Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Evaluation of the Characteristics of Asthma in Severe and Extremely Severe COPD
Authors Chen F, Lin G, Huang X , Liu Y, Zeng Z, Guo Y
Received 29 July 2019
Accepted for publication 16 November 2019
Published 3 December 2019 Volume 2019:14 Pages 2663—2671
DOI https://doi.org/10.2147/COPD.S225258
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chunxue Bai
Feng-jia Chen,* Geng-peng Lin,* Xin-yan Huang, Yang-li Liu, Zhi-min Zeng, Yu-biao Guo
Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yu-biao Guo
Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Sun Yat-Sen University, Institute of Respiratory Diseases of Sun Yat-Sen University, No. 58 Zhongshan 2nd Road, Guangzhou 510080, Guangdong, People’s Republic of China
Tel +86 20 8775 5766
Email [email protected]
Background: Biotherapy for asthma may be useful in patients suffering from chronic obstructive pulmonary disease (COPD) with asthma characteristics. Therefore, the evaluation and close monitoring of asthma characteristics in severe and extremely severe COPD can guide treatment decisions to improve prognosis.
Methods: Stable patients suffering from COPD and having a forced expiratory volume in 1 s (FEV1%) of ≤50% (GOLD 3–4) in the First Affiliated Hospital of Sun Yat-Sen University from December 2014 to June 2018 were retrospectively enrolled in this study and evaluated in terms of their asthma characteristics (blood eosinophil counts, fractional exhaled NO [FeNO] values, and reversibility).
Results: A total of 178 patients with an average age of 65.62±9.28 years were enrolled in this study. A total of 85 patients had an improvement of ≥12% in FEV1%, and 61 of these patients had an absolute increase of >200 mL. Of 122 patients, 68 had blood eosinophil counts of ≥150 cells/μl, whereas 27 showed blood eosinophil counts ≥300 cells/μl. The blood eosinophil of ≥2% was found in 66/122 (54.10%) patients, whereas ≥3% was found in 51/122 (41.80%) patients. A total of 46 of 58 patients had an increased serum IgE level of ≥30 IU/mL, and 32 patients had an IgE of ≥100 IU/mL. The FeNO value of ≥25 ACO (ppb) was found in 51/155 (32.90%) patients. Furthermore, 43 patients had asthma–COPD overlap (ACO), and the FeNO values in the ACO group was 26.13±14.91 ppb, which was significantly higher than that in the COPD alone group (20.99±9.16 ppb; P=0.016). A total of 12 patients with ACO had a negative response after bronchodilation. In the COPD alone group, 34 patients had an absolute increase of >200 mL, whereas 55 of the 95 patients had blood eosinophil counts of ≥150 cells/μl. The blood eosinophilia of ≥2% was found in 54/95 (56.84%) patients. A total of 36 of 45 patients had an increased serum IgE level of ≥30 IU/mL. The FeNO value of 34/123 (27.64%) patients was ≥25 ppb.
Conclusion: The characteristics of asthma are common findings in patients with severe and extremely severe COPD. Biomarkers should be actively used to evaluate the characteristics of asthma in these patients. If the characteristics of asthma exist, then anti-IgE or anti-IL-5 therapy should be considered to reduce exacerbation.
