Back to Journals » Journal of Experimental Pharmacology » Volume 13
Evaluation of the Diuretic Activity of Aqueous and 80% Methanol Extracts of Croton macrostachyus(Euphorbiaceae) Leaves in Saline-Loaded Rats
Authors Tufer S, Engidawork E , Ayele AG , Bashea C
Received 25 November 2020
Accepted for publication 10 February 2021
Published 1 March 2021 Volume 2021:13 Pages 213—221
DOI https://doi.org/10.2147/JEP.S294062
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bal Lokeshwar
Sara Tufer,1 Ephrem Engidawork,1 Akeberegn Gorems Ayele,1 Chala Bashea2
1Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, College of Health Science, Addis Ababa University, Addis Ababa, Ethiopia; 2Department of Medical Laboratory Sciences, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Akeberegn Gorems Ayele
Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, College of Health Sciences, Addis Ababa University, Zambia Street, PO Box 9086, Addis Ababa, Ethiopia
Tel +251-98-540-8322
Email [email protected]
Background: Croton macrostachyus (Euphorbiaceae) extract is a folk medicine traditionally used for treating a number of disorders, including edematous conditions. The present study aimed to evaluate the diuretic effects of aqueous and 80% methanol leaf extracts of Croton macrostachyus in saline-loaded rats.
Methods: Rats of either sex were randomly assigned into eight groups of eight animals per group. The animals were treated with vehicle (distilled water), standard (furosemide 10 mg/kg), and three doses (100, 200, and 400 mg/kg) of aqueous and 80% methanol leaf extracts after loading of normal saline (15 mL/kg). Then, urine volume, electrolyte concentration, and pH were measured as parameters of evaluation. Concentrations of urinary Na+ K+, and Cl– were determined and Na+:K+ and Cl−:Na+ + K+ ratios calculated to reveal possible mechanisms.
Results: The aqueous extract at 200 mg/kg had produced significant diuresis by hour 3, while the same dose of 80% methanol extract had produced substantial diuresis by the end of hour 4. Both extracts at 400 mg/kg produced significant diuresis from hour 2 to hour 5. In terms of effect on electrolysis, 400 mg/kg aqueous extract produced significant natriuresis, and a kaliuresis effect was observed for both extracts at higher doses and 200 mg/kg aqueous extract.
Conclusion: The findings collectively indicated that both aqueous and 80% methanol extract showed significant diuretic activity, thereby justifying the plant’s traditional use as a diuretic agent.
Keywords: Croton macrostachyus, diuretic activity, electrolyte, leaf, rats
Background
Since ancient times to this date, medicinal plants have been commonly used as a source of treatment for human disorders. Particularly in developing countries, a majority of people depend on herbal medicines to treat various illnesses.1 Diuretic effect is one of the fields of application for botanicals, and herbal medicines are used to treat edematous disorders, such as heart failure, cirrhosis, and nephritic syndrome, that contribute to body-fluid overload.2
Croton macrostachyus (C.macrostachyus) is a well-known medium-sized multipurpose tree that grows up to 30 m, and is in the family Euphorbiaceae, commonly known as the spurge family.3 The genus Croton contains around 1,300 species, and eight of these, including C. macrostachyus, are found in Ethiopia. C. macrostachyus is commonly known as bisana in Amharic,6 tanbuk in Tigrigna,7 and bakanisa in Afaan Oromoo.8
There is tremendous interest in the medicinal uses and pharmacological properties of C. macrostachyus. It has a long role in traditional use for the management of hypertension and edematous conditions worldwide, including Africa, Asia, and South America,4,5 particularly throughout its distributional range in tropical Africa.3 C. macrostachyus is used for the management of different disease ailments. For example, its roots and fruit are used for constipation, diabetes, and malaria in Cameroon.9 In Kenya, a concoction made by boiling its bark is used to treat respiratory disorders.10 The plant has many traditional medicinal uses in various regions of Ethiopia. In the Tigray region, decoction of leaves of the plant is used for urinary retention treatment.7,11 In other areas, stem-bark powder is mixed with milk and given orally to treat heart failure.12
Although C. macrostachyus is well recognized in traditional Ethiopian medicine as having numerous medicinal effects on various ailments, there are no scientific data regarding its diuretic effect to support the claimed ethnomedical use. Therefore, this study aimed to provide scientific evidence for this claim and help in obtaining an alternative diuretic by identifying active compounds that can be used as a potential drug or lead compound through evaluating the diuretic effects of orally administered aqueous and 80% methanol extracts of C. macrostachyus leaves in rats.
