Back to Journals » Drug Design, Development and Therapy » Volume 13
Evaluation of the effect of long-term use of antidepressants in the SSRI group on bone density with dental volumetric tomography
Authors Agacayak KS , Guler R , Ilyasov B
Received 23 May 2019
Accepted for publication 26 August 2019
Published 3 October 2019 Volume 2019:13 Pages 3477—3484
DOI https://doi.org/10.2147/DDDT.S216822
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Kamil Serkan Agacayak,1 Rıdvan Guler,1 Bekir Ilyasov2
1Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Dicle University, Diyarbakır, Turkey; 2Private Dental Clinic, Diyarbakır, Turkey
Correspondence: Kamil Serkan Agacayak
Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Dicle University, Diyarbakir 21280, Turkey
Tel +90 412 241 1017
Email [email protected]
Aim: The present study aims to employ dental volumetric tomography to examine bone mineral density among men that used antidepressants in the SSRI group for a long time.
Method: The present study was conducted through the utilisation of data related to patients that presented to the Faculty of Dentistry of Dicle University and had a dental volumetric tomography (DVT) scan for any reason. The patients were divided into 2 groups based on the use of antidepressants: Group 1 included 68 patients as the control group, and Group 2 consisted of 68 patients that used antidepressants. Radiomorphometric measurements were performed on DVT data: DVT-Mandibular Index (DVT-MI), DVT-Cortical Index (DVT-CI), Hounsfıeld Unit (HU) CORTICAL, and HU SPONGIOSIS values were calculated.
Results: The group of patients that used antidepressants exhibited a significant increase in DVT CI and a significant decrease in HU CORTICAL, HU SPONGIOSIS and DVT MI values. These findings were suggestive of osteoporosis.
Conclusion: Long-term use of antidepressants should be taken into consideration as a risk factor for osteoporosis in men.
Keywords: antidepressant, bone density, dental tomography
Introduction
Osteoporosis is the most common form of metabolic bone diseases and is characterised by an equal decrease in bone mineral and matrix below the normal value, thereby leading to an increase in bone frailty.1,2 Increased average longevity around the world has led to a higher level of importance being attached to chronic diseases observed in later stages of life.3 Approximately 7% of men and 22% of women in Europe are affected by osteoporosis. Similarly, 6% of men and 23% of women in Australia fall within the risk group.4 Osteoporosis is gaining further importance also by reason of increased longevity in Turkey. Osteoporosis increases the risk of fracture and is among the leading causes of morbidity, mortality, and disability especially among the elderly.5 Women are at a higher risk in terms of osteoporosis and fracture than men, while men are faced with more severe consequences of osteoporosis and carry a two-fold risk of mortality.6 Risk factors for low BMD and possible bone fractures include low body mass index, history of fractures, sex and menopause, low intake of calcium and vitamin D, smoking, high alcohol consumption, physical inactivity, and history of use of certain drugs including glucocorticoids and some psychotropic drugs.7–9 A series of diseases are also associated with low bone density along with factors concerning lifestyle and nutrition. As an example, such diseases as type 1 diabetes, inflammatory bowel disease, and schizophrenia have been associated with reduced bone mineral density. Certain studies established that such association also applied to depression without any distinction between sexes in addition to the aforementioned diseases.10,11 Beside clinical depression, less severe depressive symptoms, stress, anxiety, and poor welfare were shown to have a negative effect on bones.12,13
Furthermore, use of antidepressants, especially of selective serotonin reuptake inhibitors (SSRIs), has been associated with lower bone mineral density despite the lack of any exact explanation concerning the relevant mechanism of action.14,15
Osteoporosis is diagnosed through the determination of reduced bone mineral density (BMD). Diagnosis may be secured through histopathological examinations, imaging methods, and biochemical analyses. These methods and equipment that are necessary for the diagnosis may not be available at every healthcare institution. This fact adds difficulties and delays to the diagnosis of osteoporosis.
Cone beam computed tomography (CBCT) is employed specifically for the three-dimensional imaging of the head-and-neck area and has become increasingly common today by reason of its short scanning duration and significantly lower dose of radiation when compared to conventional CT.
