Back to Journals » Drug Design, Development and Therapy » Volume 14
Evaluation of the Effect of Hypericum triquetrifolium Turra on Memory Impairment Induced by Chronic Psychosocial Stress in Rats: Role of BDNF
Authors Alzoubi KH , Abdel-Hafiz L, Khabour OF , El-Elimat T , Alzubi MA, Alali FQ
Received 21 August 2020
Accepted for publication 14 November 2020
Published 1 December 2020 Volume 2020:14 Pages 5299—5314
DOI https://doi.org/10.2147/DDDT.S278153
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Karem H Alzoubi,1,* Laila Abdel-Hafiz,2,3,* Omar F Khabour,4 Tamam El-Elimat,3 Mohammad A Alzubi,5 Feras Q Alali6,7
1Department of Clinical Pharmacy, Faculty of Pharmacy, Jordan University of Science and Technology, Irbid 22110, Jordan; 2Institute of Anatomy II, Medical Faculty, Heinrich Heine Universität, Düsseldorf, Germany; 3Department of Medicinal Chemistry and Pharmacognosy, Faculty of Pharmacy, Jordan University of Science and Technology, Irbid 22110, Jordan; 4Department of Medical Laboratory Sciences, Jordan University of Science and Technology, Irbid 22110, Jordan; 5Integrative Life Sciences Doctoral Program, Department of Pathology, Virginia Commonwealth University, Richmond, VA, USA; 6College of Pharmacy, QU Health, Qatar University, Doha Qatar; 7Biomedical and Pharmaceutical Research Unit, QU Health, Qatar University, Doha, Qatar
*These authors contributed equally to this work
Correspondence: Feras Q Alali
College of Pharmacy, Qatar University, Doha 2713, Qatar
Email [email protected]
Background: Chronic psychosocial stress impairs memory function and leads to a depression-like phenotype induced by a persistent status of oxidative stress. Hypericum perforatum L. (St. John’s wort) is widely used to relieve symptoms of anxiety and depression; however, its long-term use is associated with adverse effects. Hypericum triquetrifolium Turra is closely related to H. perforatum. Both plants belong to Hypericaceae family and share many biologically active compounds. Previous work by our group showed that methanolic extracts of H. triquetrifolium have potent antioxidant activity as well as high hypericin content, a component that proved to have stress-relieving and antidepressant effects by other studies. Therefore, we hypothesized that H. triquetrifolium would reduce stress-induced cognitive impairment in a rat model of chronic stress.
Objective: To determine whether chronic treatment with H. triquetrifolium protects against stress-associated memory deficits and to investigate a possible mechanism.
Methods: The radial arm water maze (RAWM) was used to test learning and memory in rats exposed to daily stress using the resident–intruder paradigm. Stressed and unstressed rats received chronic H. triquetrifolium or vehicle. We also measured levels of brain-derived neurotrophic factor (BDNF) in the hippocampus, cortex and cerebellum.
Results: Neither chronic stress nor chronic H. triquetrifolium administration affected performance during acquisition. However, memory tests in the RAWM showed that chronic stress impaired different post-encoding memory stages. H. triquetrifolium prevented this impairment. Furthermore, hippocampal BDNF levels were markedly lower in stressed animals than in unstressed animals, and chronic administration of H triquetrifolium chronic administration protected against this reduction. No significant difference was observed in the effects of chronic stress and/or H. triquetrifolium treatment on BDNF levels in the cerebellum and cortex.
Conclusion: H. triquetrifolium extract can oppose stress-associated hippocampus-dependent memory deficits in a mechanism that may involve BDNF in the hippocampus.
Keywords: Hypericum triquetrifolium, methanolic extract, stress, learning, memory, hippocampus, BDNF
Introduction
In biological terms, stress can be defined as any condition that interrupts the equilibrium between an organism and its living environment and causes the release of stress mediators, including glucocorticoid hormones.1,2 Stress triggers a sequence of events in the brain and peripheral nervous system to help the organism cope with and adapt to challenging situations.3 However, when stress is maintained for an extended period, most physiological systems will be negatively affected.4
The complicated relationship between stress and cognition has been a major focus of research over the past century. Studies have shown that stress can variably impair, enhance, or have no effect on learning and memory processes in rodents, depending on the kind of stressor and its duration, the task used to evaluate cognition, and the age, species and strain of the animal.5–8
However, most studies agreed that prolonged stress negatively affects brain functions and impacts nearly every brain region.9 Chronic stress impairs cognition in several aspects such as memory acquisition, consolidation and recall.10 Most studies have focused on the relationship between prolonged stress and spatial ability. Decades of research have identified the hippocampus as an essential part for spatial ability, and showed that chronic stress influences hippocampal function, thus affecting spatial learning and memory.11 The structure of the hippocampus was found to be particularly sensitive to stress because of its high density of glucocorticoid receptors.1 Under situations of prolonged stress, the hippocampus undergoes disturbances in neurogenesis,12 as well as neuronal atrophy,13,14 leading to a corresponding reduction in the total number of neurons and their ramifications.15,16
Stress also has a crucial role in the etiology of anxiety and mood disorders such as depression.17,18 In rats, chronic stress leads to a depression-like phenotype induced by a persistent state of oxidative stress,19 which causes oxidative damage, specifically in the hippocampus.20 Many studies have shown that chronic stress and depression can influence the hippocampus in a highly dynamic manner. For instance, depression shrinks hippocampal volume,21 and causes deficits in hippocampus-dependent declarative memory.22 At the molecular level, depression is associated with a decline in the expression of brain-derived neurotrophic factor (BDNF).23 Moreover, reduced hippocampal plasticity in depressed individuals supports the literature on spatial ability and dendritic structure in the hippocampus following prolonged exposure to the stress in rodents.24 Together, this evidence demonstrates that chronic stress and depression are closely associated, and suggests that putative antidepressant agents, such as H. perforatum, should be investigated as therapeutic options to improve the negative outcomes of chronic exposure to stress.
BDNF plays a crucial role in synaptic plasticity and neuronal survival.25 Its neurotrophic action has been implicated in its improvement of normal,26 and impaired memory function.27 BDNF levels decline as well during chronic stress situations.28
The resident–intruder psychosocial stress paradigm is an animal model that produces stress by continuously disrupting the established social hierarchy.29–31 This method elevates serum corticosterone levels,32 and blood pressure,33 proving its validity as a chronic stress induction model. Chronic social stress also induces depression-like behaviors.34 Therefore, exposing rats to long-term social stress is expected to lead not only to deficits in spatial ability, but also to a depression-like phenotype.35
H. perforatum (St. John’s wort) is a commercially available medicinal plant used to relieve symptoms of depression.36 Studies using various behavioral learning and memory paradigms indicate it has nootropic activity,37,38 but further investigations are required to confirm this. H. perforatum extract also enhanced BDNF expression in a human-derived cell line.39 However, several clinical studies revealed that its long-term administration resulted in several side effects such as anxiety, restlessness, insomnia, and gastrointestinal disorders including diarrhea.40–42 One recent study in rats showed that long-term treatment with H. perforatum led to impairments in short- and long-term memory and decreased hippocampal BDNF levels.43 Therefore, a safer alternative to H. perforatum is needed that improves or has no effect on memory function under unstressed conditions, while counteracting the deleterious effect of chronic stress on spatial working and reference memory. Such an agent would provide us with an alternative treatment to be used daily to help us cope with the repeated, persistent stresses of everyday life. H. triquetrifolium also gained considerable scientific interest in the last years, due to its richness of a variety of bioactive compounds that make H. triquetrifolium to be a medicinal herb with a wide range of medicinal applications.44 H. triquetrifolium grows wild in Jordan,45 and it shares many active compounds with H. perforatum, but the plants display considerable phytochemical diversity.46 Generally, the therapeutic activity observed in medicinal plants is not attributed to a single active compound, and in most cases, extracts show activity based on the synergistic or antagonistic effects of their different components. Therefore, we used a holistic approach to investigating H. triquetrifolium by testing the effects of the methanolic extract, which contains many biologically active components and in preliminary work by our group, showed potent antioxidant activity.46–48
Despite the well-studied effect of H. perforatum on cognitive functions, H. triquetrifolium was poorly explored. For this purpose, H. triquetrifolium aerial parts were collected from the wild nature in Jordan, dried and subsequently extracted with different solvents to get the methanolic extract in order to evaluate its possible attenuating influence on stress-induced learning and memory impairment. Here, we report the first investigation of how H. triquetrifolium Turra, a closely related species to H. perforatum, affects cognitive processes and cognitive biomarkers in the brain. To the best of our knowledge, this is the first time to link the impact of H. triquetrifolium extract to learning and memory, H. triquetrifolium extract impact on stress-associated memory impairment, and chronic psychosocial stress to different forms of consolidation processes. We investigated the impact of chronic stress on different stages of hippocampus-dependent memory processing and tested the putative memory-enhancing properties of H. triquetrifolium methanolic extract. We then purified the methanolic extract and identified some of its active ingredients. Finally, we investigated the possible underlying mechanisms by which H. triquetrifolium exerts its effects by investigating BDNF levels in multiple brain areas, especially in the hippocampus, due to its well-known role in spatial learning and memory.49
Methods
Plant Collection
The aerial parts of H. triquetrifolium Turra were collected during the flowering stage in July from Al-Mafraq and the campus of Jordan University of Science and Technology (JUST), Irbid, Jordan. The collected plant material was identified by Dr. Mohammad Gharaibeh, a plant taxonomist at the Department of Natural Resources and Environment, Faculty of Agriculture, JUST. A voucher specimen (PHS-120) was deposited in the herbarium of the Faculty of Pharmacy, JUST, Irbid, Jordan. The raw material was cleaned and air-dried at room temperature, away from direct sunlight. The dried plant material was then ground to a fine powder using a laboratory mill (RetschMühle, Retsch GmbH, Haan, Germany) and stored at room temperature (22–23°C) in paper bags protected from light and humidity until required for analysis.