Methods
Chemicals and Reagents
Chemicals and solvents used in this study were absolute methanol (Lova Chemie, India), distilled water (Social Pharmacy and Pharmaceutics Laboratory, Addis Ababa University), normal saline (Addis Pharmaceutical Factory, Ethiopia), and furosemide (Epharm, Ethiopia). All chemicals used were of analytical grade.
Plant Material
Fresh leaves of C. macrostachyus were collected in March 2019 from Sebeta, about 25 km southwest of Addis Ababa. The plant was authenticated by a taxonomist, Melaku Wondafrash, and a voucher specimen (ST001) was deposited at the National Herbarium, College of Natural and Computational Sciences, Addis Ababa University for future reference.
Experimental Animals
Healthy Sprague Dawley rats of either sex (age 6–8 weeks, body weight 180–260 g) were used for the experiment. All animals were obtained from the animal house of the School of Pharmacy, Addis Ababa University. The animals were housed in polypropylene cages (eight to ten per cage). They were acclimatized to laboratory conditions for a week, then each animal was placed in an individual metabolic cage (Techniplast, Italy) 24 hours prior to commencement of the actual experiment. Rats were fed a standard pellet diet and water ad libitum. Handlings of the animals was in accordance with internationally accepted guidelines.13,14 The protocol was approved by the ethics review board of the School of Pharmacy (ERB/SOP/11/2//02/2019).
Extraction of the Plant
Leaves of C. macrostachyus were thoroughly washed with tap water to remove dirt and soil, sliced into small pieces manually, and dried under shade. The dried leaves were then crushed using a mortar and pestle to get a fine powder. This was then divided into two portions and subjected to extraction.
Preparation of Aqueous Extract
Decoction was used for extraction. The dried and powdered leaves (200 g) were boiled in 2 L distilled water for 30 minutes, then cooled to room temperature for 15 minutes.15 It was then filtered with muslin cloth, followed by Whatman grade 1 filter paper. The filtrate was then frozen at −20°C and lyophilized (Operon, South Korea) until dried. The dried aqueous extract was then packed in a bottle and stored in a desiccator until use. The calculated percentage yield for the aqueous extract was 11.18% (w:w).
Preparation of 80% Methanol Extract
Maceration was used for extraction. The plant powder (100 g) was macerated with 400 mL 80% methanol for 72 hours. The extract was filtered using Whatman grade1 filter paper. The filtrate was then concentrated under reduced pressure using an R200 Büchi Rotavapor at 40°C. The extract obtained was frozen at −20°C and lyophilized until dried. Then, the dried plant extract was placed in a bottle and stored in a desiccator until use. The calculated yield of the 80% methanol extract was 14.44% (w:w).
Grouping and Dosing of Animals
Rats of either sex were randomly assigned into eight groups of eight animals per group. Negative controls were treated orally with the vehicle used for reconstitution (2 mL/100 g distilled water) and positive controls with the standard drug furosemide at 10 mg/kg (F10). Groups III–V received aqueous extract of plant material and groups VI–VIII 80% methanol extract. In both cases, test doses were 100, 200 and 400 mg/kg. Dose selection was based on data obtained from previous studies.16,17
Determination of Diuretic Activity
Diuretic activity was determined following methods used by earlier studies.18,19 All animals were subjected to fasting overnight with free access to water. They were pretreated with normal saline (0.9% NaCl) at an oral dose of 15 mL/kg to impose a uniform water and salt load. Each group then received furosemide, water, and various doses of the extract. After dosing, rats were placed in metabolic cages (one per cage). Urine was then collected, measured, and pH determined at 1, 2, 3, 4, and 5 hours. Finally, the urine was stored at −20°C for electrolyte analysis. The parameters measured for each rat were total urine volume, urine concentration of Na+, K+, and Cl–, and urine pH. Urinary excretion was calculated as total urinary output divided by total liquid administered (Equation 1). The ratio of urinary excretion in a test group to urinary excretion in the control group was used as a measure of diuretic action of a given dose of an agent (Equation 2). In addition, in order to measure diuretic activity, the diuretic action of the extract was compared to that of furosemide (Equation 3).