CBCT has been employed in various studies for the measurement of BMD.16,17 Marquezan et al identified a positive correlation between the BMD of a total bone block as measured by dual-energy X-ray absorptiometry and the value measured by CBCT.18
Various studies were conducted with respect to the effects of osteoporosis on jawbones and the diagnosis of osteoporosis using radiographic images, and the radiographic findings pointing at osteoporosis were identified to include a general decrease in the density of jawbones and in the number of trabeculae in the mandibula; reduced thickness in the inferior mandibular cortex and cortical bone lines; loss of alveolar bone; collagen collapse; and secondary loss of teeth. Some patients were also reported to exhibit increased thickness in lamina dura. The literature includes studies concerning the evaluation of the effects of osteoporosis on jawbones through various radiographic images.19 There are many studies addressing the findings of osteoporosis in jawbones as identified through the use of radiomorphometric indices utilising the measurements of length and angle at certain sites of the jaw.20
The present study aims to examine the differences in the effects of the long-term treatment with antidepressants in the SSRI group on the bone mineral density of jawbones as determined by DVT.
Materials and methods
The present study was conducted at the Department of Dentistry and Oral and Maxillofacial Surgery of the Faculty of Dentistry of Dicle University. The study was approved by the ethics board of the Faculty of Medicine of Dicle University (Protocol No:54/22.11.2018). In accordance with the Helsinki Declaration, consent was obtained from the patients that they wanted to participate in the study. A retrospective examination was performed on the CBCT images of male patients in the age range of 19–74 with a variety of dental indications. The CBCTs had originally been taken for routine patient clinical examination and not for the investigation of osteoporosis of the jaws. Furthermore, we excluded from the study, patients with serious pathologies in the jaw including defects, tumours, and cysts. A control group was also established through a retrospective endeavour. When choosing the control group, we behaved objectively and randomly selected the control group at similar ages and in the residence.
Images of jaws and face were obtained in an environment of 120 kVp and 3.7 mÅ by cone beam computed tomography (I-Cat; Imaging Sciences International, Hatfield, PA, USA). The images were obtained in a period of 10 seconds (the real exposure time was 9 seconds). Every scan generated small, single frames (up to 440 frames) with a voxel size of 0.300 mm (Newtom Cone Beam 3D Imaging, AFP Imaging Corporation, USA).
The present study divided the patients into two categories: The control group or Group 1 was formed with 68 healthy male patients who had not been treated with a regular administration of medicine whereas 68 male patients that had been receiving a treatment with antidepressants in the SSRI group for a period longer than 5 years were included in Group 2. Most of our patients have low sociocultural levels and most of them cannot remember how long they used in how many months. They can report how many years. Therefore, patients with SSRI use of at least 5 years were included in the study. Female patients and patients with a history of any cancer, substance abuse (smoking, alcohol and other drugs), diabetes mellitus, Paget’s disease, thyroid dysfunction, or osteoporosis or that had received chemotherapy or any treatment for osteoporosis were excluded from the study. In addition, the study did not include patients with serious pathologies in the jaw including defects, tumours, and cysts.
The CBCT procedure
The study scanned and assessed a total number of 140 dental tomography images obtained from male patients in the age range of 19–74 by reason of indications specific to each case. CBCT scans were obtained and reviewed by the same radiologist using the I-Cat Vision software. The present study checked bone mineral density through various indices. These included Hounsfıeld Unit (HU), Dental Volumetric Tomography - Cortical Index (DVT-CI) and Dental Volumetric Tomography - Mandibular Index (DVT-MI). Four patients were excluded from the study because of their on-going systemic conditions.
Measurement of radiomorphometric parameters
Hounsfield unit (HU)
The following points were designated on a coronal cross-section in implant mode:
- The site that was 3 mm lateral to the upper cortical line of the mental foramen with the inclusion of 1 mm2 of spongiosis bone (HU SPONGIOSIS)
- The site that included 1 mm2 of cortical bone on the upper cortical wall of the mental foramen (HU CORTICAL)
HU values of these sites were calculated (Figure 1).
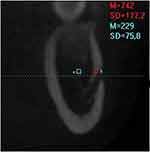 |
Figure 1 On coronal section in implant mode: a. HU SPONGIOSIS, b. HU CORTICAL. |
Assessment of hounsfield units (HU)
Bone density on CT images is expressed in Hounsfield Units (HU).1 HU values lie between the values of −1000 and +3000. Here, −1000 refers to the value of pure air whereas +3000 signifies the metal with the highest density. This assessment indicates the value for water to be 0 HU and the figure for soft tissue to be close to 0 or a negative value. Bones provide results in the range of +100 HU to +1900 HU.21 The values corresponding to various HU values under Misch Classification are as given below:
- D1 bone: >1250 HU
- D2 bone: 850–1250 HU
- D3 bone: 350 −850 HU
- D4 bone: 150–350 HU
- D5 bone: 0–150 HU (116).