Phytochemical Analysis
The powdered plant material (1.1 kg) was extracted exhaustively for 3 h using methanol in a Soxhlet extractor. The solvent was evaporated to dryness under reduced pressure using a rotary evaporator (RE 200, Bibby Steriline Ltd., Stone, UK) to yield 237 g of the MeOH extract. The MeOH fraction was dissolved in 500 mL of MeOH:H2O [9:1] and partitioned with 1 L hexane. The aqueous methanolic layer (Fraction A) was then concentrated and partitioned between CHCl3:MeOH (4:1) and water (1:1). After this, the chloroform/methanolic fraction (Fraction B) was washed with 1% saline to yield 19.9 g extract (Fraction C), of which about 1 g was further purified using normal phase successive Sephadex LH20 column chromatography and preparative thin-layer chromatography (PTLC) (Scheme 1).
About 40 g of Sephadex (bead size 25–100 μm, Sigma-Aldrich, St Louis, MO, USA) was swollen for 7 h in 100 mL HPLC-grade ethanol to obtain a final bed volume of 160 mL. Isocratic elution with 100% HPLC-grade ethanol was performed. The subtractions obtained were then purified using successive Sephadex LH20 column chromatography and PTLC. For PTLC, the following mobile phase system was used: ethyl acetate: glacial acetic acid: formic acid: water, at a ratio of 10:1.1:1.1:2.6 to yield compound 1 (Scheme 1).
Compound 2 was isolated from the methanolic extract of H. triquetrifolium based on the method of50). In brief, about 57 g of the dried methanolic extract was washed with 200 mL of diethyl ether five times at room temperature until no fluorescent pink color was observed in the ether phase in the presence of ultraviolet radiation. Successive PTLC was used for the isolation of 2 by dissolving the carotenoid pigment-free extract in ethanol. The mobile phase solvent system used was ethyl acetate: glacial acetic acid: formic acid: water, at a ratio of 10:1.1:1.1:2.6.
The chemical structures of the isolated compounds were elucidated using mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectroscopy and by comparison with an authentic standard of known compounds. NMR analysis of the isolated compounds was conducted using a 400 MHz Bruker spectrometer. Mass spectra were obtained using an ion-trap mass spectrometer (Agilent, Palo Alto, CA, USA) equipped with an electrospray ionization source and an Agilent 100 series HPLC.
Pharmacological Studies
Animals
Adult male Wistar rats (starting weight 150–250 g each) were used and housed in metal cages (2 to 5 animals per cage). The rats were housed up to five rats per cage under a 12 h light/dark cycle (light off 7AM) at 25°C, with free access to food and tap water. The study protocol was approved by the Institutional Animal Care and Use Committee of Jordan University of Science and Technology (Approval number: 74/2008). Work was in compliance with The Institutional Animal Care and Use Committee Guidebook, 2nd edition of 2002.51
Animals were randomized to four groups of 12–15 animals each: Vehicle-treated group (control); H. triquetrifolium extract (Hypericum); chronic stress (sts); chronic stress + H. triquetrifolium (sts/Hypericum). The sts and sts/Hypericum groups were subjected to chronic psychosocial stress for 9 weeks using the resident–intruder model (detailed 2.3.2). While control and Hypericum groups were handled and transferred to the experimental room and returned to their home cage every day. During this experimental period, the Hypericum and sts/Hypericum groups received H. triquetrifolium (50 mg/kg/day i.p.) once daily, dose was selected based on memory-enhancing properties of H.perforatum extract on the conditioning of avoidance learning in rats (Klusa et al, 2001). Both H. triquetrifolium and H. perforatum belong to Hypericaceae family and share many of biologically active compounds.46 The control and sts groups received equivalent volumes of the vehicle (0.3% DMSO and 0.1 M NaOH at a ratio of 1:1) without plant extract, on the same schedule (Figure 1). All experimental protocols were conducted during the dark period in a dimly lit room.
Induction of Chronic Psychosocial Stress
Chronic stress was established in sts and sts/Hypericum using the intruder model as previously described (Alzoubi et al 2013 a, b). Briefly, two groups of animals, marked as stressed only “sts” and stressed- H. perforatum “sts/Hypericum”, were housed with the same cage mates for 2 weeks to establish a social hierarchy. Stress was then induced by swapping two rats (chosen pseudo-randomly) daily between those cages from each cage to the other for a period of 9 weeks. Thus, animals were forced to continually adapt to new stressful situations as daily swapping disrupts the social hierarchy among animals.52,53 This method was shown by our group to elevate serum corticosterone levels,32 and blood pressure,33 indicating that it induces chronic stress.
Radial Arm Water Maze (RAWM)
After 9 weeks of sts and/or Hypericum treatment, learning and memory performance in the RAWM was tested in all experimental groups. We used the same procedure for the spatial working memory version of RAWM as described by.32,54 The RAWM is a black circular water tank with six V-shaped stainless steel plates arranged to form a swimming field with an open central area and six arms filled with water (24 ± 1°C). A transparent escape platform was hidden/submerged 2 cm beneath the water surface at the far end of one of the swim arms (goal arm). Animals had to find this hidden platform. Briefly, there were four phases in the procedure: acquisition, followed by three memory tests.
To begin a trial, each animal was placed into the pool facing the wall at one of the five possible starting points or “start arm” randomly chosen (any arm other than the goal arm), and allowed to freely swim in the maze until it found the hidden platform. A correct choice scored when the animal swam into the goal arm, climbed onto the hidden escape platform and allowed to remain on it for 15 s. However, every time the animal entered incorrect arm, an error was counted. The number of errors made before the animal found the platform was recorded. The maximum time allowed to perform the task was 60 sec.
The RAWM phases were the learning phase (acquisition phase), followed by three memory tests. In the learning phase, each animal was tested by conducting 12 learning-trials (two sessions, each session consisted of 6 trials with 5 min rest in-between; after the first six trials). After completion of the acquisition sessions (the learning phase), the animal’s memory was tested at 30 min, 5 h, and 24 h time periods after ending the learning phase (1 trial per test session). The final memory test (24h test) was conducted just before the next acquisition session. The location of the platform was fixed in one arm “goal arm” for each day, but was relocated to another arm on the subsequent day in order to determine if the rat learned a problem-solving strategy and cognitive flexibility.