Analytical Procedure
Levels of sodium, potassium, and chloride in urine and the plant extract were analyzed. Urinary sodium, potassium, and chloride concentrations were determined using an ion-selective electrode analyzer (AVL 9181; Roche, Germany) at the Ethiopian Public Health Institute. Ratios of electrolytes; — Na+:K+ and Cl−:K+ + Na+ — were calculated. pH was directly determined on fresh urine samples using a pH meter (Mettler Toledo, Columbus, OH, USA). The salt content of the different doses of both extracts was determined to rule out its contribution to urinary electrolyte concentration.
Statistical Analysis
All statistical analyses were performed using SPSS version 25. Statistically significant differences among groups were evaluated by one-way ANOVA followed by Tukey post hoc tests. Results are expressed as means ± SEM. Statistical significance was set at p<0.05.
Results
Diuretic Activity: Effect on Urine Volume
Aqueous Extract
The lower dose (CMAE100) of the extract produced a significant difference in urine volume compared to the negative group at hour 5 (44%, p<0.05). On the other hand, the middle dose (CMAE200) started to produce significant effects from hour 3(51.7%, p<0.05) to hour 5 (77%, p<0.001). The maximum effect was observed with the higher dose (CMAE400), which started showing an effect from hour 2 (128%, p<0.001), which continued to hour 5 (108.54%, p<0.001; Table 1). Comparison among the different doses of the aqueous extract showed that CMAE400 produced significant diuresis compared to lower and middle doses at different time points.
 |
Table 1 Effect of aqueous leaf extract of Croton macrostachyus on 5-hour urine volume in rats |
The diuretic actions of CMAE200 and CMAE400 were 1.77 and 2.03, with urinary excretion of 77.68% and 91.41%, respectively (Table 1). Urinary excretion with the last two doses was higher than the negative control (52.86%).
Methanol Extract
Both 100 mg/kg (CMME100) and 200 mg/kg (CMME200) of the 80% methanol extract produced significant diuresis at hours 4 (p<0.01) and 5 (p<0.05). In contrast, 400 mg/kg of the extract (CMME400) produced significant diuresis from hour 2 (109.26%, p<0.01) onward. There was no statistically significant difference in diuresis when the different doses of 80% methanol extract were compared to one another, with the exception of CMME400, which exhibited significant diuresis compared to CMME100 at hour 5 (p<0.05; Table 2).
 |
Table 2 Effect of 80% methanol leaf extract of Croton macrostachyus on 5-hour urine volume in rats |
The aqueous extract had slightly better diuretic activity than the 80% methanol extract, yet there was no apparent difference between equivalent doses of aqueous and 80% methanol extracts when compared to each other. When different doses were compared, CMAE400 produced significant diuresis at the hours 3 (p<0.05), 4 (p<0.05), and 5 (p<0.05) compared to CMME200 and CMME100. On the other hand, CMME400 significantly increased urine volume at hours 4 (p<0.01) and 5 (p<0.05) compared to CMAE100, while a statistically significant difference in diuresis was observed only at hour 2 (p<0.01) compared to CMAE200 (Table 1).
