Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Evaluation of the Efficacy and Safety of Apremilast in the Management of Lichen Planus
Authors Viswanath V , Joshi P, Dhakne M, Dhoot D , Mahadkar N, Barkate H
Received 28 September 2022
Accepted for publication 26 November 2022
Published 2 December 2022 Volume 2022:15 Pages 2593—2600
DOI https://doi.org/10.2147/CCID.S390591
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Vishalakshi Viswanath,1 Pradnya Joshi,1 Mayuri Dhakne,1 Dhiraj Dhoot,2 Namrata Mahadkar,2 Hanmant Barkate2
1Department of Dermatology, Rajiv Gandhi Medical College, Thane, Maharashtra, India; 2Global Medical Affairs, Glenmark Pharmaceuticals Ltd, Mumbai, Maharashtra, India
Correspondence: Namrata Mahadkar, Email [email protected]
Introduction: Lichen planus is a chronic disease with often disappointing and less than optimal treatment options. Apremilast modulates inflammatory signalling pathways which play a central role in the pathogenesis of lichen planus, thus making it useful in the management of such patients.
Materials and Methods: The present study was an investigator-initiated, single-centre, non-randomized, open-label, pilot study of the efficacy and safety of Apremilast in the treatment of lichen planus. All the patients were prescribed Apremilast 30mg, twice daily, for 12 weeks. Patients were evaluated for improvement in their lesions, based on the physician’s global assessment (PGA), subject global assessment (SGA), and target area lesion symptom score (TALSS).
Results: A total of 34 patients were included in the study; 26 patients completed the study duration and were considered for the final analysis. After 12 weeks, 34.61% (n = 9/26) patients showed 2 or more grade improvement in their disease as per PGA. About 42.30% (n = 11/26) patients achieved more than 50% improvement in their lesions based on the subject global assessment of their disease. There was a significant improvement in TALSS during the study period (p < 0.0001). Only 23.07% (n = 6/26) patients developed one or more adverse events because of Apremilast with headache being the commonest side effect.
Conclusion: The results obtained in our study justify that Apremilast is efficacious and safe in the management of patients with lichen planus. Based on these results, Apremilast can be considered as a promising alternative treatment option in patients with lichen planus.
Keywords: Apremilast, lichen planus, efficacy, safety
Introduction
Lichen planus is an idiopathic chronic inflammatory disease, affecting the skin, mucous membranes, hair, and nails.1–4 Lichen planus is usually associated with significant morbidity, including severe pruritus and pain. Pathogenesis of Lichen planus mainly involves skin and mucosal damage by T-cell mediated inflammatory agents, such as tumour necrosis factor-α and interferon-γ.1–4
Mainstay treatment options for the management of lichen planus are topical and oral corticosteroids, retinoid, cyclosporine, griseofulvin, dapsone, and phototherapy.5,6 These therapies can be used either as monotherapy or in combinations. The management of lichen planus is often disappointing and controversial because of less-than-optimal results and significant adverse events associated with the conventional agents.5,6 Thus, there is a need for a novel treatment approach in the management of lichen planus.
Apremilast is a novel phosphodiesterase type IV (PDE4) inhibitor approved for the management of moderate-to-severe psoriasis and oral ulcers associated with Behcet's disease.7 By inhibiting PDE4, Apremilast increases levels of cyclic adenosine monophosphate (cAMP), thus activating protein kinase A. This effectively inhibits proinflammatory cytokine transcription, neutrophil degranulation, chemotaxis, and adhesion to endothelial cells. Ultimately, Apremilast inhibits the production of various inflammatory mediators, such as tumour necrosis factor-α, interferon-γ, leukotriene B4, and interleukin (IL)-2, IL-5, IL-8, and IL-12.8 Because of this novel immunomodulatory mechanism of action, Apremilast modulates inflammatory signalling pathways which also play a central role in pathogenesis of lichen planus. In various clinical studies and case reports, Apremilast was associated with significant improvement in signs and symptoms of lichen planus.9–13
Apremilast modulates inflammatory signalling pathways which play a central role in the pathogenesis of lichen planus; hence, it is plausible that Apremilast may be an effective treatment for lichen planus. This study was conducted with the objective to evaluate the overall efficacy and safety of oral Apremilast in patients with lichen planus.
Materials and Methods
Study Design
This study was an investigator-initiated, prospective, single-centre, non-randomized and an open-label to evaluate the safety and efficacy of Apremilast in the treatment of lichen planus. Participants were recruited from July 2020 till April 2021 and followed up for 12 weeks, and all patients provided informed written consent.