Keywords: chronic obstructive pulmonary disease, asthma–COPD overlap, fractional exhaled nitric oxide, blood eosinophil counts, bronchodilator reversibility
Introduction
According to 2019 GOLD guidelines, chronic obstructive pulmonary disease (COPD) is a preventable and treatable disease characterized by a persistent and progressive airflow limitation.1 COPD is a heterogeneous disease, and this characteristic has led to the development of classifications based on the following clinical phenotypes: chronic bronchitis, emphysema, and mixed pattern or asthma–COPD overlap (ACO).1,2 The characteristics of asthma in patients with COPD has been gaining increasing attention. These patients are associated with a poor quality of life,3 a rapid decline in pulmonary function,4 a high risk of exacerbation,5,6 and a high economic burden.7 They may benefit from targeted therapy or inhaled corticosteroid treatment compared with patients with COPD without any asthma characteristics.8
ACO is a distinct clinical phenotype that represents a subset of patients who have COPD and concomitant asthma with a prevalence ranging from 2.1% to 55% depending on different diagnostic criteria.9 ACO should be suspected in patients with COPD based on symptoms such as wheezing, paroxysmal dyspnea, and airway reversibility. In actual clinical practice, patients with COPD present with eosinophilic airway inflammation without asthma characteristics, while some patients present with asthma characteristics without meeting the ACO criteria.10 About 60% of patients with COPD may have bronchial hyperresponsiveness or reversibility without asthma.11 Patients who have ACO or COPD with asthma characteristics should be treated intensively because they predict a higher exacerbation in future.12 GINA/GOLD documents on ACO recommend that fractional exhaled NO (FeNO) and blood eosinophil counts can be used as inflammatory biomarkers in differentiating ACO from COPD.12 FeNO value does not differ significantly between patients with COPD and healthy volunteers,13 whereas FeNO value increases in a subgroup of patients who have COPD and share several common characteristics with asthma.14 Taking 25 ppb as a cut-off value to differentiate ACO from COPD, FeNO indicated a sensitivity of 60.6% and a specificity of 87.7%.15 COPD is an inflammatory disease characterized by a predominant neutrophilic inflammation.1 However, when the peripheral blood eosinophil counts of 150 cells/μl is used as the cut-off value, the eosinophilic phenotype is found in up to 40% of patients who have COPD but have no history of asthma.16 High blood eosinophilia is one of the asthma characteristics associated with a high risk of COPD exacerbation.17 Therefore, in clinical practice, blood eosinophil counts and FeNO values can be used as biomarkers to evaluate asthmatic features in COPD patients.18
FeNO is related to interleukin (IL)-4 and IL-13-mediated inflammatory pathways, while blood eosinophils are related to IL-5-mediated inflammatory pathways, respectively; target-specific inflammatory pathways are the bases of biotherapy in patients with COPD.19–21 Treatments targeting eosinophilic airway inflammation are the most promising for COPD. IL-5 is an important cytokine in the CD4-T helper2 (Th2) pathway and implicated in the function and survival of eosinophils.22 Clinical trials have shown that mepolizumab, which is a monoclonal antibody against IL-5, can reduce the exacerbation rate of COPD by inhibiting blood eosinophilia.23 Omalizumab, which is an IgE antibody, has shown effective treatment results in patients with severe allergic asthma and COPD overlap because of the effect of binding to serum IgE.24 FeNO is the most important biomarker in anti-IgE treatment.19
Data about the characteristics of asthma in patients with COPD, especially severe and extremely severe groups, are lacking because most clinical trials on COPD exclude patients with asthma characteristics. Therefore, differentiating asthma characteristics in patients with severe and extremely severe COPD is clinically urgent because these patients are likely to have severe exacerbations that may lead to death. The close monitoring of asthma characteristics of patients with COPD can guide treatment decisions to improve prognosis.
Patients and Methods
Patient Population
Patients aged > 40 years and suffering from severe and extremely severe COPD in the First Affiliated Hospital of Sun Yat-Sen University from December 2014 to June 2018 were retrospectively enrolled in this study in accordance with the following criteria: (1) stable patients with COPD having forced expiratory volume in 1 s (FEV1%) ≤ 50% (GOLD 3–4; stability was defined as no exacerbation in the least 4 weeks); (2) patients underwent bronchodilator reversibility (BDR), blood, and FeNO tests without receiving treatment of any oral or inhaled corticosteroid and bronchodilator therapy within 72 h; and (3) nonsmokers or ex-smokers with smoking cessation for at least 6 months prior to BDR and FeNO tests. Exclusion criteria included patients with classes III and IV congestive heart failure, history of lung resection, gastroesophageal reflux disease, Parkinson’s disease, dementia, and immunodeficiency diseases. In our study, ACO is defined as follows: patients with COPD, a history of asthma, and persistent partially reversible airflow limitation.1,9
The study was approved by the Institutional Research Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University. Patients data were maintained with confidentiality or anonymized in accordance with the Declaration of Helsinki. The institutional review board waived the requirement for approval and signed informed consent form because this study was retrospective in nature and not an intervening trial.