Saluretic Activity
Aqueous Extract
Electrolyte contents (Na+, K+, and Cl–) from the urine samples over the 5-hour period are presented in Table 3. CMAE400 caused significantly increased sodium (78.8%, p<0.05) and chloride (72.9%, p<0.05) loss compared to the negative control. On the other hand, both CMAE200 (187.5%, p<0.001) and CMAE400 (199.9%, p<0.001) caused increased potassium loss compared to the negative control. F10 produced the maximum sodium excretion (133.5%, p<0.001), significantly greater than the negative control and the first two doses of the extract (CMAE100, p<0.001; CMAE200, p<0.05). A similar pattern was observed for F10 in terms of potassium (157.9%, p<0.01) and chloride (94.6%, p<0.001) excretion when compared to the negative control, though no apparent difference was noted when compared to the middle and higher doses of the extract for chloride excretion. As shown in Table 3, the saluretic indices of F10 for Na+ (2.33) and Cl− (1.95) were slightly higher than CMAE400 (1.79 for Na+ and 1.73 for Cl−). Interestingly, saluretic indices of K+ for CMAE200 (2.87) and CMAE400 (2.99) were somewhat higher than F10 (2.58). Furthermore, Na+:K+ ratios of CMAE100 (0.65), CMAE200 (0.62), and CMAE 400 (0.69) were lower than furosemide (1.10). The carbonic anhydrase–inhibitory activity of CMAE200 and CMAE400 was 0.51 and 0.57, respectively, which appeared to be closer to that of F10 (0.61).
 |
Table 3 Effect of aqueous leaf extract of Croton macrostachyus on 5-hour urinary electrolytes in rats |
Methanol Extract
Electrolyte contents (Na+, K+, and Cl–) from the urine samples over the 5-hour period are presented in Table 4. No apparent differences were observed between CMME100/CMME200 and negative controls in loss of measured electrolytes. On the other hand, CMME400 produced significant K+ (193.1%, p<0.001) and Cl– (67.2%, p<0.05) loss, with a lesser effect on Na+ excretion. In contrast, F10 caused a significant loss of all ions compared to the negative control. Saluretic indices of F10 for Na+ (2.33) and Cl– (1.95) were higher than all three doses of 80% methanol extract, while the K+ index for CMME400 (2.93) was slightly higher than F10 (2.58). F10 and CMME400 had identical carbonic anhydrase–inhibitory activity (0.61).
 |
Table 4 Effect of 80% methanol leaf extract of Croton macrostachyus on 5-hour urinary electrolyte in rats |
Higher doses of the aqueous and 80% methanol extracts exhibited a relatively comparable effect on urinary electrolyte excretion. CMAE400 (p<0.05) produced a significant difference in chloride excretion when compared to CMME100 and CMME200.
Electrolyte Content of Extracts
Water-soluble salts can present in extracts and consequently interfere with the urinary excretion of electrolytes. In order to rule out this possibility, Na+, K+, and Cl– content in both extracts was determined. There were detectable levels of the three ions in both extracts. However, the K+ level was higher than the other two ions at different doses of both extracts. K+ content (from the lowest to highest dose) was found to be 59.9, 90.2, and 171.1 mmol/L for aqueous and 33.2, 71.6, and 132.2 mmol/L for 80% methanol extracts of C. macrostachyus.
Urinary pH
Urinary pH measurement revealed that both aqueous and 80% methanol leaf extracts produced comparatively alkaline urine (Figure 1). CMAE100 (p<0.01), CMAE200 (p<0.001), and CMAE 400 (p<0.01) produced significantly higher pH than the negative control. The same was true of 80% methanol extract. Furthermore, furosemide (7.92) produced alkaline urine intermediate between the treatment and control groups (7.41).
Discussion
Diuretics are mainly used to adjust the volume and composition of body fluids in a variety of disorders, including hypertension, congestive heart failure, hepatic cirrhosis, and nephrotic syndrome.20,21 This adjustment is mainly achieved through inhibition of reabsorption of water and electrolytes across tubular epithelial cells into the bloodstream.22 In this study, urine volume and electrolyte concentrations were measured to evaluate the diuretic activity of the leaf of C. macrostachyus. Regarding toxicity of the experimental plant, previous acute-toxicity studies have shown that aqueous and 80% methanol extracts of the plant are safe at doses of 2 g and 5 g/kg, suggesting that the LD50 of the extract is >5 g/kg.16,17
Several medicinal plants have been proven to improve conditions of volume overload resulting from retention of electrolytes and water, with better safety profiles.23,24 Therefore, it is very necessary to demonstrate effectiveness of plant extracts in the presence of electrolytes and water,25 and so normal saline was loaded to simulate edema.