Hounsfield Units (HU) offer a system to identify density on the basis of the transmission of X-rays through the site under examination on computer tomography (CT).22 The digital imaging system available with CT consists of picture elements termed as pixels. Every pixel has a certain volume, namely voxel, depending on the thickness of the selected cross-section. Voxel converts the X-ray absorbed by the organism into a numeric value. This system is termed as HU. BD is determined as the HU unit expressed for the site under examination.23
DVT is quite precise in linear measurements and has exhibited a correlation with bone density. However, the HU values determined by way of DVT do not reveal the actual bone density despite a low correlation with bone density values measured through micro-CT and histological analyses.24 There are a large number of studies regarding DVT as a reliable method for the measurement of bone density.25 Nevertheless, certain research studies debate the accuracy in the measurement of HU through DVT.26
Dental volumetric tomography - mandibular index (DVT-MI)
Measurements were obtained of the length of the perpendicular from the lower cortical line of the right mental foramen to the basis mandibular (MFU) in the coronal cross-section of the implant planning mode and of the thickness of the basis mandibula (BMD) at the same site and the DVT-MI value was calculated in line with the formula below (Figure 2).
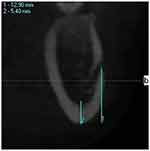 |
Figure 2 DVT-MI measurement: 1-MFU, 2-BMC. |
Dental volumetric tomography – cortical index (DVT-CI)
- DVT-CI =1: Intact cortical bone (Figure 3).
- DVT-CI =2: Cortical bone exhibiting a small resorbed cavity or residue between one and three (Figure 4).
- DVT-CI =3: Visibly porous cortical bone with multiple resorptive cavities (Figure 5).
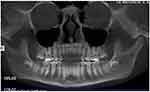 |
Figure 3 DVT-CI=1, integrity of the cortical bone. |
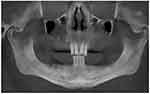 |
Figure 4 DVT-CI=2, cortical bone with resorbed cavities or residues of one to three sizes and small size. |
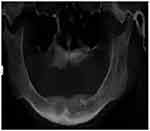 |
Figure 5 DVT-CI=3, cortical bone, which is apparent porosal, and seen in many resorbent cavities. |
Statistical analysis
Statistical analyses were performed using the SPSS Statistics 18.0 software (IBM Corporation, Armonk, NY, USA). The Shapiro-Wilks test was employed to determine the homogeneity and normality of distribution among the variables of comparison. An independent student t-test was conducted for data exhibiting a normal distribution. Data not exhibiting a normal distribution were made subject to the Mann-Whitney U test. The level for statistical significance was P<0.05. Spearman correlation analysis was employed to check whether there was an association between each parameter and age. Multivariate linear regression analysis was performed to evaluate the effect of age on parameters measured by DVT.
Results
The study included male patients in the age range of 19–74. The patients’ average age was 38. The radiographic characteristics of all patients were demonstrated in Table 1. Considering the average values measured through DVT-CI and DVT-MI, patients that had used antidepressants had a significant increase in DVT-CI and a significant decrease in DVT MI, HU CORTICAL and HU SPONGIOSIS values (Table 2). In addition, DVT-CI correlated positively with age (Figure 6). Multivariate linear regression analysis showed no effect on HU CORTICAL, HU SPONGIOSIS, DVT MI values. However, age was significantly influenced on DVT CI (Table 3).
 |
Table 1 Evaluation of the radiographic parameters in the patients |
 |
Table 2 Comparison of radiographic parameters between patients receiving antidepressants and those who did not |
 |
Figure 6 Positive correlation between age and DVT-CI. Abbreviation: DVT-CI, Dental Volumetric Tomography – Cortical Index |
 |
Table 3 Results of multivariant analysis evaluating the effect of age on parameters |
Discussion
The association between depression and low BMD and risk of fracture is currently not known for certain. This potential association should be explored further with a view to evaluating the effects of pathophysiological pathways and other factors, as well as the effects of antidepressants used for treatment. We, in the present study, evaluated the effects of antidepressants on BMD and determined that the use of antidepressants leads to a significant decrease in BMD.