Ethical Considerations
This research was undertaken in compliance with the ICH harmonized tripartite guidelines for good clinical practice (GCP) adherence to the Helsinki declaration of ethical standards. The research was initiated after approval by the institutional ethics committee of “Rajiv Gandhi Medical College and Chhatrapati Shivaji Maharaj Hospital” and was registered with CTRI (CTRI/2020/05/025197).
Study Participants
Patients (≥18 years) fulfilling either of three below mentioned inclusion criteria, were recruited from the tertiary care hospital in Thane.
- Patients with biopsy confirmed cutaneous or mucosal lichen planus with a PGA score of 3 or more,
- Patients who were candidates for systemic therapies,
- Patients who non-responded to treatment with topical corticosteroids.
Patients with clinical history and lesion distribution suspicious of a lichenoid drug eruption were excluded from the study. Patients with other skin diseases or clinically significant systemic diseases (eg, cardiac, respiratory, gastrointestinal, and renal disease) that in the opinion of the investigator could affect the subject’s safety or interfere with the study assessments, were also excluded from the study.
Patient Follow-Up
All patients were treated with 30 mg of Apremilast orally, twice a day, for 12 weeks after initial standard titration. The patients were followed up on days 28, 56 and 84. All patients were subjected to evaluation of Physician Global Assessment (PGA) of disease and Target Area Lesion Symptom Score (TALSS) at baseline and all follow-up visits. Subject Global Assessment (SGA) of disease was done on days 28, 56 and 84.
Efficacy Assessment
The primary efficacy end point was the proportion of patients who achieve a significant clinical response to cutaneous disease, defined as a 2-grade or more improvement in PGA score after 12 weeks of treatment. The secondary end points were the proportion of patients achieving improvement in their disease based on SGA at week 12, and change in the mean target area lesion symptom score from baseline, at week 12. (The “target area” was defined as the part of the body with the greatest disease severity; its boundaries were clearly defined and documented at the baseline to facilitate future assessments.)
The PGA consisted of the evaluation of the disease score by investigators on a 5-point Likert scale, with 0 representing complete clearance of lesions while 4 being severe disease. SGA consisted of evaluation of disease improvement by patients on a 6-point Likert scale with 0 being complete resolution of lesions while 5 being worsening of lesions. For TALSS assessment investigator evaluated 3 symptoms ie, erythema, elevation and pruritus in the target area as defined earlier. The final TALSS consisted of the sum of all of the 3 elements of the target lesion score. Assessment scores range from 0 to 9 on a Likert scale, with higher numbers meaning more severe disease in the target skin lesion.
Safety Assessments
Safety was assessed by monitoring the incidences of treatment emergent adverse events (TEAEs), treatment-related AEs and AEs/SAEs, leading to study withdrawal.
Statistical Analyses
Descriptive statistics were computed for demographic characteristics, including the patients’ age, sex, and race. The proportion of patients who achieved a 2-grade or more improvement in the PGA score after 12 weeks of treatment was calculated. Test results with p < 0.05 were considered statistically significant. Statistical analyses were performed using GraphPad Prism version 8 (San Diego, California: GraphPad Software Inc., 20057).
Results
A total of 34 patients were included in the study; 26 patients completed the study duration and were considered for the final analysis. Eight patients were lost to follow-up and hence were excluded from the final analysis. The baseline characteristics of the patients are depicted in Table 1.
 |
Table 1 Baseline Characteristics |
Physician Global Assessment of Disease
At baseline, the mean PGA score was 3.35 ± 0.48, suggesting moderate to severe grade of lichen planus. Apremilast was associated with a significant improvement in the disease based on PGA of the disease from 1 month onwards, as shown in Table 2. After 12 weeks of therapy with Apremilast, 34.61% (n = 9/26) patients showed 2 or more grade improvement in their disease based on PGA of the disease. One grade improvement was seen in 34.61% (n = 9/26) patients, while 30.76% (n = 8/26) patients showed no improvement in their disease.
 |
Table 2 Improvement in the Patients’ Disease Condition Based as per the Physician’s Global Assessment |
Subject Global Assessment of the Disease
Similar to PGA, there was significant improvement in the patient’s disease condition based on SGA of the disease. After 12 weeks of Apremilast therapy, 7.69% (n = 2/26) patients showed more than 75% improvement in their lesions, 34.61% (n = 9/26) patients showed 50–75% improvement, 34.61% (n = 9/26) showed <25% improvement while in 19.23% (n = 5/26) patients there was no change in their lesions. In 1 patient, there was worsening of lesions after 12 weeks of therapy with Apremilast (Figure 1).