The present study reports the aquaretic and pronounced kaliuretic effects of aqueous and 80% methanol leaf extracts of C. macrostachyus. With regard to urine output, both extracts resulted in an increase in urine excretion in a dose-dependent manner. The effect turned out to be more significant at higher doses tested compared to the negative control, possibly due to increased concentration of active components. Compared to the 80% methanol extract, the aqueous extract produced slightly better diuresis. The slight increment in diuresis observed with the aqueous extract could possibly be explained by polar ingredients in the plant material, which may be responsible for increasing urine output. On the other hand, the medium dose of the aqueous extract was able to produce apparent effects from hour 3, while the same dose of the 80% methanol extract produced effects from hour 4.However, both extracts at lower doses did not produce any appreciable effect, probably owing to insufficient active components responsible for induction of diuresis.
In terms of onset of action, delayed onset of diuresis was observed for both extracts when compared to furosemide, which induced rapid and significant diuresis within 60 minutes of administration. This was expected, as extracts are crude and no match for pure substances like furosemide. In addition, slightly better values were obtained for the aqueous than the 80% methanol extract regarding diuretic action and activity. The higher doses of both extracts resulted mild diuretic activity: 0.91 and 0.87 for CMAE400 and CMME400, respectively. Diuretic activity is considered good if it is >1.50, moderate if 1.00–1.50, mild if 0.72–0.99, and nil if<0.72.19
In view of electrolyte composition of urine, patterns of effect on ion excretion of the aqueous and 80% methanol extracts were a bit different. The higher dose of aqueous extract produced significant increases in urinary excretion of all ions compared to the negative control, but a similar dose of the 80% methanol extract promoted less Na+ excretion with notable effects on K+ and Cl– levels. Urinary excretion of Na+, K+, and Cl– was not elevated significantly at the lower and middle doses of aqueous and 80% methanol extract, with the exception of CMME200 which significantly increased urinary K+ excretion. The excessive K+ excretion observed in this study might have been due to high K+ concentration in the extracts.
The of Na+:K+ ratio was calculated for natriuretic activity. Values >2 indicate a favorable natriuretic effect, whereas ratios >10 indicate a potassium-sparing effect.26 Increased Na+:K+ implies more Na+ excretion than K+, which is regarded a very good profile for diuretic agents. However, neither aqueous nor 80% methanol extracts increased the Na+:K+ ratio, indicating that the plant has low natriuretic potential but a pronounced kaliuretic effect. The observed K+-wasting effects of the extracts may not be enough to comment on whether the plant has a potassium-sparing effect or not, since when there is an increase in potassium intake, there will be an increase in its excretion as well.27 When potassium overloading occurs, the kidney tubules are unable to absorb it and consequently produce urinary excretion of the osmotic type, which promotes water diuresis.28
It was evident that both leaf extracts had the potential to augment the volume of urine; however, they did not have much effect on electrolyte excretion, as seen from the saliuretic indices of the extracts. Therefore, it is possible that the diuretic effect of the extracts of C. macrostachyus is more of an aquaretic than saluretic type.29 Aquaretics are believed to work by enhancing glomerular filtration rate via increasing renal blood flow.30
The Cl−:Na+ + K+ ratio is used to estimate carbonic anhydrase inhibition. Carbonic anhydrase inhibition can be excluded for ratios of 0.8–1. With decreasing ratios, slight to strong carbonic anhydrase inhibition can be assumed.26 CMAE200 and CMAE400 had carbonic anhydrase indices of 0.51 and 0.57, respectively. Likewise, the higher dose of the 80% methanol extract had a carbonic anhydrase index of 0.61. Therefore, it is possible to presume that these extracts act by inhibiting carbonic anhydrase enzymes in the renal tubule. These findings, together with the significant increment in urinary pH values compared to controls, strengthen the notion that carbonic anhydrase inhibition as one of the possible mechanisms of action of the plant.
With regard to the patterns of urine output and excretion of electrolytes (K+, Na+, and Cl–), together with the diverse bioactive principles present in the crude aqueous and 80% methanol extracts, it appears that the plant may have mechanisms of action similar to some herbal medications suggested to have a wide range of diuretic mechanisms.31,32 As such, in addition to the suggested carbonic anhydrase–inhibitory activity, osmosis-like effects and increased glomerular filtration rate might be another mode of action that contributes to the diuretic effect of the plant.