Collection procedure and data analysis
Medical history (particularly atopy or asthma and allergy history), family history, patients’ characteristics, and data (including height, weight, body surface area, smoking history, years of tobacco use, current medication information, FeNO values, blood tests, and pulmonary function test [PFT] data), were obtained from the patients’ medical charts written by physicians.
Measurements
PFT
PFT was performed through body plethysmography in accordance with the 2014 recommendations of the Chinese National Guidelines of PFT.25 The percentage of the predicted values was calculated on the basis of healthy Chinese adults in accordance with previously determined reference values. All the subjects were required to take a PFT in a reproducible manner by a trained technician, and the best values from the acceptable tests of each subject were selected. The forced vital capacity (FVC), FEV1%, FEV1/FVC, forced expiratory flow at 25%, 50%, and 75% of FVC (MEF25%–75%), maximum forced peak expiratory flow, vital capacity, and diffusion capacity of the lung for CO (DLCO) were measured.
BDR
BDR test should be performed between 15 and 20 min after patients receive 400 μg of inhalations of a salbutamol-metered dose inhaler with a spacer. Positive response was defined as an improvement of ≥12% in FEV1 and an increase in ≥200 mL.25
FeNO Measurement
FeNO values were measured using an electrochemical analyzer (NIOX MINO Analytical Instruments, Aerocrine AB, Solna, Sweden) in accordance with the American Thoracic Society/European Respiratory Society recommendations. The participants were instructed to inhale air without NO to the total lung capacity (TLC) and immediately exhale fully into the device at a constant expiratory flow rate of 50 mL/s for 10 s.26
The cut-off points of FeNO for clinical applications as recommended by An Official ATS Clinical Practice Guideline are as follows: values more than 50 ppb indicate a high probability of eosinophilic inflammation, values less than 25 ppb denote a low probability of eosinophilic inflammation, and values between 25 and 50 ppb correspond to an intermediate probability of eosinophilic inflammation.26
Statistical Analysis
Data were analyzed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). Results were shown as median (interquartile range) for quantitative or non-normal variables when appropriate, mean±standard deviation (SD), or number (%) of patients. Differences between groups were assessed with Student’s t-test for normally distributed data and Mann–Whitney U-test for non-normally distributed data. Categorical variables were compared via a chi-squared test. The relationships between FeNO levels and IgE and between blood eosinophil counts and percentage were assessed by determining Spearman’s rank correlation coefficients. Statistical significance was defined as P<0.05.
Results
Study Subject Characteristics
A total of 178 patients who were in the stable phase of COPD were enrolled in this study. The majority of the patients were males (80%), the average age was 65.62±9.28 years, and the mean FEV1% as 35.86%±9.53%. This patient group had increased (TLC) and residual volume (RV) of 104.98±21.77% and 181.02%±51.61%, respectively. DLCO also decreased, and many patients in this group could not be examined because of their severely reduced vital capacity. The FeNO value was 22.05±10.75 ppb. The clinical characteristics and PFT data of the study population are shown in Table 1.
 |
Table 1 Clinical Characteristics and PFT Data |
Asthma Characteristics
In our study, 43 patients had a history of asthma. 21 patients had atopy and allergy history, among these patients 18 cases had a definite history of asthma. 85 patients had an improvement of ≥12% in FEV1 after they used 400 μg of inhalations of salbutamol. However, 61 of these patients had an absolute increase of >200 mL, and 9 patients had an increase of >400 mL. Among the 68 patients from 122 patients with blood eosinophil counts of ≥150 cells/μl, 27 showed blood eosinophil counts of ≥300 cells/μl. The blood eosinophilia of 66 of the 122 patients (54.10%) was ≥2%, the blood eosinophilia of 51 of the 122 patients (41.80%) was ≥3%. A total of 46 of the 58 patients had an increased serum IgE level of ≥30 IU/mL, whereas 32 patients had an IgE level of ≥100 IU/mL. The FeNO value of 51 of the 155 patients (32.90%) was ≥25 ppb, and 4 of these 155 patients showed the FeNO value of ≥50 ppb (Table 2).