Depression can be regarded as a risk factor for osteoporosis.27 Animal experiments showed that serotonin affected bone mass especially during periods of growth.28 It was also expressed that osteoblasts had serotonin-transporting receptors and selective serotonin reuptake inhibitors (SSRIs) exerted a direct impact on bone mass through this pathway.29 Two previous studies found no association between the use of antidepressants and low bone mass whereas another study established the presence of similar bone mass losses among users of the same SSRIs.30 The literature also indicated an association between the use of SSRIs by older women and bone loss, while such an effect was not reported with tricyclic antidepressants (TCAs).31
The effects of antidepressants on the bone metabolism is reported to be potentially associated with menopause in addition to the period of growth. One study addressing the effects of antidepressants on humans found that the on-going use of drugs in the SSRI group was associated with concurrent changes of BMD in the lower back, spine, and hips, while there was no such association with the group of tricyclic antidepressants.32 Therefore, we excluded female patients from the study and considered only male patients.
One prospective study associated the use of selective serotonin reuptake inhibitors (SSRIs) with a two-fold increase in the risk of clinical fragility fractures.33 Our patients did not exhibit a history of fractures. Similarly, our group of patients did not have previously diagnosed osteoporosis.
A review of the mechanism of impact of drugs in the SSRI group reported the presence of the blocked serotonin-transporters also in the bone and hence a possible effect on the bone metabolism.34 In a previous study, a total of 137 patients using citalopram, fluoxetine, fluvoxamine, paroxetine or sertraline within the SSRI group were followed up for a period of 5 years. The study indicated that the use of SSRIs caused a significant decrease in BMD and a significant increase in the risk of falls, thereby leading to a significant increase in the risk of fractures. Drugs in the SSRI group were reported to have the potential to cause significant health problems due to their possibility of increasing the risk of fractures depending on the dosage and the observation that the daily use of SSRIs increased the risk of clinical fragility fractures by 1.5–2 times.35 A review of the results of our patients showed a significant increase in DVT-CI values. In other words, a significant decrease was observed in BMD. The value concerned here is 1.47 and points to a cortical bone exhibiting a resorbed cavity or residue.
The authors of certain studies conducted through PMI analysis argued that the value of 0.4 was the critical value for this analysis and individuals exhibiting values below this threshold should be examined for osteoporosis. One research study established a significant difference in terms of PMI values between individuals with osteoporosis and individuals without any systemic diseases, but did not define 0.4 as a critical value, but observed the average value for healthy individuals to be 0.32 and the average value for the osteoporotic group to be 0.27.36 In the present study, DVT-MI was identified to be lower in the group treated with antidepressants than in the control group and to range below 0.4 despite the absence of any difference between the two groups.
A previous study comparing the bone resistance felt by the dentist during the placement of an implant and respective HU values was conducted with the dentist assessing the bone resistance with a score between 0 and 10.HU values were calculated by DVT in a range between 0 and 1000. The study revealed a direct proportion between bone resistance and HU values.37 The present study indicated a significantly higher HU CORTICAL values in the control group, i.e. a significantly higher BMD was observed in the control group.
One of the limitations of our study consists of a very wide age group of patients, another limitation is that patients cannot express the duration of SSRI usage in months.
Conclusion
Considering the results of the present study, we established with radiomorphometric methods that the long-term use of antidepressants in the SSRI group created negative effects on bone tissue.
Based on the data we examined and the results we established with respect to the low bone density, we suggest that a comparison is necessary with DEXA results for the diagnosis of osteoporosis or osteopenia. We aimed to evaluate the effect of antidepressants on bone tissue by way of DVT and became the first to work on this subject in the field of dentistry. The conduct of more studies of a similar nature and comparisons with DEXA results will offer more data on bone density.