The training was continued for five consecutive days or until the animal reached days to criterion (DTC) in the last acquisition trial (trial number 12) and all memory tests (30 min, 5 h, and 24 h). The DTC is the number of days the animal took to make no errors (or zero error) in two consecutive days. All experiments were carried out during the animals’ active phase. Numerous extra-maze cues were pasted on the walls around the maze to serve as spatial cues including one white circle with diameter of 52 cm and one rectangle with black-white stripes with 50 x 43 cm dimensions. Groups were compared based on DTC, number of errors per acquisition trial, and number of errors per memory test. It is important to note that animals were time staggered into four stages, each stage had 3–4 animals from each experimental group, so that in each day only 15 animals needed to go through the RAWM paradigm.
Biochemical Analysis
Brain Tissue Dissection
Twenty four hours after the last memory test brains were dissected over a frosted glass plate (4°C) containing glucose solution, placed on top of crushed ice. The hippocampus, cortex, and cerebellum were obtained from each brain. They were then moved into separate tubes that were immersed in liquid nitrogen and stored at −80ºC until analysis.
Determination of BDNF Levels
Brain tissues were homogenized in lysis buffer (137 mM NaCl, 20 mM Tris-HC1 pH 8, 0.1% NP-40, 10% glycerol, 0.5 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, and protease inhibitor cocktail (Sigma-Aldrich)). Homogenates were centrifuged (14,000 × g for 5 min at 4°C) to remove insoluble material. Total protein concentrations were estimated using a Bradford assay kit (BioRAD, Hercules, CA, USA) according to the manufacturer’s instructions. To quantify BDNF protein, samples were diluted 1:4 in Dulbecco’s phosphate-buffered saline (0.2 g KC1, 8.0 g NaCl, 0.2 g KH2PO, 1.15 g Na2HPO, 100 mg MgCl2.6H2O and 133 mg CaCl2) and then BDNF protein was measured using an enzyme-linked immunosorbent assay (ELISA; Human/Mouse BDNF DuoSet kit, R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. A BDNF standard curve (0–1000 pg/mL) was freshly prepared in a mixture of lysis buffer and Dulbecco’s phosphate-buffered saline (1:4). Incubation times and washes were as described in Adlard and Cotman, 2004. ELISA plates were read at 450 nm using an automated plate reader (Stat Fax 2100, Awareness Technology, Palm City, FL, USA).
Statistical Analysis
The number of errors on acquisition in the RAWM were compared via two-way repeated measures ANOVAs followed by post test for multiple comparisons. The repeated measures factor was time. Whereas treatment and stress as between-subject factors. For DTC in the RAWM and the biochemical assays results, two-way ANOVA and Tukey’s post hoc test were used. The significance level was set as α ≤ 0.05. All analyses were performed in GraphPad Prism 6. Values all over the study were reported as mean ± SEM.
Results
Phytochemical Analysis
Fraction C of the methanolic extract of the aerial parts of H. triquetrifolium afforded the known flavonoid rutin (1) (obtained as a yellowish powder in 1.8% (wt/wt) yield, calculated based on the dry weight of the whole plant). The spectral data of the compound were in agreement with those for rutin standard as well as those reported in the literature.55,56
Carotenoid-free methanolic extract of the aerial parts of H. triquetrifolium afforded the known naphthodianthrone hypericin (2) (Figure 2). The compound was obtained as a reddish powder in 0.417% (wt/wt) yield, calculated from the dry weight of the whole plant. The spectral data of the compound were in agreement with those for hypericin standard as well as those reported in the literature.57
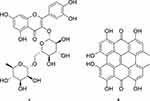 |
Figure 2 Structures of rutin (A) and hypericin (B). |
Evaluation of Plant Extract (H. triquetrifolium)
Combined Effect of H. triquetrifolium on Learning and Memory in Chronically Stressed Rats
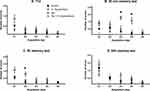
Animals were trained on a new goal every day; thus, they all started with a high number of errors at the beginning of each training day. The number of errors declined as the number of training trials increased, indicating that the animals were learning the task effectively, by recalling the within-day platform location.
In the comparison of the number of errors committed at the end of each daily acquisition session (last acquisition trial, T12) between the sts and control groups, a significant main effect of day F4196 = 5.36 (p = 0.004) was found. However, neither the main effect of group nor the day × group interaction reached significance: day, F1169 = 3.626 (p > 0.05); group × day, F4196 = 0.45 (p > 0.05). When the Hypericum and sts/Hypericum groups were each compared to the control group, there was a significant main effect of day (F4161 = 4.081, p = 0.004) but not group (F1161 = 0.218; p > 0.05), with no group × day interaction (F4161 = 2.135; p > 0.05) (Figure 3, T12).
However, in the comparison of the average number of errors committed in each daily acquisition session (the means of 12 trials per day) between the sts and control groups, a significant main effect of day F4164 = 28.83 (p <0.001) and group (F1164 = 19.41; p < 0.001) was found, but not their interaction; group × day, F4164 = 1.602 (p > 0.05). Post hoc tests revealed significant differences in errors committed to reach the platform on acquisition days 1, 2 and 3 (p = 0.016, 0.0114 and 0.001, respectively), while day 4 revealed a near significant value (p= 0.0551). When the Hypericum and sts/Hypericum groups were each compared to the control group, there was a significant main effect of day (F4161 = 29.05, p < 0.001) but not group (F1161 = 3.197; p =0.076), with no group × day interaction (F4161 = 0.730; p > 0.05). These results indicate that chronic stress may have impaired learning process, and that this effect was prevented by chronic H. triquetrifolium administration.
In the short-term (30 min) memory test, the number of errors committed by animals in the sts group was higher than those in the other experimental groups. Results revealed a significant main effect of group (F1,66 = 17.33; p < 0.001) and day (F4,66 = 3.54; p = 0.011), but not their interaction (F4,66 = 2.137; p > 0.05). Post hoc tests revealed significant differences in errors committed to reach the platform on acquisition days 3 and 4 (p = 0.001 and 0.017, respectively). However, when the sts/Hypericum group was compared to the control group, a significant main effect of day (F4,61 = 4.57; p = 0.003) but not group (F1,61 = 1.533; p > 0.05) was found, as well as a group × day interaction (F4,61 = 0.729; p > 0.05) (Figure 3; 30 min test). Together, these results indicate that chronic stress impaired short-term memory, and that this effect was prevented by chronic H. triquetrifolium administration.
In the 5 h memory test, when comparing the sts and control groups, there was a significant main effect of group (F1164 = 4.64; p = 0.033) and day (F4164 = 5.212; p < 0.001) but no group × day interaction (F4164 = 2.186; p > 0.05) (Figure 3). Post hoc tests revealed significant differences in time to reach the platform location on acquisition day 2 (p = 0.019). Comparing the sts/Hypericum group with the control group revealed a main effect of day (F4161 = 4.781; p = 0.001) but not group (F1161 = 1.95; p > 0.05) and no group × day interaction (F4161 = 0.75; p > 0.05), indicating that chronic stress also induced long-term memory deficits in the 5 h memory test, and that this was prevented by chronic H. triquetrifolium administration. In the long-term (24 h) memory tests, no significant differences were detected in performance between any groups on any day (Figure 3). Notably, chronic H. triquetrifolium administration did not affect memory performance in unstressed animals in any of the memory tests, indicated by the comparable number of errors committed in the Hypericum and control groups (p > 0.05).