 |
Table 2 Asthma Characteristics in Patients with Severe and Extremely Severe COPD |
According to the diagnostic criterion, 43 patients who suffered from COPD and had a history of asthma were defined ACO. The FeNO value in the ACO group was 26.13±14.91 ppb, which was significantly higher than that of the COPD alone group (20.99±9.16 ppb, P=0.016), but no significant differences existed in the absolute blood eosinophils and PFT values between these groups (Table 3).
 |
Table 3 Asthma Characteristics in Patients with Asthma–COPD Overlap (ACO) and COPD Alone |
 |
Table 4 Asthma Characteristics in Patients with Severe and Extremely Severe COPD |
In the ACO group, 12 patients had a negative response after bronchodilation and 31 patients had an improvement of ≥12% in FEV1. Of these 31 patients, 27 had an absolute increase of >200 mL, 6 had an increase of >400 mL. 11 of 27 patients were observed to have blood eosinophi counts of ≥150 cells/μl and 6 had ≥300 cells/μl. Blood eosinophilia of ≥2% and ≥3% were found in 12/27 (44.44%) and 10/27 (37.03%) patients, respectively. Moreover, 10 of the 13 patients had a raised serum IgE level of ≥30 IU/mL, whereas 6 patients had an IgE level of ≥100 IU/mL. The FeNO value of ≥25 ppb was found in 17/32 (53.12%) patients, and only 3 of these patients showed FeNO values of ≥50 ppb (Table 3).
In the COPD group, 54 patients had an improvement of ≥12% in FEV1. Of these patients, 34 had an absolute increase of >200 mL and 3 had an increase of >400 mL. A total of 55 of 95 patients had blood eosinophil counts of ≥150 cells/μl, whereas 21 patients had blood eosinophil counts of ≥300 cells/μl. Furthermore, 54/95 (56.84%) and 41/95 (43.16%) patients had blood eosinophilia of ≥2% and ≥3%, respectively. In addition, 36 of the 45 patients had an increased serum IgE level of ≥30 IU/mL, whereas 26 patients had ≥100 IU/mL. Moreover, 34/123 (27.64%) had a FeNO value of ≥25 ppb, and only 1 of these patients showed FeNO value of ≥50 ppb (Table 3).
According to the severity of airway obstruction, the patients were divided into severe (30% ≤ FEV1 < 50%) and extremely severe (FEV1 < 30%) cases with COPD. The differences in FeNO values, bronchodilator reversibility, serum IgE, and blood eosinophil counts with regard to the severity of airway obstruction were not significant (Table 4).
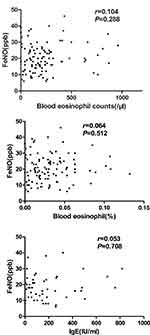
The correlations between FeNO values and blood eosinophil counts in patients with severe and extremely severe COPD were not significant (Figure 1).
In this retrospective study, nine patients were reviewed for PFT and FeNO measurements 3 months after treatment (ICS+LABA+LAMA). Most patients showed an improved lung function and a decreased FeNO, but the differences were statistically not significant. Figure 2 presents the FeNO values and FEV1 before and after treatment during follow-up.
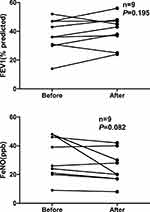 |
Figure 2 FeNO values and FEV1 during follow-up. Abbreviation: FeNO, fractional exhaled NO. |
Discussion
We performed a retrospective study to observe the asthmatic characteristics in stable patients with COPD and having FEV1% ≤ 50% (GOLD 3–4). TLC, RV and RV/TLC significantly increased, and DLCO decreased significantly. The patients in this category were at a high risk of acute exacerbations, had complex conditions and history, and required detailed evaluation and treatment even during the stable phase.