The exact nature of the active principle responsible for the observed activities of C. macrostachyus extracts is not well known, but it is may be that secondary metabolites are responsible for this action. For example, saponins, tannins, and terpenoids are responsible for diuretic activity by exerting favorable effects on physiological processes of the kidney, such as increasing potassium-sparing capacity, blockade of adenosine A1 receptor or possibly by inhibiting tubular reabsorption of water and accompanying anions.30 Flavonoids and saponins are presumed to be responsible for the observed diuretic effect of the plant via promoting vasodilatation in the afferent arterioles of the renal vasculature, thereby increasing the rate of glomerular filtration, which in turn promotes increased urine formation.33,34
Phytochemical screening of the aqueous extract of the current medicinal plant revealed the presence of alkaloids, steroids, terpenoids, saponins, tannins, and flavonoids,35 whereas 80% methanol extract constitutes tannins and anthocyanins.36 The slight superiority of aqueous extracts over 80% methanol extract of C. macrostachyus might be due to the presence of more water-soluble active phytoconstituents responsible for diuretic activity.
Conclusion
Collectively, the results of this study revealed that that both aqueous and 80% methanol extracts of C. macrostachyus possessed significant diuretic activity. Specifically, the larger doses of both extracts produced notable diuresis. Urinary pH and electrolyte analysis showed that the extracts have many modes of action. This study thus substantiates this plant’s traditional claim as a diuretic agent.
Data Sharing Statement
The data sets used for this study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The protocol was approved by the institutional review board of the School of Pharmacy, College of Health Sciences, Addis Ababa University (ERB/SOP/11/2//02/2019).
Acknowledgments
The authors would like to thank Addis Ababa University for financial support for this study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, took part in drafting the article or revising it critically for important intellectual content, agreed to submit to the current journal, gave final approval to the version to be published, and agree to be accountable for all aspects of the work.
Funding
The study was done with funding obtained from Addis Ababa University for MSc dissertation research. The role of the funder was providing animals used in the experiment and financial support for purchasing materials and chemicals.
Disclosure
The authors declare that they have no financial and/or nonfinancial competing interests.
References
1. Zhang X, Organization WH. Traditional medicine strategy 2002 2005; 2002.
2. Vazir A, Cowie MR The use of diuretics in acute heart failure: evidence based therapy? 2013.
3. Maroyi A. Ethnopharmacological uses, phytochemistry, and pharmacological properties of Croton macrostachyus Hochst. Ex Delile: a comprehensive review. Evid Based Compl Alt Med. 2017;2017.
4. Salatino A, Salatino MLF, Negri G. Traditional uses, chemistry and pharmacology of Croton species (Euphorbiaceae). J Braz Chem Soc. 2007;18(1):11–33. doi:10.1590/S0103-50532007000100002
5. Getu A. Ethnobotanical profile of Croton macrostachyus (Euphorbiaceae) in Ethiopia: review of the literature. Int J Res Pharm Pharm Sci. 2018;3(1):209–222.
6. Teklehaymanot T, Giday M. Ethnobotanical study of medicinal plants used by people in Zegie Peninsula, Northwestern Ethiopia. J Ethnobiol Ethnomed. 2007;3(1):12. doi:10.1186/1746-4269-3-12
7. Araya S, Abera B, Giday M. Study of plants traditionally used in public and animal health management in Seharti Samre District, Southern Tigray, Ethiopia. J Ethnobiol Ethnomed. 2015;11(1):22. doi:10.1186/s13002-015-0015-5
8. Suleman S, Alemu T. A survey on utilization of ethnomedicinal plants in Nekemte town, East Wellega (Oromia), Ethiopia. J Herbs Spices Med Plants. 2012;18(1):34–57. doi:10.1080/10496475.2011.645188
9. Mbiantcha M, Nguelefack T, Ndontsa B, Tane P, Kamanyi A. Preliminary assessment of toxicity of Croton macrostachyus stem bark (Euphorbiaceae) extracts. Int J Pharm Chem Biol Sci. 2013;3:2–113.