Two-way ANOVA revealed no difference in DTC between groups at T12 (F3,67 = 0.184; p > 0.05), 5 h (F3,40 = 0.611; p > 0.05) and 24 h (F3,63 = 0.921; p > 0.05) (Figure 4A, C and D). In contrast, there was a significant difference in DTC between groups in the 30 min memory test (F3,24 = 13.01; p < 0.001) (Figure 4B). A post hoc independent samples Tukey’s test showed that rats in the sts group needed significantly more days to reach criterion than those in the control, Hypericum, and sts/Hypericum groups for the 30 min memory test (p < 0.05) (Figure 4B), indicating impaired short-term memory in the sts group that was prevented in the sts/Hypericum group.
Combined Effect of H. triquetrifolium on Hippocampal BDNF Levels in Chronically Stressed Rats
Two-way ANOVA revealed a significant difference in BDNF levels in the hippocampus between groups (F3,50 = 5.982; p = 0.001). Accordingly, a post hoc Tukey’s test for independent samples showed significantly lower BDNF levels in chronically stressed rats than in the control, Hypericum and sts/Hypericum groups (p > 0.05) (Figure 5A). Moreover, chronic administration of H. triquetrifolium extract normalized the reduction in BDNF levels and was comparable to the control group (p > 0.05). No significant difference was observed in the effects of chronic stress and/or H. triquetrifolium treatment on BDNF levels in the cerebellum and cortex (Figure 5B and C).
Discussion
In the present study, we examined the influence of chronic stress on hippocampus-dependent spatial learning and memory, and investigated whether the effects could be prevented by chronic administration of a methanolic extract of H. triquetrifolium Turra. Behavioural effects were tested in the RAWM, a modified version of a standard task commonly used to evaluate hippocampus-dependent spatial learning and memory.58,59 Chronic administration of H. triquetrifolium prevented chronic psychosocial stress-induced impairments in spatial learning and memory performance and normalized the stress-induced reduction in hippocampal BDNF levels.
BDNF links stress and mood disorders with somatic diseases,60 and BDNF expression is regulated by stress.61 BDNF is a crucial mediator of neuronal events underlying learning and memory processes.62 Its expression is elevated in the hippocampus of animals that learn a spatial memory task, and reducing this expression results in spatial learning and memory deficits.25 Correspondingly, in the present study, we observed a significant reduction in hippocampal BDNF levels combined with spatial learning and memory deterioration after prolonged stress exposure, in agreement with previous reports.63,64
Current findings are consistent with the negative effect of prolonged stress on spatial learning and memory tested in the RAWM and BDNF levels in the hippocampus.52 Additionally, they shed light on the negative influence nine weeks of chronic stress on hippocampus-dependent memory processes. Herein, we will discuss possible mechanisms underlying the ameliorating effect of chronic H. triquetrifolium administration on stress-associated memory impairment.
Stress, Spatial Acquisition and Retrieval
Long-term production of stress hormones challenges homeostatic mechanisms in the brain by over-activating stress systems that lead to negative morphological and functional changes in the brain,30,65 including hippocampal damage,14,66 and learning and memory deficits.67 The significance of the hippocampus in spatial learning and memory,68,69 and the negative influence of prolonged stress on hippocampal structure are well documented.14,66
The influence of chronic stress on spatial learning appears to be specific to the learning task used. The deterioration in spatial learning and memory performance exhibited by stressed rats in the present study is mostly related to the acquired within-day acquisition strategy used in which the platform location was changed daily. We deduced this because this protocol efficiently revealed stress-induced performance deficits in spatial learning and memory.70–73
Chronic stress had a negative impact on spatial learning, short-term (30 min) and 5 h memory retrieval. However, long-term (24 h) memory appeared to be preserved, with the performance of the chronically stressed group being comparable to that of the control group at this time point. This disparity is not surprising, since the stabilization and strengthening process for the initially labile memory goes through a complex process following the learning experience,74,75 occurs in stages,76,77 and is time-dependent.77 Post-encoding consolidation processes depend on the biological state of the tested animal, ie, whether it is active (awake) or inactive (asleep).78,79
Early studies on consolidation implicated the importance of sleep, and even classified memory according to biological state. For instance, according to the memory model introduced by Lewis (1979), formation of memory during the active and inactive states was suggested to be analogous to the formation of short-term (seconds to hours) and long-term (days to weeks) memories, respectively. This reflects the importance of sleep in long-term memory formation. Indeed, memory replay during sleep is crucial to long-term memory consolidation.80
Classically, consolidation was considered a process by which an initially labile memory trace was strengthened. Recently, sleep has been found to have a more significant role than merely stabilizing original memories. It involves a more complex process integrating the initially labile memory trace into established cortical memory networks,81,82 to extract meaning,83 and develop insight,84 leading to superior memory performance in later tests.
For declarative memory, accumulating evidence strongly implicates the importance of sleep in consolidation and in reactivation processes.79,85 Memory replay in sleep is crucial for the declarative form of memory consolidation.86 Hippocampal place cells, which encode the spatial context for memories, show enhanced firing during deep sleep after a session of spatial learning in the preceding waking periods.87,88 During subsequent sleep, improvement in hippocampus-mediated spatial memory occurs,89 and newly encoded spatial information gradually transits from short-term hippocampus dependence to long-term hippocampus independence by integrating into already-existing memory networks.90,91 Some studies suggest that the hippocampus-independent consolidation process develops up to 6 hours after the initial encoding.74 Others show that memory is enhanced in discrete stages of sleep via the “offline replay” of the newly encoded information, and integrated into larger associative networks in a process called “active system consolidation” during sleep.75,92
Current findings highlight the negative influence of chronic stress on hippocampus-based short-term (30 min) and 5 h memory retrieval. However, memory retrieval outside the hippocampus-based domain (24 h memory test) was re-normalized, which was probably due to the offline replay of the spatial information obtained in the preceding waking period during sleep. Together with the comparable performance of the sts and control groups in the 24 h memory test, it is suggested that an overnight improvement occurred in hippocampus-mediated spatial memory, thus normalizing memory in stressed animals. It also suggests that the hippocampus-independent long-term memory consolidation process remained intact despite the chronic elevation of stress hormones in the present study. In agreement with these results, BDNF protein expression was impaired only in the hippocampus and not in the other brain structures examined.
Combined Effect of H. triquetrifolium on Learning and Memory in Chronically Stressed Rats
Chronic administration of H. triquetrifolium extract prevented the reduction in BDNF levels in chronically stressed rats, which could also explain behavioral findings from the current study. In addition to a BDNF protein-mediated mechanism, other postulated mechanisms could involve amelioration of oxidative stress status triggered by prolonged exposure to stress hormones by H. triquetrifolium extract. Alternatively, it could be due to the putative antidepressant activity of H. triquetrifolium extract, since it shares many biologically active components with H. perforatum, a herb widely used to relieve symptoms of depression.36
Chronic mild stress leads to a depression-like phenotype induced by a persistent state of oxidative stress.19 Depression is often associated with cognitive deficits and memory disturbances. A stress-triggered depressive phenotype also shows altered levels of BDNF protein.93 Furthermore, chronic stress impairs synaptic transmission, leading to downregulation of BDNF and resulting in sustained oxidative stress; this ultimately leads to a depression-like phenotype,94,95 and learning and memory deficits.5,96 This can be prevented by antioxidant treatment.19 H. triquetrifolium is an excellent free radical-scavenging herb,47,48 and the two compounds isolated in the present study, rutine and hypericin, are potent antioxidants.97,98 Rutin was previously described in H. triquetrifolium.99 The Antioxidant effect of rutin was already confirmed by a number of studies.100–102 It was shown to significantly elevate the activities of the antioxidant enzymes such as superoxide dismutase and catalase, and the levels of the antioxidant glutathione.102 Rutin effectively upregulated BDNF level resulting in alleviation of oxidative stress in hippocampal neurons.102 The high hypericin content of the H. triquetrifolium that is growing wild in Jordan was described by a previous study from our group.103 Hypericins are one of the main compounds in many species of the genus Hypericum. They are responsible for a wide variety of biological effects of Hypericum such as its antimicrobial, and antiviral effects.104 Importantly previous studies have reported stress-relieving and antidepressant effects for hypericin.105 The isolation of these compounds was part of a phytochemical study for quality purposes in the current project. Moreover, the amounts of the isolated compounds were not sufficient to design experiments to verify their action. Our future work will be covering this point.