We recommend all patients that have been on a long course of any antidepressant to undergo bone scanning for osteoporosis. Patients using SSRIs that are at risk for osteoporosis should be offered preventive treatment against osteoporosis at the beginning of treatment. In addition, patients should be recommended to follow a healthy diet, to engage in regular physical activity, to benefit from exposure to sunlight, and to stay away from such risk factors as alcohol consumption and smoking.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Akyüz G, Ofluoğlu D. Osteoporozda ağrı ve yaşam kalitesi. In: Gökçe Kutsal Y, editor. Osteoporoz., Modern Tıp Seminerleri,19, Ed. Ankara: Güneş Kitabevi; 2001:
2. Eskiyurt N, Akyüz G. Osteoporoz: genel bir değerlendirme. In: Karaaslan Y, Akyüz G, editors. Osteoporoz Top 40. Ankara: MD Yayıncılık; 2002:
3. Eryavuz Sarıdoğan M. Osteoporoz epidemiyolojisi. In: Gökçe Kutsal Y, editor. Osteoporoz. Modern Tıp Seminerleri,19, Ed. Ankara: Güneş Kitabevi; 2001:
4. Henry MJ, Pasco JA, Nicholson GV, Kotowicz MA. Prevalence of osteoporosis in Australian men and women: geelong Osteoporosis Study. Med J Aust. 2011;195(6):321–322. doi:10.5694/mja11.10571
5. Seeman E, Allen T. Risk factors for osteoporosis. Aust NZ J Med. 1989;19:69–75. doi:10.1111/j.1445-5994.1989.tb01682.x
6. Haentjens P, Magaziner J, Colón-Emeric CS, et al. Meta analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152(6):380–390. doi:10.7326/0003-4819-152-6-201003160-00008
7. Cizza G, Primma S, Csako G. Depression as a risk factor for osteoporosis. Trends Endocrinol Metab. 2009;20(8):367–373. doi:10.1016/j.tem.2009.05.003
8. Williams LJ, Pasco JA, Henry MJ, et al. (acetaminophen) use, fracture and bone mineral density. Bone. 2011;48(6):1277–1281. doi:10.1016/j.bone.2011.03.435
9. Bolton JM, Targownik LE, Leung S, Sareen J, Leslie WD. Risk of low bone mineral density associated with psychotropic medications and mental disorders in postmenopausal women. J Clin. Psychopharmacol. 2011;31(1):56–60. doi:10.1097/JCP.0b013e3182075587
10. Wong SY, Lau EM, Lynn H, et al. Depression and bone mineral density: is there a relationship in elderly Asian men? Results from Mr. Os (Hong Kong). Osteoporos Int. 2005;16(6):610–615. doi:10.1007/s00198-004-1730-2
11. Mussolino ME, Jonas BS, Looker AC. Depression and bone mineral density in young adults: results from NHANES III. Psychosom Med. 2004;66(4):533–537. doi:10.1097/01.psy.0000132873.50734.7d
12. Rauma PH, Koivumaa-Honkanen H, Williams LJ, Tuppurainen MT, Kröger H, Honkanen RJ. Life satisfaction and bone mineral density among postmenopausal women - Cross-sectional and longitudinal associations. Psychosom Med. 2014;76(9):709–715. doi:10.1097/PSY.0000000000000114
13. Oikonen M, Hintsanen M, Laaksonen M, et al. Depressive symptoms are associated with lower bone mineral density in young adults with high job strain. The Cardiovascular Risk in Young Finnsstudy. Int J Behav. Med. 2014;21(3):464–469. doi:10.1007/s12529-013-9327-9
14. Williams LJ, Henry MJ, Berk M, et al. Selective serotonin reuptake inhibitoruse and bone mineral density in women with a history of depression. Int Clin Psychopharmacol. 2008;23(2):84–87. doi:10.1097/YIC.0b013e3282f2b3bb
15. Hodge JM, Wang Y, Berk M, et al. Selective serotonin reuptake inhibitors inhibit human osteoclast and osteoblast formation and function. BiolPsychiatry. 2013;74(1):32–39.
16. González-Martín O, Lee EA, Veltri M. CBCT fractal dimension changes at the apex of immediate implants placed using under sized drilling. Clin Oral Implants Res. 2012;23(8):954–957. doi:10.1111/clr.2012.23.issue-8
17. Hohlweg-Majert B, Pautke C, Deppe H, Metzger MC, Wagner K, Schulze D. Qualitative and quantitative evaluation of bony structures based on DICOM dataset. J Oral Maxillofac Surg. 2011;69(11):2763–2770. doi:10.1016/j.joms.2011.02.076
18. Marquezan M, Osório A, Sant’Anna E, Souza MM, Maia L. Does bone mineral density influence the primary stability of dental implants? A systematic review. Clin Oral Implants Res. 2012;23(7):767–774. doi:10.1111/j.1600-0501.2011.02228.x
19. White S, Pharaoh M. Charter 5. Projection Geometry in Oral Radiology: Principles and Interpretation.
20. Dutra V, Devlin H, Susin C, Yang J, Horner K, Fernandes AR. Mandibular morphological changes in low bone mass edentulous females: evaluation of Panoramic radiographs. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(5):663–668. doi:10.1016/j.tripleo.2006.02.023
21. Romeo E, Lops D, Margutti E, Ghisolfi M, Chiapasco M, Vogel G. Long-term survival and success of oral implants in the treatment of full and partial arches: a 7-year prospective study with the ITI dental implant system. Int J Oral Maxillofac Implants. 2004;19:247–259.
