in the US Department of Health and Human Services (IRB00011046). The study was conducted in accordance with the revised Declaration of Helsinki.
Acknowledgments
The clinical trial was carried out by the clinical research organization Vedic Lifesciences Pvt. Ltd. The study products and placebo were provided by the Mibelle Group.
Disclosure
The study was funded by the Mibelle Group. The authors report no other conflicts of interest in this work.
References
1. Fore J. A review of skin and the effects of aging on skin structure and function. Ostomy Wound Manage. 2006;52(9):24–35.
2. Global anti-aging market report and forecast (2021–2026): imarc; 2021. Available from: https://www.imarcgroup.com/anti-aging-market.
3. Rawlings AV. The stratum corneum and aging. In: Farage M, Miller K, Maibach H, editors. Textbook of Aging Skin. Berlin, Heidelberg: Springer; 2017.
4. Green M, Kashetsky N, Feschuk A, Maibach HI. Transepidermal water loss (TEWL): environment and pollution—A systematic review. Skin Health Dis. 2022;2(2):e104. doi:10.1002/ski2.104
5. Jose SS, Bendickova K, Kepak T, Krenova Z, Fric J. Chronic inflammation in immune aging: role of pattern recognition receptor crosstalk with the telomere complex? Front Immunol. 2017;8:1078. doi:10.3389/fimmu.2017.01078
6. Alexander H, Brown S, Danby S, Flohr C. Research techniques made simple: transepidermal water loss measurement as a research tool. J Invest Dermatol. 2018;138(11):2295–2300.e1. PMID: 30348333. doi:10.1016/j.jid.2018.09.001
7. Mukherjee S, Date A, Patravale V, Korting HC, Roeder A, Weindl G. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging. 2006;1(4):327. doi:10.2147/ciia.2006.1.4.327
8. Park BS, Ka J, Sung JH, et al. Adipose‐derived stem cells and their secretory factors as a promising therapy for skin aging. Dermatol Surg. 2008;34(10):1323–1326. doi:10.1111/j.1524-4725.2008.34283.x
9. Raj U, Sharma G, Dang S, Gupta S, Gabrani R. Impact of dietary supplements on skin aging. In: Textbook of Aging Skin. Springer; 2016:1–13.
10. Michalak M, Pierzak M, Kręcisz B, Suliga E. Bioactive compounds for skin health: a review. Nutrients. 2021;13(1):203. doi:10.3390/nu13010203
11. Monti DM, Rigano MM, Monti SM, Peixoto HS. Role of antioxidants in the protection from aging-related diseases. Hindawi. Oxid Med Cell Longev. 2019;2019:1–2. doi:10.1155/2019/7450693
12. Schaefer K. Fenugreek support for skin structure; 2011. Available from: https://www.cosmeticsandtoiletries.com/formulating/function/active/119496199.html.