10. Kigen G, Kipkore W, Wanjohi B, Haruki B, Kemboi J. Medicinal plants used by traditional healers in Sangurur, Elgeyo Marakwet County, Kenya. Pharmacogn Res. 2017;9(4):333. doi:10.4103/pr.pr_42_17
11. Yirga G. Use of traditional medicinal plants by indigenous people in Mekele town, capital city of Tigray regional state of Ethiopia. J Med Plants Res. 2010;4(17):1799–1804.
12. Enyew A, Asfaw Z, Kelbessa E, Nagappan R. Ethnobotanical study of traditional medicinal plants in and around Fiche District, Central Ethiopia. Current Res J Biol Sci. 2014;6(4):154–167. doi:10.19026/crjbs.6.5515
13. OECD-425. Organization for Economic Cooperation and Development Guidelines for the Testing of chemicals.Acute Oral Toxicity-Up and Down Procedure.
14. Council NR. Guide for the Care and Use of Laboratory Animals. Washington, DC: The National Academies Press; 2011.
15. Evans WC. Trease and Evans Pharmacognosy, International Edition E-Book. Elsevier Health Sciences; 2009.
16. Degu A, Engidawork E, Shibeshi W. Evaluation of the anti-diarrheal activity of the leaf extract of Croton macrostachyus Hocsht. ex Del. (Euphorbiaceae) in mice model. BMC Compl Altern Med. 2016;16(1):379. doi:10.1186/s12906-016-1357-9
17. Bantie L, Assefa S, Teklehaimanot T, Engidawork E. In vivo antimalarial activity of the crude leaf extract and solvent fractions of Croton macrostachyus Hocsht. (Euphorbiaceae) against Plasmodium berghei in mice. BMC Complement Altern Med. 2014;14(1):79. doi:10.1186/1472-6882-14-79
18. Lahlou S, Tahraoui A, Israili Z, Lyoussi B. Diuretic activity of the aqueous extracts of Carum carvi and Tanacetum vulgare in normal rats. J Ethnopharmacol. 2007;110(3):458–463. doi:10.1016/j.jep.2006.10.005
19. Hailu W, Engidawork E. Evaluation of the diuretic activity of the aqueous and 80% methanol extracts of Ajuga remota Benth (Lamiaceae) leaves in mice. BMC Compl Altern Med. 2014;14(1):135. doi:10.1186/1472-6882-14-135
20. Roush GC, Kaur R, Ernst ME. Diuretics: a review and update. J Cardiovasc Pharmacol Ther. 2014;19(1):5–13. doi:10.1177/1074248413497257
21. Hullatti K, Manjunatha J, Kuppasth I. Comparative Study on Diuretic Effect of Buchanania angustifolia Roxb., and Buchanania lanzan Spreng. Fruit Extracts and Fractions. J Appl Pharm Sci. 2014;4(8):59.
22. Wright C, Van-buren L, Kroner C, Koning M. Herbal medicines as diuretics: a review of the scientific evidence. J Ethnopharmacol. 2007;114(1):1–31. doi:10.1016/j.jep.2007.07.023
23. Ahmad W, Zeenat F, Ahmad M, Ansari N. Medicinal plants as potent diuretic: a review. Int J Adv Pharm Med Bioallied Sci. 2017;2017(2017):1–8.
24. Dutta KN, Chetia P, Lahkar S, Das S. Herbal plants used as diuretics: a comprehensive review. J Pharm Chem Biol Sci. 2014;2(1):27–32.