The main findings of our study included the following: FeNO was important for phenotype assessment with significantly higher levels detected in the ACO phenotype than in COPD alone group. In COPD, CD8+ lymphocytes, macrophages, and neutrophils are predominant.27 Therefore, the FeNO values and blood eosinophil counts did not increase in our cohort. Several patients with a mixed phenotype could present the characteristics of asthma with predominant eosinophils and TH2 lymphocytes, which could be measured indirectly with FeNO produced by epithelial cells in response to ILs, such as IL-4 and IL-13.20,21 Few studies have assessed the FeNO value in patients suffering from COPD and having severe and extremely severe airflow obstruction. Patients with ACO have higher rates of exacerbation and poorer quality of life than those with COPD alone.5,6 In patients with severe COPD, a large proportion had frequent exacerbations despite the intensive inhaled bronchodilator therapy, systemic corticosteroids, or additional treatments. Therefore, patients with severe and extremely severe COPD combined with asthma must be closely monitored and treated. Our study confirmed the potential role of FeNO as a biomarker in identifying asthma in groups with severe and extremely severe COPD. The PFT values did not significantly differ between the COPD alone and ACO groups (P>0.05).
We divided the patients into two groups: ACO and COPD alone. Of the 43 patients with ACO, 27 (62.79%) had true-positive BDR reversibility (improvement of ≥12% in FEV1% and an increase of >200 mL). Some patients with ACO had severe airflow limitation as FEV1% increased by 12%, but the absolute value hardly exceeded 200 mL. In 12 patients with long-term asthma, airflow was completely irreversible. We conclude that patients with ACO after long-term treatment with dual or triple inhalation, the degree of airway reversibility is reduced.28 Previous study has suggested that approximately 60% of patients with COPD could have airway hyperresponsiveness or reversibility without asthma.11 For the COPD alone group, 34 patients had significant bronchodilator reversibility, with an improvement of ≥12% in FEV1% and an increase of >200 mL without asthma history. These values were lower than those reported by Sandhya Matthes18 because we enrolled patients with extremely severe obstruction and achieved 200 mL of BDR. Hence, reversibility is a distinguishing characteristic between asthma and COPD to some extent, but it is not the most important factor in distinguishing ACO in patients with severe and extremely severe COPD.
Eosinophils can play a critical role in 10–40% of patients with COPD.29 Other markers, such as eosinophils in peripheral blood, may be significant and easily determined. Eosinophils are cells that generally exist in small percentages in the peripheral blood and have considerable variability.27,30 In our study, blood eosinophil counts were acquired from 122 of the 178 enrolled patients. A total of 54.10% of the patients had levels higher than 2%, and 55.74% patients had blood eosinophil counting of ≥150 cells/μl, which was higher than those reported in other studies. Of the 58 patients, 32 had an increased serum IgE level of ≥100 IU/mL. 51 of the 155 patients (32.90%) the FeNO value was ≥25 ppb. Approximately 54.1% of patients with COPD showed blood eosinophilia of ≥2%, thereby suggesting that this criterion was unsuitable to be used alone in differentiating the asthma phenotype with any clinical relevance. Many patients with COPD might present with eosinophilic airway inflammation without asthma.
A study has shown that FeNO is not better than blood eosinophil counts in patients with COPD.31 Considering that FeNO and blood eosinophil counts show similar diagnoses in determining ACO phenotypes, Ramírez et al thought that blood eosinophil counts are enough, and FeNO does not need to be measured. However, the results of our study did not agree with this conclusion. First, they enrolled patients with AECOPD, but we enrolled stable patients with GOLD 3–4. Second, the differences in airway inflammation were based on different cell profiles. In our study, the correlations between FeNO values and blood eosinophil counts in patients with severe and extremely severe COPD were not significant. FeNO and blood eosinophil counts should be both evaluated in patients with COPD.
In this retrospective study, nine patients were reviewed for PFT and FeNO measurements 3 months after the treatment. Although lung function and FeNO improved, the differences between these variables were not significant. Therefore, larger sample size and more observations are needed to confirm the results.