At the molecular level, NMDA receptor dysregulation and BDNF downregulation in chronic stress are major contributors to the etiology of depression.95 The NMDA receptor sensitization is critical in the early phase of spatial acquisition.106 Even though hyperforin was not among isolated compounds in this study, H. triquetrifolium was found to have a high level of hyperforin.107 Hyperforin is an NMDA receptor antagonist,108 and is therefore potentially neuroprotective.108,109 When the antidepressant H. perforatum lacks hyperforin, it also lacks antidepressant-like properties, suggesting that hyperforin is the main active component responsible for the antidepressant properties of H. perforatum.110
Chronic stress and depression are closely associated and influence the hippocampus in a highly dynamic manner. Rats subjected to prolonged social stress show a broad spectrum of depression-related behaviors.34,111 Chronic stress and depression negatively influence memory formation, as well as cognitive and emotional processing. The hyperforin in H. triquetrifolium might attenuate depression- and anxiety-like behaviors in this rat model of chronic stress, thus, ameliorating the negative impact of chronic stress on memory processing.
Chronic stress that leads to a depression-like phenotype is also associated with reduced hippocampal BDNF levels.112–114 This effect was normalized by antidepressants,112,115 which also rescue stress hormone-triggered impairments in spatial memory.116 Therefore, in addition to the powerful antioxidant properties of hyperforin in H. triquetrifolium, we propose that its NMDA antagonist action prevents BDNF downregulation and improves depression- and anxiety-like behaviors. This could prevent chronic stress-induced disruption to learning and memory processes.
To our best knowledge, this is the first study to show that methanolic extract of H. triquetrifolium ameliorates stress-associated hippocampal-dependent memory deficits via a mechanism involving BDNF in the hippocampus. In humans, the vast majority of research has focused on St. John’s wort (Hypericum perforatum L.) extract, a closely related species to H. triquetrifolium. H. perforatum, that used for centuries to clinically treat a number of common ailments, eg, neuralgia, sleep pattern disturbances, wound healing and hemorrhoids.117,118 However, it gained popularity for its use to relieve symptoms of mild to moderate depression.119 Both H. triquetrifolium and H. perforatum belong to Hypericaceae family and share many of biologically active compounds.46
Multiple doses of Hypericum perforatum L neither enhanced nor deteriorated cognitive functions in healthy volunteers when tested in various behavioral paradigms such as; choice reaction, psychomotor coordination, short-term memory and responsiveness to distractive stimuli.120 This is in line with the current study’s findings, which revealed that the restorative effect on memory function of H. triquetrifolium is more prominent in deficit models but not in non-deficit models.
Further research with more behavioural dimensions is needed to test the hypothesis that H. triquetrifolium extract can rescue hippocampal function from the negative effect of chronically elevated stress hormones. Indeed, a more comprehensive behavioural profile is needed to extract emotional, attention and anxiety and reveal the impact of chronic administration of H. triquetrifolium extract. Moreover, investigations of the pharmacological activities of H. triquetrifolium on other forms of learning and memory impairment in psychiatric diseases are highly recommended. One limitation of this study is that we did not examine oxidative stress biomarkers such as reduced/oxidized glutathione ratio, glutathione peroxidase and catalase activity, and oxidized glutathione levels. This would be worth doing in a future study since H. triquetrifolium extract has already been shown to have excellent antioxidant properties.47 Besides the amelioration effect of oxidative stress by rutin when tested in differentiated neuronal cells.101 Another limitation of the current study is the test of a single dose of H. triquetrifolium extract, and the need for positive control as part of the study. Future work should also investigate the effect of multiple doses of H. triquetrifolium extract on hippocampus-dependent learning and memory with incorporation of positive controls as needed.
Conclusion
The data from this study indicate that chronic psychosocial stress can impair hippocampus-dependent memory processing. Chronic administration of H. triquetrifolium extract attenuated the negative impact on spatial learning and retrieval and normalized BDNF levels in the hippocampus. However, long-term treatment with H. triquetrifolium neither improved nor reduced spatial ability when tested in the aversively motivated water maze task. Thus, the restorative effect on memory function of H. triquetrifolium could be more prominent in deficit models than in non-deficit models, which is in line with the positive impact of chronic H. triquetrifolium on memory function and BDNF levels in chronically stressed, but not unstressed, rats. These properties of H. triquetrifolium extract could be of interest for future intervention studies.
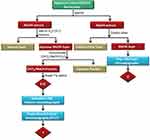 |
Scheme 1 Extraction and fractionation scheme of the methanolic extract of the aerial parts of H. triquetrifolium. |
Acknowledgment
This research was supported by the Deanship of Research, Jordan University of Science and Technology, Irbid, Jordan (Grant No. 74/2008). The publication of this article was funded by the Qatar National Library.
Disclosure
The authors report no conflicts of interest for this work.
References
1. De Quervain D, Schwabe L, Roozendaal B. Stress, glucocorticoids and memory: implications for treating fear-related disorders. Nat Rev Neurosci. 2017;18:7–19.
2. Ranabir S, Reetu K. Stress and hormones. Indian J Endocrinol Metab. 2011;15(1):18–22. doi:10.4103/2230-8210.77573
3. Sandi C. Stress, cognitive impairment and cell adhesion molecules. Nat Rev Neurosci. 2004;5(12):917–930. doi:10.1038/nrn1555
4. Huang RR, Hu W, Yin YY, Wang YC, Li WP, Li WZ. Chronic restraint stress promotes learning and memory impairment due to enhanced neuronal endoplasmic reticulum stress in the frontal cortex and hippocampus in male mice. Int J Mol Med. 2015;35(2):553–559. doi:10.3892/ijmm.2014.2026
5. Liu D, Wang Z, Gao Z, et al. Effects of curcumin on learning and memory deficits, BDNF, and ERK protein expression in rats exposed to chronic unpredictable stress. Behav Brain Res. 2014;271:116–121. doi:10.1016/j.bbr.2014.05.068
6. Xu Y, Pan J, Sun J, et al. Inhibition of phosphodiesterase 2 reverses impaired cognition and neuronal remodeling caused by chronic stress. Neurobiol Aging. 2015;36(2):955–970. doi:10.1016/j.neurobiolaging.2014.08.028
7. Moreira PS, Almeida PR, Leite-Almeida H, Sousa N, Costa P. Impact of chronic stress protocols in learning and memory in rodents: systematic review and meta-analysis. PLoS One. 2016;11(9):e0163245. doi:10.1371/journal.pone.0163245
8. Conrad CD. A critical review of chronic stress effects on spatial learning and memory. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(5):742–755. doi:10.1016/j.pnpbp.2009.11.003
9. Finsterwald C, Alberini CM. Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: from adaptive responses to psychopathologies. Neurobiol Learn Mem. 2014;112:17–29. doi:10.1016/j.nlm.2013.09.017
10. Sandi C. Stress and cognition. Wiley Interdiscip Rev Cogn Sci. 2013;4(3):245–261.
11. Conrad CD, Ortiz JB, Judd JM. Chronic stress and hippocampal dendritic complexity: methodological and functional considerations. Physiol Behav. 2017;178:66–81. doi:10.1016/j.physbeh.2016.11.017
12. Schoenfeld TJ, McCausland HC, Morris HD, Padmanaban V, Cameron HA. Stress and loss of adult neurogenesis differentially reduce hippocampal volume. Biol Psychiatry. 2017;82(12):914–923. doi:10.1016/j.biopsych.2017.05.013
13. Yagi S, Galea LAM. Sex differences in hippocampal cognition and neurogenesis. Neuropsychopharmacology. 2019;44(1):200–213. doi:10.1038/s41386-018-0208-4
14. Kim EJ, Pellman B, Kim JJ. Stress effects on the hippocampus: a critical review. Learn Mem. 2015;22(9):411–416. doi:10.1101/lm.037291.114
15. McEwen BS. Stress, sex, hippocampal plasticity: relevance to psychiatric disorders. Clin Neurosci Res. 2001;1(1):19–34. doi:10.1016/S1566-2772(00)00004-9
16. Galea LAM, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81(3):689–697. doi:10.1016/S0306-4522(97)00233-9
17. Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160(1):1–12. doi:10.1677/joe.0.1600001
18. Holsboer F. Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. J Affect Disord. 2001;62(1–2):77–91. doi:10.1016/S0165-0327(00)00352-9
19. Bouvier E, Brouillard F, Molet J, et al. Nrf2-dependent persistent oxidative stress results in stress-induced vulnerability to depression. Mol Psychiatry. 2017;22(12):1701–1713. doi:10.1038/mp.2016.144
20. Wang Y, Kan H, Yin Y, et al. Protective effects of ginsenoside Rg1 on chronic restraint stress induced learning and memory impairments in male mice. Pharmacol Biochem Behav. 2014;120:73–81.