25. Nedi T, Mekonnen N, Urga K. Diuretic effect of the crude extracts of Carissa edulis in rats. J Ethnopharmacol. 2004;95(1):57–61. doi:10.1016/j.jep.2004.06.017
26. Krishnakanth K, Kumar P, Neeraja K, Cheekavolu C. Effect of Sesbania grandiflora Linn leaf extracts on diuresis in wistar rats. Int J Basic Clin Pharmacol. 2017;6(6):1305. doi:10.18203/2319-2003.ijbcp20172081
27. Haji H, Makonnen E, Debella A, Geleta B. Evaluation of diuretic and antihypertensive activity of leaf extracts of Thymus schimperi in rats. British J Pharmacol Toxicol. 2016;7(1):1–8. doi:10.19026/bjpt.7.2779
28. Ramin S, David P, E H. Diuretic Agents Katzung, Basic and Clinical Pharmacology.
29. Al-Saikhan FI, Ansari MN. Evaluation of the diuretic and urinary electrolyte effects of methanolic extract of Peganum harmala L. in Wistar albino rats. Saudi J Biol Sci. 2016;23(6):749–753. doi:10.1016/j.sjbs.2016.01.025
30. Kebamo S, Makonnen E, Debella A, Geleta B. Evaluation of diuretic activity of different solvent fractions of methanol extract of Carissa edulis root bark in rats. Med Chem. 2015;5:472–478. doi:10.4172/2161-0444.1000304
31. Jayakody J, Ratnasooriya W, Fernando W, Weerasekera K. Diuretic activity of leaves extract of hot water infusion of ruta graveolens L. in rats. JPT. 2011;6(5):525–532.
32. Hullatti K, Gopikrishna U, Kuppast I. Phytochemical investigation and diuretic activity of Cyclea peltata leaf extracts. J Adv Pharm Technol Res. 2011;2(4):241. doi:10.4103/2231-4040.90880
33. Getahun M, Engidawork E. Evaluation of the diuretic activity and phytochemical screening of different solvent fractions of the rhizomes of rumex abyssinicus Jacq (Polygonaceae) in rats. Ethiopian Pharm J. 2015;31(1):35–48. doi:10.4314/epj.v31i1.4
34. Abdala S, Martin-Herrera D, Benjumea D, Perez-Paz P. Diuretic activity of smilax canariensis, an endemic canary Island species. J Ethnopharmacol. 2008;119(1):12–16. doi:10.1016/j.jep.2008.05.025
35. Arika W, Abdirahman Y, Mawia M, et al. In vivo antidiabetic activity of the aqueous leaf extract of Croton macrostachyus in alloxan induced diabetic mice. Pharm Anal Acta. 2015;6(11):1–5.
36. Sd CR, Fokom R, Az PH. Antifungal and antioxidant activity of crude extracts of three medicinal plants from Cameroon pharmacopeia. J Med Plants Res. 2013;7(21):1537–1542.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

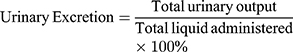
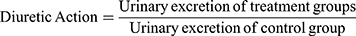
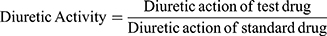

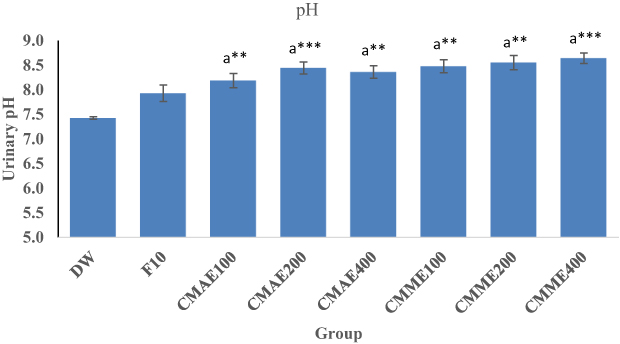 Notes: Each value represents mean ± SEM (n=8), Analysis was performed by one-way ANOVA. aCompared to negative control; **p<0.01; ***p<0.001. Numbers following F, CMAE, and CMME indicate dose/kg.Abbreviations: DW, distilled water; F, furosemide; CMME, Croton macrostachyus methanol extract; CMAE, Croton macrostachyus aqueous extract.
Notes: Each value represents mean ± SEM (n=8), Analysis was performed by one-way ANOVA. aCompared to negative control; **p<0.01; ***p<0.001. Numbers following F, CMAE, and CMME indicate dose/kg.Abbreviations: DW, distilled water; F, furosemide; CMME, Croton macrostachyus methanol extract; CMAE, Croton macrostachyus aqueous extract.