 |
Figure 1 Improvement in the patients’ disease condition based on subject global assessment. |
Target Area Lesion Symptom Score
At baseline, the mean TALSS was 6.35 ± 1.64 which was gradually improved during the treatment period. Apremilast was associated with significant improvement in TALSS of patients after 8 weeks of therapy (6.35 ± 1.64 vs 4.77 ± 1.76; p < 0.012) which was further improved after 12 weeks of therapy (6.35 ± 1.64 vs 3.88 ± 2.14; p < 0.0001) (Table 3). Clinical improvement in the disease before and after treatment is shown in Figure 2.
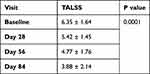 |
Table 3 Improvement in the Target Area Lesion Symptom Score |
Safety Assessment
Overall, Apremilast was well tolerated during the study period. About 23.07% (n = 6/26) patients developed 1 or more adverse events because of Apremilast. Headache (n = 4, 15.38%) was the most common side effect followed by diarrhoea (n = 3, 11.53%) and abdominal pain (n = 1, 3.84%). All the side effects were of a mild degree and appeared within the first 2 weeks after starting Apremilast treatment. None of the patients discontinued Apremilast because of adverse events.
Discussion
Lichen planus is a chronic debilitating disease with often disappointing, less than optimal treatment options, associated with a significant side effect profile. Thus, there is a need for a novel efficacious and safer treatment option for the management of patients with lichen planus. Apremilast can be considered in the management of patients with lichen planus because of its novel immunomodulatory activity. In multiple clinical studies, Apremilast was proven to be effective and safe in the management of various chronic inflammatory conditions, such as psoriasis, psoriatic arthritis, cutaneous sarcoidosis, and atopic dermatitis.14–17
Apremilast is a novel PDE4 inhibitor, thus resulting in increased accumulation of cAMP leading to the activation of protein kinase A, which ultimately inhibits the transcription of proinflammatory cytokines. Because of this novel immunomodulatory activity, Apremilast effectively inhibits the production of tumour necrosis factor-α, interferon-γ, leukotriene B4, and IL-2, IL-5, IL-8, and IL-12, all of which contribute to the pathogenesis of LP, psoriasis, and other chronic inflammatory conditions.18
In our study, after 12 weeks of therapy with Apremilast, 34.61% (n = 9/26) patients showed 2-grade or more improvement in their disease, thus achieving the primary end point. Because of the lack of established and validated clinical assessment scales and consensus regarding the management of lichen planus, PGA seems to be the appropriate evaluation parameter in such patients. Our results were in accordance with the results obtained in a previous study conducted by Paul et al. In their study, 30% patients achieved 2-grade or more improvement in the PGA score.9 Similar results were reported in a few case studies by Bettencourt et al, AbuHilal et al, and Hafner et al.10–12 Even though the primary end point was achieved by only 34.61% (n = 9/26) patients, clinically significant improvement in lichen planus was seen in all the patients during the study period, compared to baseline (p < 0.006).
In our study, 42.30% (n = 11/26) patients achieved more than 50% improvement in their lesions based on the subject global assessment of their disease. In 34.61% (n = 9/26) patients, there was <25% improvement in their lesions, while in 19.23% (n = 5/26) patients, there was no change in their lesions. In 1 patient, there was a worsening of lesions after 12 weeks of therapy with Apremilast. These results are also in accordance with previous studies.9–13
We observed that 12-week therapy with Apremilast was associated with significant improvement in common signs and symptoms of lichen planus like erythema, pruritus and elevation based on significant improvement in target area lesion symptom score compared to baseline (p < 0.0001). Similar results were seen in earlier studies by Paul et al, Bettencourt et al, AbuHilal et al, and Hafner et al.9–13
In landmark clinical studies and real-world studies, Apremilast was associated with adverse events in around 30–70% patients.19 Headache, diarrhoea, and abdominal pain were the most common adverse events.19 However, in our study, only 23.07% (6/26) patients reported 1 or more adverse events. This low incidence of AEs in our study may be attributed to a lack of awareness amongst patients regarding the reporting of AE, which is usually seen in India with small sample size. All the side effects were of mild degree and appeared within first 2 weeks after initiating Apremilast treatment. None of the patient discontinued Apremilast because of adverse events.
Limitations
Small sample size and lack of control group were the main limitations of our study, which may limit the generalizability of our study.