21. Sawyer K, Corsentino E, Sachs-Ericsson N, Steffens DC. Depression, hippocampal volume changes, and cognitive decline in a clinical sample of older depressed outpatients and non-depressed controls. Aging Ment Health. 2012;16(6):753–762. doi:10.1080/13607863.2012.678478
22. Vasic N, Walter H, Hose A, Wolf RC. Gray matter reduction associated with psychopathology and cognitive dysfunction in unipolar depression: a voxel-based morphometry study. J Affect Disord. 2008;109(1–2):107–116. doi:10.1016/j.jad.2007.11.011
23. Erickson KI, Miller DL, Roecklein KA. The aging hippocampus: interactions between exercise, depression, and BDNF. Neuroscientist. 2012;18(1):82–97. doi:10.1177/1073858410397054
24. Czeh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? Eur Arch Psychiatry Clin Neurosci. 2007;257(5):250–260.
25. Bekinschtein P, Cammarota M, Medina JH. BDNF and memory processing. Neuropharmacology. 2014;76(Pt):
26. Alonso M, Vianna MR, Depino AM, et al. BDNF-triggered events in the rat hippocampus are required for both short- and long-term memory formation. Hippocampus. 2002;12(4):551–560. doi:10.1002/hipo.10035
27. Kiprianova I, Freiman TM, Desiderato S, et al. Brain-derived neurotrophic factor prevents neuronal death and glial activation after global ischemia in the rat. J Neurosci Res. 1999;56(1):21–27. doi:10.1002/(SICI)1097-4547(19990401)56:1<21::AID-JNR3>3.0.CO;2-Q
28. Zaletel I, Filipovic D, Puskas N. Hippocampal BDNF in physiological conditions and social isolation. Rev Neurosci. 2017;28(6):675–692.
29. Abdul-Razzak KK, Alzoubi KH, Abdo SA, Hananeh WM. High-dose vitamin C: does it exacerbate the effect of psychosocial stress on liver? Biochemical and histological study. Exp Toxicol Pathol. 2012;64(4):367–371. doi:10.1016/j.etp.2010.09.011
30. Aleisa AM, Alzoubi KH, Gerges NZ, Alkadhi KA. Chronic psychosocial stress-induced impairment of hippocampal LTP: possible role of BDNF. Neurobiol Dis. 2006;22(3):453–462. doi:10.1016/j.nbd.2005.12.005
31. Alzoubi KH, Abdul-Razzak KK, Khabour OF, Al-Tuweiq GM, Alzubi MA, Alkadhi KA. Adverse effect of combination of chronic psychosocial stress and high fat diet on hippocampus-dependent memory in rats. Behav Brain Res. 2009;204(1):117–123. doi:10.1016/j.bbr.2009.05.025
32. Gerges NZ, Alzoubi KH, Park CR, Diamond DM, Alkadhi KA. Adverse effect of the combination of hypothyroidism and chronic psychosocial stress on hippocampus-dependent memory in rats. Behav Brain Res. 2004;155(1):77–84. doi:10.1016/j.bbr.2004.04.003
33. Alkadhi KA, Alzoubi KH, Aleisa AM, Tanner FL, Nimer AS. Psychosocial stress-induced hypertension results from in vivo expression of long-term potentiation in rat sympathetic ganglia. Neurobiol Dis. 2005;20(3):849–857. doi:10.1016/j.nbd.2005.05.020
34. Kukel’ova D, Bergamini G, Sigrist H, Seifritz E, Hengerer B, Pryce CR. Chronic social stress leads to reduced gustatory reward salience and effort valuation in mice. Front Behav Neurosci. 2018;12:134. doi:10.3389/fnbeh.2018.00134
35. Mohammadi HS, Goudarzi I, Lashkarbolouki T, Abrari K, Elahdadi Salmani M. Chronic administration of quercetin prevent spatial learning and memory deficits provoked by chronic stress in rats. Behav Brain Res. 2014;270:196–205. doi:10.1016/j.bbr.2014.05.015
36. Ng QX, Venkatanarayanan N, Ho CY. Clinical use of Hypericum perforatum (St John’s wort) in depression: a meta-analysis. J Affect Disord. 2017;210:211–221. doi:10.1016/j.jad.2016.12.048
37. Kumar V, Singh PN, Muruganandam AV, Bhattacharya SK. Effect of Indian Hypericum perforatum Linn on animal models of cognitive dysfunction. J Ethnopharmacol. 2000;72(1–2):119–128. doi:10.1016/S0378-8741(00)00216-6
38. Klusa V, Germane S, Noldner M, Chatterjee SS. Hypericum extract and hyperforin: memory-enhancing properties in rodents. Pharmacopsychiatry. 2001;34(Suppl 1):S61–69. doi:10.1055/s-2001-15451
39. Gonulalan EM, Nemutlu E, Bayazeid O, Kocak E, Yalcin FN, Demirezer LO. Metabolomics and proteomics profiles of some medicinal plants and correlation with BDNF activity. Phytomedicine. 2019;152920.
40. Gryzlak BM, Wallace RB, Zimmerman MB, Nisly NL. National surveillance of herbal dietary supplement exposures: the poison control center experience. Pharmacoepidemiol Drug Saf. 2007;16(9):947–957. doi:10.1002/pds.1445
41. Rychlik R, Siedentop H, von den Driesch V, Kasper S. General practice research study of St. Johns wort extract WS 5572. Normally 600 mg per day is enough. MMW Fortschr Med. 2001;143(47):48.
42. Lecrubier Y, Clerc G, Didi R, Kieser M. Efficacy of St. John’s wort extract WS 5570 in major depression: a double-blind, placebo-controlled trial. Am J Psychiatry. 2002;159(8):1361–1366. doi:10.1176/appi.ajp.159.8.1361
43. Valvassori SS, Borges C, Bavaresco DV, et al. Hypericum perforatum chronic treatment affects cognitive parameters and brain neurotrophic factor levels. Braz J Psychiatry. 2018;40(4):367–375. doi:10.1590/1516-4446-2017-2271
44. Aleisa AM. Cytological and biochemical effects of St. John’s wort supplement (a complex mixture of St. John’s wort, Rosemary and Spirulina) on somatic and germ cells of Swiss Albino mice. Int J Environ Res Public Health. 2008;5(5):408–417. doi:10.3390/ijerph5050408
45. Al-Eisawi DM. Field Guide to Wild Flowers of Jordan and Neighbouring Countries. Amman, Jordan: Jordan Press Founation, Al-Rai; 1998.
46. Nurk NM, Crockett SL. Morphological and phytochemical diversity among hypericum species of the mediterranean basin. Med Aromat Plant Sci Biotechnol. 2011;5(SpecialIssue 1):14–28.