13. Diao P, He H, Tang J, Xiong L, Li L. Natural compounds protect the skin from airborne particulate matter by attenuating oxidative stress. Biomed Pharmacother. 2021;138:111534. doi:10.1016/j.biopha.2021.111534
14. Moon IJ, Kim W, Kim SY, et al. Saponins of Korean red ginseng may protect human skin from adipokine-associated inflammation and pigmentation resulting from particulate matter exposure. Nutrients. 2022;14(4):845. doi:10.3390/nu14040845
15. Benayad Z, Gómez-Cordovés C, Es-Safi NE. Characterization of flavonoid glycosides from fenugreek (Trigonella foenum-graecum) crude seeds by HPLC-DAD-ESI/MS analysis. Int J Mol Sci. 2014;15(11):20668–20685. doi:10.3390/ijms151120668
16. Kang SS, Cordell GA, Soejarto DD, Fong HH. Alkaloids and Flavonoids from Ricinus communis. J Nat Prod. 1985;48:155–156. doi:10.1021/np50037a041
17. Baraibar MA, Ladouce R, Friguet B. Proteomic quantification and identification of carbonylated proteins upon oxidative stress and during cellular aging. J Proteomics. 2013;92:63–70. doi:10.1016/j.jprot.2013.05.008
18. Shoshani D, Markovitz E, Monstrey SJ, Narins DJ. The modified fitzpatrick wrinkle scale: a clinical validated measurement tool for nasolabial wrinkle severity assessment. Dermatol Surg. 2008;34:S85–S91. doi:10.1111/j.1524-4725.2008.34248.x
19. Ezure T, Hosoi J, Amano S, Tsuchiya T. Sagging of the cheek is related to skin elasticity, fat mass and mimetic muscle function. Skin Res Technol. 2009;15(3):299–305. doi:10.1111/j.1600-0846.2009.00364.x
20. Chowjarean V, Phiboonchaiyanan PP, Harikarnpakdee S, Tengamnuay P. A natural skin anti-ageing serum containing pseudobulb ethanolic extract of Grammatophyllum speciosum: a randomized double-blind, placebo-controlled trial. Int J Cosmet Sci. 2019;41(6):548–557. doi:10.1111/ics.12571
21. Naidu MM, Shyamala B, Naik JP, Sulochanamma G, Srinivas P. Chemical composition and antioxidant activity of the husk and endosperm of fenugreek seeds. LWT - Food Sci Technol. 2011;44(2):451–456. doi:10.1016/j.lwt.2010.08.013
22. Ravikumar C. Therapeutic potential of Brassica oleracea (broccoli)–A review. Int J Drug Dev Res. 2015;7:009–10.
23. Sanlier N, Guler S. The benefits of Brassica vegetables on human health. J Hum Health Res. 2018;1:1–13.
24. Al-Dabbagh B, Elhaty IA, Al Sakkaf R, El-Awady R, Ashraf SS, Amin A. Antioxidant and anticancer activities of Trigonella foenum-graecum, Cassia acutifolia and Rhazya stricta. BMC Complement Altern Med. 2018;18(1):1–12. doi:10.1186/s12906-018-2285-7
25. Nobile V, Schiano I, Peral A, Giardina S, Spartà E, Caturla N. Antioxidant and reduced skin-ageing effects of a polyphenol-enriched dietary supplement in response to air pollution: a randomized, double-blind, placebo-controlled study. Food Nutr Res. 2021;65:10. doi:10.29219/fnr.v65.5619
26. Hu M, Chen Z, Cui H, Wang T, Zhang C, Yun K. Air pollution and critical air pollutant assessment during and after COVID-19 lockdowns: evidence from pandemic hotspots in China, the Republic of Korea, Japan, and India. Atmos Pollut Res. 2021;12(2):316–329. doi:10.1016/j.apr.2020.11.013
27. Mumbai Air Quality Index (AQI) and India Air Pollution. AirVisual. Available from: https://www.iqair.com/india/maharashtra/mumbai.