Conclusion
The results obtained in our pilot, proof of concept study justify that Apremilast may be efficacious and safe in the management of patients with lichen planus. In our study, Apremilast was associated with significant improvement in signs and symptoms of lichen planus with a good safety profile. Based on these results, Apremilast can be considered as a promising alternative treatment option in patients with lichen planus.
Data Sharing Statement
The datasets are available only on request due to privacy/ethical restrictions and can be requested from [email protected].
Acknowledgment
Authors would like to acknowledge the medical team of Glenmark Pharmaceuticals Ltd for compiling all data and its analysis and manuscript preparation.
Funding
No funding was received for this clinical study, but drugs were supplied by Glenmark Pharmaceuticals Ltd, Mumbai for conduct of this study.
Disclosure
Dr Dhiraj Dhoot, Dr Namrata Mahadkar and Dr Hanmant Barkate are employees of Glenmark Pharmaceuticals Ltd, India. The other authors declare no conflicts of interest in this work.
References
1. Bolognia JL, Jorizzo JL, Rapini RP. Dermatology.
2. James WD, Berger TG, Elston DM, editors. Andrew’s Diseases of the Skin: Clinical Dermatology.
3. Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53–60.
4. Lavanya N, Jayanthi P, Rao UK, Ranganathan K. Oral lichen planus: an update on pathogenesis and treatment. J Oral Maxillofac Pathol. 2011;15:127–132.
5. Husein-ElAhmed H, Gieler U, Steinhoff M. Lichen planus: a comprehensive evidence-based analysis of medical treatment. J Eur Acad Dermatol Venereol. 2019;33(10):1847–1862.
6. Gupta S, Jawanda MK. Oral lichen planus: an update on etiology, pathogenesis, clinical presentation, diagnosis and management. Indian J Dermatol. 2015;60(3):222–229. doi:10.4103/0019-5154.156315
7. Apremilast prescribing information. Available from: https://www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/otezla/otezla_pi_english.ashx.
8. Schett G, Sloan VS, Stevens RM, Schafer P. Apremilast: a novel PDE4 inhibitor in the treatment of autoimmune and inflammatory diseases. Ther Adv Musculoskelet Dis. 2010;2(5):271–278.
9. Paul J, Foss CE, Hirano SA, et al. An open-label pilot study of Apremilast for the treatment of moderate to severe lichen planus: a case series. J Am Acad Dermatol. 2013;68:255–261. doi:10.1016/j.jaad.2012.07.014
10. Bettencourt M. Oral lichen planus treated with Apremilast. J Drugs Dermatol. 2016;15:1026–1028.
11. AbuHilal M, Walsh S, Shear N. Treatment of recalcitrant erosive oral lichen planus and desquamative gingivitis with oral Apremilast. J Dermatol Case Rep. 2016;10:56–57. doi:10.3315/jdcr.2016.1232
12. Hafner J, Gubler C, Kaufmann K, et al. Apremilast is effective in lichen planus mucosae-associated stenotic esophagitis. Case Rep Dermatol. 2016;8:224–226. doi:10.1159/000447051
13. Ravichandran S, Kheterpal MK. Apremilast for the off-label treatment of lichenoid and interface dermatoses. J Am Acad Dermatol. 2020;83(5):1489–1491. doi:10.1016/j.jaad.2020.05.112
14. Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a Phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73(1):37–49. doi:10.1016/j.jaad.2015.03.049
15. Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a Phase 3 randomised, placebo-controlled trial with Apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73(6):1020–1026. doi:10.1136/annrheumdis-2013-205056
16. Baughman RP, Judson MA, Ingledue R, Craft NL, Lower EE. Efficacy and safety of Apremilast in chronic cutaneous sarcoidosis. Arch Dermatol. 2012;148(2):262–264. doi:10.1001/archdermatol.2011.301
17. Samrao A, Berry TM, Goreshi R, Simpson EL. A pilot study of an oral phosphodiesterase inhibitor (Apremilast) for atopic dermatitis in adults. Arch Dermatol. 2012;148(8):890–897. doi:10.1001/archdermatol.2012.812
18. Schafer PH, Parton A, Gandhi AK, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol. 2010;159:842–855. doi:10.1111/j.1476-5381.2009.00559.x
19. Rajagopalan M, Dogra S, Saraswat A, Varma S, Banodkar P. The use of apremilast in psoriasis: an Indian perspective on real-world scenarios. Psoriasis. 2021;14(11):109–122. doi:10.2147/PTT.S320810
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