47. Alali FQ, Tawaha K, El-Elimat T, et al. Antioxidant activity and total phenolic content of aqueous and methanolic extracts of Jordanian plants: an ICBG project. Nat Prod Res. 2007;21(12):1121–1131. doi:10.1080/14786410701590285
48. Tawaha K, Alali FQ, Gharaibeh M, Mohammad M, El-Elimat T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007;104(4):1372–1378. doi:10.1016/j.foodchem.2007.01.064
49. Mizuno M, Yamada K, He J, Nakajima A, Nabeshima T. Involvement of BDNF receptor TrkB in spatial memory formation. Learn Mem. 2003;10(2):108–115. doi:10.1101/lm.56003
50. Duru B. Isolation of a Bioactive Compound Hypericin from a Medicinal Plant Hypericum perforatum L. Using Basic Chromatography Methods. Department of Chemistry, The Middle East Technical University; 2003.
51. Office of Laboratory Animal Welfare. AR. The Institutional Animal Care and Use Committee Guidebook.
52. Alzoubi KH, Abdul-Razzak KK, Khabour OF, Al-Tuweiq GM, Alzubi MA, Alkadhi KA. Caffeine prevents cognitive impairment induced by chronic psychosocial stress and/or high fat-high carbohydrate diet. Behav Brain Res. 2013;237:7–14. doi:10.1016/j.bbr.2012.09.018
53. Alzoubi KH, Srivareerat M, Aleisa AM, Alkadhi KA. Chronic caffeine treatment prevents stress-induced LTP impairment: the critical role of phosphorylated CaMKII and BDNF. J Mol Neurosci. 2013;49(1):11–20. doi:10.1007/s12031-012-9836-z
54. Srivareerat M, Tran TT, Alzoubi KH, Alkadhi KA. Chronic psychosocial stress exacerbates impairment of cognition and long-term potentiation in beta-amyloid rat model of Alzheimer’s disease. Biol Psychiatry. 2009;65(11):918–926. doi:10.1016/j.biopsych.2008.08.021
55. Exarchou V, Fiamegos YC, van Beek TA, Nanos C, Vervoort J. Hyphenated chromatographic techniques for the rapid screening and identification of antioxidants in methanolic extracts of pharmaceutically used plants. J Chromatogr A. 2006;1112(1):293–302. doi:10.1016/j.chroma.2005.11.077
56. Abdullah Y, Schneider B, Petersen M. Occurrence of rosmarinic acid, chlorogenic acid and rutin in Marantaceae species. Phytochem Lett. 2008;1(4):199–203.
57. Tatsis EC, Exarchou V, Troganis AN, Gerothanassis IP. 1H NMR determination of hypericin and pseudohypericin in complex natural mixtures by the use of strongly deshielded OH groups. Anal Chim Acta. 2008;607(2):219–226. doi:10.1016/j.aca.2007.11.040
58. Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. doi:10.1038/297681a0
59. Stewart S, Cacucci F, Lever C. Which memory task for my mouse? A systematic review of spatial memory performance in the Tg2576 Alzheimer’s mouse model. J Alzheimers Dis. 2011;26(1):105–126. doi:10.3233/JAD-2011-101827
60. Licinio J, Wong M. Brain-derived neurotrophic factor (BDNF) in stress and affective disorders. Mol Psychiatry. 2002;7(6):519.
61. Smith MA, Makino S, Kim SY, Kvetnansky R. Stress increases brain-derived neurotropic factor messenger ribonucleic acid in the hypothalamus and pituitary. Endocrinology. 1995;136(9):3743–3750. doi:10.1210/endo.136.9.7649080
62. Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab. 2014;25(2):89–98. doi:10.1016/j.tem.2013.10.006
63. Bulygina VV, Shishkina GT, Berezova IV, Dygalo NN. BDNF protein expression in the hippocampus following exposure of rats to forced swimming stress. Dokl Biol Sci. 2011;437(1):82–84. doi:10.1134/S0012496611020116
64. Filho CB, Jesse CR, Donato F, et al. Chronic unpredictable mild stress decreases BDNF and NGF levels and Na(+), K(+)-ATPase activity in the hippocampus and prefrontal cortex of mice: antidepressant effect of chrysin. Neuroscience. 2015;289:367–680. doi:10.1016/j.neuroscience.2014.12.048
65. McEwen BS. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583(2–3):174–185. doi:10.1016/j.ejphar.2007.11.071
66. Amri H, Drieu K, Papadopoulos V. Ex vivo regulation of adrenal cortical cell steroid and protein synthesis, in response to adrenocorticotropic hormone stimulation, by the Ginkgo biloba extract EGb 761 and isolated ginkgolide B. Endocrinology. 1997;138(12):5415–5426. doi:10.1210/endo.138.12.5604
67. Schoenfeld TJ, Gould E. Stress, stress hormones, and adult neurogenesis. Exp Neurol. 2012;233(1):12–21. doi:10.1016/j.expneurol.2011.01.008
68. Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35(4):625–641.
69. Kosaki Y, Lin TC, Horne MR, Pearce JM, Gilroy KE. The role of the hippocampus in passive and active spatial learning. Hippocampus. 2014;24(12):1633–1652. doi:10.1002/hipo.22343
70. Arbel I, Kadar T, Silbermann M, Levy A. The effects of long-term corticosterone administration on hippocampal morphology and cognitive performance of middle-aged rats. Brain Res. 1994;657(1–2):227–235.
71. Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110(6):1321–1334. doi:10.1037/0735-7044.110.6.1321
72. Dachir S, Kadar T, Robinzon B, Levy A. Cognitive deficits induced in young rats by long-term corticosterone administration. Behav Neural Biol. 1993;60(2):103–109. doi:10.1016/0163-1047(93)90173-F
73. Stillman MJ, Shukitt-Hale B, Levy A, Lieberman HR. Spatial memory under acute cold and restraint stress. Physiol Behav. 1998;64(5):605–609. doi:10.1016/S0031-9384(98)00091-2
74. Stickgold R, Walker MP. Memory consolidation and reconsolidation: what is the role of sleep? Trends Neurosci. 2005;28(8):408–415. doi:10.1016/j.tins.2005.06.004
75. Born J, Wilhelm I. System consolidation of memory during sleep. Psychol Res. 2012;76(2):192–203. doi:10.1007/s00426-011-0335-6
76. Dudai Y. The restless engram: consolidations never end. Annu Rev Neurosci. 2012;35(1):227–247. doi:10.1146/annurev-neuro-062111-150500
77. McGaugh JL. Memory – a century of consolidation. Science. 2000;287(5451):248–251. doi:10.1126/science.287.5451.248
78. Nader K. Memory traces unbound. Trends Neurosci. 2003;26(2):65–72. doi:10.1016/S0166-2236(02)00042-5
79. Wamsley EJ, Stickgold R. Memory, sleep and dreaming: experiencing consolidation. Sleep Med Clin. 2011;6(1):97–108. doi:10.1016/j.jsmc.2010.12.008
80. Dupret D, O’Neill J, Pleydell-Bouverie B, Csicsvari J. The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat Neurosci. 2010;13(8):995–1002. doi:10.1038/nn.2599
81. Tamminen J, Payne JD, Stickgold R, Wamsley EJ, Gaskell MG. Sleep spindle activity is associated with the integration of new memories and existing knowledge. J Neurosci. 2010;30(43):14356–14360. doi:10.1523/JNEUROSCI.3028-10.2010
82. Dumay N, Gaskell MG. Sleep-associated changes in the mental representation of spoken words. Psychol Sci. 2007;18(1):35–39. doi:10.1111/j.1467-9280.2007.01845.x
83. Payne JD, Schacter DL, Propper RE, et al. The role of sleep in false memory formation. Neurobiol Learn Mem. 2009;92(3):327–334. doi:10.1016/j.nlm.2009.03.007
84. Wagner U, Gais S, Haider H, Verleger R, Born J. Sleep inspires insight. Nature. 2004;427(6972):352–355. doi:10.1038/nature02223
85. Zhang Y, Gruber R. Can slow-wave sleep enhancement improve memory? A review of current approaches and cognitive outcomes. Yale J Biol Med. 2019;92(1):63–80.