28. Choi SY, Ko EJ, Lee YH, et al. Effects of collagen tripeptide supplement on skin properties: a prospective, randomized, controlled study. J Cosmet Laser Ther. 2014;16(3):132–137. doi:10.3109/14764172.2013.854119
29. Neukam K, De Spirt S, Stahl W, et al. Supplementation of flaxseed oil diminishes skin sensitivity and improves skin barrier function and condition. Skin Pharmacol Physiol. 2011;24(2):67–74. doi:10.1159/000321442
30. Schwartz S, Frank E, Gierhart D, Simpson P, Frumento R. Zeaxanthin‐based dietary supplement and topical serum improve hydration and reduce wrinkle count in female subjects. J Cosmet Dermatol. 2016;15(4):e13–e20. doi:10.1111/jocd.12226
31. Foolad N, Vaughn AR, Rybak I, et al. Prospective randomized controlled pilot study on the effects of almond consumption on skin lipids and wrinkles. Phytother Res. 2019;33(12):3212–3217. doi:10.1002/ptr.6495
32. Genovese L, Corbo A, Sibilla S. An insight into the changes in skin texture and properties following dietary intervention with a nutricosmeceutical containing a blend of collagen bioactive peptides and antioxidants. Skin Pharmacol Physiol. 2017;30(3):146–158. doi:10.1159/000464470
33. Phetcharat L, Wongsuphasawat K, Winther K. The effectiveness of a standardized rose Hip powder, containing seeds and shells of Rosa canina, on cell longevity, skin wrinkles, moisture, and elasticity.. Clin Interv Aging. 2015;10:1849. doi:10.2147/CIA.S90092
34. De Luca C, Mikhal’chik EV, Suprun MV, Papacharalambous M, Truhanov AI, Korkina LG. Skin antiageing and systemic redox effects of supplementation with marine collagen peptides and plant-derived antioxidants: a single-blind case-control clinical study. Oxid Med Cell Longev. 2016;2016:1–14. doi:10.1155/2016/4389410
35. Thornfeldt CR. Chronic inflammation is etiology of extrinsic aging. J Cosmet Dermatol. 2008;7(1):78–82. doi:10.1111/j.1473-2165.2008.00366.x
36. Rinnerthaler M, Bischof J, Streubel MK, Trost A, Richter K. Oxidative stress in aging human skin. Biomolecules. 2015;5(2):545–589. doi:10.3390/biom5020545
37. Fuller B. Role of PGE-2 and other inflammatory mediators in skin aging and their inhibition by topical natural anti-inflammatories. Cosmetics. 2019;6(1):6. doi:10.3390/cosmetics6010006
38. Qin Z, Okubo T, Voorhees JJ, Fisher GJ, Quan T. Elevated cysteine-rich protein 61 (CCN1) promotes skin aging via upregulation of IL-1β in chronically sun-exposed human skin. Age. 2014;36(1):353–364. doi:10.1007/s11357-013-9565-4
39. Borg M, Brincat S, Camilleri G, Schembri-Wismayer P, Brincat M, Calleja-Agius J. The role of cytokines in skin aging. Climacteric. 2013;16(5):514–521. doi:10.3109/13697137.2013.802303
40. Vincent Morrisette-Thomas AAC, Fülöp T, Riesco É, et al. Inflamm-aging does not simply reflect increases in pro-inflammatory markers. Mech Ageing Dev. 2014;139(1):49–57. doi:10.1016/j.mad.2014.06.005
41. Tumsutti P, Maiprasert M, Sugkraroek P, Wanitphakdeedecha R, Bumrungpert A. Effects of a combination of botanical actives on skin health and antioxidant status in post‐menopausal women: a randomized, double‐blind, placebo‐controlled clinical trial. J Cosmet Dermatol. 2021;21:2064–2072. doi:10.1111/jocd.14345
42. Lin ZC, Lee CW, Tsai MH, et al. Eupafolin nanoparticles protect HaCaT keratinocytes from particulate matter-induced inflammation and oxidative stress. Int J Nanomedicine. 2016;11:3907–3926. doi:10.2147/IJN.S109062
43. Wang D, Lu J, Miao A, Xie Z, Yang D. HPLC-DAD-ESI-MS/MS analysis of polyphenols and purine alkaloids in leaves of 22 tea cultivars in China. J Food Compost Anal. 2008;21(5):361–369. doi:10.1016/j.jfca.2008.01.002
44. Lee W, Ku SK, Kim JE, Cho SH, Song GY, Bae JS. Inhibitory effects of protopanaxatriol type ginsenoside fraction (Rgx365) on particulate matter-induced pulmonary injury. J Toxicol Environ Health A. 2019;82(5):338–350. doi:10.1080/15287394.2019.1596183
45. Piao MJ, Ahn MJ, Kang KA, et al. Particulate matter 2.5 damages skin cells by inducing oxidative stress, subcellular organelle dysfunction, and apoptosis. Arch Toxicol. 2018;92(6):2077–2091. doi:10.1007/s00204-018-2197-9
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