22. De Vos J, Casselman GRJ. Swennen: cone-beam computerized tomography (CBCT) imaging of the oral and maxillofacial region: a systematic review of the literature. Int J Oral Maxillofac Surg. 2009;38:609–625. doi:10.1016/j.ijom.2009.02.028
23. Gur A, Nas K, Kayhan O, et al. The relation between tooth loss and bone mass in postmenopausal osteoporotic Women in Turkey: a multicenter study. J Bone Miner Metab. 2003;21(1):43–47. doi:10.1007/s007740300007
24. Suttapreyasri S, Suapear P, Leepong N. The accuracy of cone-beam computed tomography for evaluating bone density and cortical bone thickness at the implant site: micro-computed tomography and histologic analysis. J Craniofac Surg. 2018;1. doi:10.1097/SCS.0000000000004672
25. Parsa A, Ibrahim N, Hassan B, Motroni A, van der Stelt P, Wismeijer D. Influence of cone beam CT scanning parameters on gray value measurements at implant site. Dentomaxillofac Radiol. 2013;42:79884780. doi:10.1259/dmfr/79884780
26. Molteni R. Prospects and challenges of rendering tissue density in Hounsfield units for cone beam computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:105–119. doi:10.1016/j.oooo.2013.04.013
27. Onat ŞŞ, Delialioğlu SÜ, Biçer S, Özel S. Osteoporozda Depresyon ve Yaşam Kalitesine Etkisi. Turk J Osteoporos. 2013;19(3).
28. Warden SJ, Robling AG, Sanders MS, Bliziotes MM, Turner CH. Inhibition of the serotonin (5-hydroxytryptamine) transporter reduces bone accrual during growth. Endocrinology. 2005;146(2):685–693. doi:10.1210/en.2004-1259
29. Bliziotes MM, Eshleman AJ, Zhang XW, Wiren KM. Neurotransmitter action in osteoblasts: expression of a functional system for serotonin receptor activation and reuptake. Bone. 2001;29(5):477–486.
30. Petronijević M, Petronijević N, Ivković M, et al. Low bone mineral density and high bone metabolism turnover in premenopausal women with unipolar depression. Bone. 2008;42(3):582–590. doi:10.1016/j.bone.2007.11.010
31. Diem SJ, Blackwell TL, Stone KL, . Depressive symptoms and rates of bone loss at the hip in older women. J Am Geriatr Soc. 2007;55(6):824–831. doi:10.1111/j.1532-5415.2007.01194.x
32. Bonnet N, Bernard P, Beaupied H, et al. Various effects of antidepressant drugs on bone microarchitecture, mechanical properties and bone remodeling. Toxicol Applied Pharmacol. 2007;221:111–118. doi:10.1016/j.taap.2007.02.005
33. Licinio J, Wong M. The role of inflammatory mediators in the biology of major depression: central nervous system cytokines mdulate the biological substrate of depressive symptoms, regulate stress-response systems, and contribute to neurotoxicity and neuroprotection. Mol Psychiatry. 1999;4:317–327.
34. Richards JB, Papaioannou A, Adachi JD, et al. Effect of selective serotonin reuptake inhibitors on the risk of fracture. Arch Intern Med. 2007;167:188–194. doi:10.1001/archinte.167.2.188
35. Bolton JM, Metge C, Lix L, Prior H, Sareen J, Leslie WD. Fracture risk from psychotropic medications: A population-based analysis. J Clin Psychopharmacol. 2008;28:384–391. doi:10.1097/JCP.0b013e31817d5943
36. Hastar E, Yılmaz HH, Orhan H. Evaluation of mental index, mandibular cortical index and panoramic mandibular index on dental panoramic radiographs in the elderly. Eur J Dent. 2011;5:60–67.
37. Bilhan H, Arat S, Geçkili O. Hekimin implant cerrahisi sırasında hissettiği kemik direnci ile radyografiden elde edilen Hounsfield Ünitesi değerlerinin karşılaştırılması: bir pilot çalışma. GÜ Dis Hek Fak Derg. 2012;29(1):19–24.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