86. Smith C. Sleep states and memory processes in humans: procedural versus declarative memory systems. Sleep Med Rev. 2001;5(6):491–506. doi:10.1053/smrv.2001.0164
87. Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271(5257):1870–1873. doi:10.1126/science.271.5257.1870
88. Kudrimoti HS, Barnes CA, McNaughton BL. Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics. J Neurosci. 1999;19(10):4090–4101. doi:10.1523/JNEUROSCI.19-10-04090.1999
89. Peigneux P, Laureys S, Fuchs S, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44(3):535–545. doi:10.1016/j.neuron.2004.10.007
90. Sutherland GR, McNaughton B. Memory trace reactivation in hippocampal and neocortical neuronal ensembles. Curr Opin Neurobiol. 2000;10(2):180–186. doi:10.1016/S0959-4388(00)00079-9
91. Stickgold R. EMDR: a putative neurobiological mechanism of action. J Clin Psychol. 2002;58(1):61–75. doi:10.1002/jclp.1129
92. Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11(2):114–126. doi:10.1038/nrn2762
93. Phillips C. Brain-derived neurotrophic factor, depression, and physical activity: making the neuroplastic connection. Neural Plast. 2017;2017:7260130. doi:10.1155/2017/7260130
94. Tordera RM, Garcia-Garcia AL, Elizalde N, et al. Chronic stress and impaired glutamate function elicit a depressive-like phenotype and common changes in gene expression in the mouse frontal cortex. Eur Neuropsychopharmacol. 2011;21(1):23–32. doi:10.1016/j.euroneuro.2010.06.016
95. Vasquez CE, Riener R, Reynolds E, Britton GB. NMDA receptor dysregulation in chronic state: a possible mechanism underlying depression with BDNF downregulation. Neurochem Int. 2014;79:88–97. doi:10.1016/j.neuint.2014.09.007
96. Wang C, Guo J, Guo R. Effect of XingPiJieYu decoction on spatial learning and memory and cAMP-PKA-CREB-BDNF pathway in rat model of depression through chronic unpredictable stress. BMC Complement Altern Med. 2017;17(1):73. doi:10.1186/s12906-016-1543-9
97. Enogieru AB, Haylett W, Hiss DC, Bardien S, Ekpo OE. Rutin as a potent antioxidant: implications for neurodegenerative disorders. Oxid Med Cell Longev. 2018;2018:1–17. doi:10.1155/2018/6241017
98. Orcic DZ, Mimica-Dukic NM, Franciskovic MM, Petrovic SS, Jovin ED. Antioxidant activity relationship of phenolic compounds in Hypericum perforatum L. Chem Cent J. 2011;5:34. doi:10.1186/1752-153X-5-34.
99. Çirak C, Radusiene J, Janulis V, Ivanauskas L, Camas N, Ayan A. Phenolic constituents of Hypericum triquetrifolium Turra (Guttiferae) growing in Turkey: variation among populations and plant parts. Turk J Biol. 2011;35:449–456.
100. Nassiri-Asl M, Naserpour T, Abbasi E, et al. Effects of rutin on oxidative stress in mice with kainic acid-induced seizure. J Integr Med. 2013;11(5):337–342. doi:10.3736/jintegrmed2013042
101. Sivanantham B, Krishnan U, Rajendiran V. Amelioration of oxidative stress in differentiated neuronal cells by rutin regulated by a concentration switch. Biomed Pharmacother. 2018;108:15–26. doi:10.1016/j.biopha.2018.09.021
102. Song K, Na JY, Kim S, Kwon J. Rutin upregulates neurotrophic factors resulting in attenuation of ethanol-induced oxidative stress in HT22 hippocampal neuronal cells. J Sci Food Agric. 2014;95.
103. Alali F, Tawaha K, El-Elimat T. Determination of hypericin content in Hypericum triquetrifolium Turra (Hypericaceae) growing wild in Jordan. Nat Prod Res. 2004;18(2):147–151. doi:10.1080/14786410310001608046
104. Garcia I, Ballesta S, Gilaberte Y, Rezusta A, Pascual Á. Antimicrobial photodynamic activity of hypericin against methicillin-susceptible and resistant Staphylococcus aureus biofilms. Future Microbiol. 2015;10(3):347–356. doi:10.2217/fmb.14.114
105. Karioti A, Bilia AR. Hypericins as potential leads for new therapeutics. Int J Mol Sci. 2010;11(2):562–594. doi:10.3390/ijms11020562
106. Bannerman DM, Good MA, Butcher SP, Ramsay M, Morris RG. Distinct components of spatial learning revealed by prior training and NMDA receptor blockade. Nature. 1995;378(6553):182–186. doi:10.1038/378182a0
107. Tawaha K, Sadi R, Qa’dan F, Matalka KZ, Nahrstedt A. A bioactive prodelphinidin from Mangifera indica leaf extract. Z Naturforsch C J Biosci. 2010;65(5–6):322–326. doi:10.1515/znc-2010-5-603
108. Kumar V, Mdzinarishvili A, Kiewert C, et al. NMDA receptor-antagonistic properties of hyperforin, a constituent of St. John’s Wort. J Pharmacol Sci. 2006;102(1):47–54. doi:10.1254/jphs.FP0060378
109. Palmer GC. Neuroprotection by NMDA receptor antagonists in a variety of neuropathologies. Curr Drug Targets. 2001;2(3):241–271. doi:10.2174/1389450013348335
110. Mennini T, Gobbi M. The antidepressant mechanism of Hypericum perforatum. Life Sci. 2004;75(9):1021–1027. doi:10.1016/j.lfs.2004.04.005
111. Wei S, Ji XW, Wu CL, et al. Resident intruder paradigm-induced aggression relieves depressive-like behaviors in male rats subjected to chronic mild stress. Med Sci Monit. 2014;20:945–952. doi:10.12659/MSM.890200
112. Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59(12):1116–1127. doi:10.1016/j.biopsych.2006.02.013
113. Russo-Neustadt A, Ha T, Ramirez R, Kesslak JP. Physical activity-antidepressant treatment combination: impact on brain-derived neurotrophic factor and behavior in an animal model. Behav Brain Res. 2001;120(1):87–95. doi:10.1016/S0166-4328(00)00364-8
114. Zhang R, Peng Z, Wang H, et al. Gastrodin ameliorates depressive-like behaviors and up-regulates the expression of BDNF in the hippocampus and hippocampal-derived astrocyte of rats. Neurochem Res. 2014;39(1):172–179. doi:10.1007/s11064-013-1203-0
115. Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64(2):238–258. doi:10.1124/pr.111.005108
116. Conboy L, Tanrikut C, Zoladz PR, et al. The antidepressant agomelatine blocks the adverse effects of stress on memory and enables spatial learning to rapidly increase neural cell adhesion molecule (NCAM) expression in the hippocampus of rats. Int J Neuropsychopharmacol. 2009;12(3):329–341. doi:10.1017/S1461145708009255
117. Yadollah-Damavandi S, Chavoshi-Nejad M, Jangholi E, et al. Topical Hypericum perforatum improves tissue regeneration in full-thickness excisional wounds in diabetic rat model. Evid Based Complement Alternat Med. 2015;2015:245328. doi:10.1155/2015/245328
118. Klemow KM, Bartlow A, Crawford J, Kocher N, Shah J, Ritsick M. Medical attributes of St. John’s wort (Hypericum perforatum). In: Benzie IFF, Wachtel-Galor S, editors. Herbal Medicine: Biomolecular and Clinical Aspects. Boca Raton (FL);2011.
119. Whiskey E, Werneke U, Taylor D. A systematic review and meta-analysis of Hypericum perforatum in depression: a comprehensive clinical review. Int Clin Psychopharmacol. 2001;16(5):239–252. doi:10.1097/00004850-200109000-00001
120. Siepmann M, Krause S, Joraschky P, Muck-Weymann M, Kirch W. The effects of St John’s wort extract on heart rate variability, cognitive function and quantitative EEG: a comparison with amitriptyline and placebo in healthy men. Br J Clin Pharmacol. 2002;54(3):277–282. doi:10.1046/j.1365-2125.2002.01658.x
 © 2020 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
© 2020 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.