This work is a retrospective study; hence, it has several limitations. First, many patients were under treatment with dual- or triple-inhaled treatments (bronchodilator therapy and corticosteroids). Although patients stopped these treatments within 72 h before they underwent a blood test, a PFT, and a FeNO test, data might have been partially affected.32 Second, induced sputum could directly reflect the characteristics of airway inflammation, but the technical requirements of sputum processing and cell counting might limit its feasibility in routine clinical practice. The correlation between sputum and blood eosinophil is moderate, blood test is easily conducted, and cell counting is standardized in laboratories.27,29,30 Therefore, in this study, blood eosinophilia was used instead of sputum eosinophilia. Third, we standardized the FeNO test and PFT and excluded patients with other diseases that might affect FeNO values. For example, we excluded current smokers, but we were unsure how the coexistence of other diseases affected our study. Lastly, different diagnostic criteria slightly influenced the definition of ACO population, and the definition of patients with a history of asthma in our study was reasonable.
In this study, a certain number of patients with severe and extremely severe COPD can have asthma or present with asthma characteristics (high blood eosinophil counts, high FeNO values, and reversibility). Asthma and its characteristics in patients with COPD represent a treatable trait due to targeted therapy. This study carefully analyzed patients with severe and extremely severe COPD and highlighted a subgroup of patients who had asthma characteristics and were more likely to suffer from severe exacerbation. This aspect should be close monitored in clinical practice.
Conclusion
Asthma characteristics, such as airway reversibility, high FeNO values, and blood eosinophil counts, are common findings in patients with severe and extremely severe COPD, but clinically relevant values need to be further determined. Biomarkers should be actively used to evaluate the asthma characteristics in these patients. If the characteristics of asthma exist, then anti-IgE or anti-IL-5 therapy should be considered for these patients to reduce exacerbation.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Global Initiative for Chronic Obstructive Lung Disease. Report global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease 2019 report. Available from: www.goldcopd.org.
2. Miravitlles M, Soler-Cataluna JJ, Calle M, et al. Spanish Guidelines for Management of Chronic Obstructive Pulmonary Disease (GesEPOC) 2017. Pharmacological treatment of stable phase. Arch Bronconeumol. 2012;48:247–257. doi:10.1016/j.arbres.2012.04.001
3. Kauppi P, Kupiainen H, Lindqvist A, et al. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma. 2011;48:279–285. doi:10.3109/02770903.2011.555576
4. McDonald VM, Simpson JL, Higgins I, Gibson PG. Multidimensional assessment of older people with asthma and COPD: clinical management and health status. Age Ageing. 2011;40:42–49. doi:10.1093/ageing/afq134
5. Bateman ED, Reddel HK, van Zyl-smit RN, Agusti A. The asthma-COPD overlap syndrome: towards a revised taxonomy of chronic airways diseases? Lancet Respir Med. 2015;3:719–728. doi:10.1016/S2213-2600(15)00254-4
6. Nielsen M, Barnes CB, Ulrik CS. Clinical characteristics of the asthma-COPD overlap syndrome-a systematic review. Int J Chron Obstruct Pulmon Dis. 2015;10:1443–1454. doi:10.2147/COPD.S85363
7. Shaya FT, Dongyi D, Akazawa MO, et al. Burden of concomitant asthma and COPD in a Medicaid population. Chest. 2008;134:14–19. doi:10.1378/chest.07-2317
8. Bafadhel M, McKenna S, Terry S, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012;186:48–55. doi:10.1164/rccm.201108-1553OC
9. Corlateanu A, Covantev S, Mathioudakis A, Botnaru V, Siafakas N. Asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): current evidence and future research directions. COPD Res Prac. 2017;3:6. doi:10.1186/s40749-017-0025-x
10. Leigh R, Pizzichini MM, Morris MM, Maltais F, Hargreave FE, Pizzichini E. Stable COPD: predicting benefit from high-dose inhaled corticosteroid treatment. Eur Respir J. 2006;27:964–981. doi:10.1183/09031936.06.00072105
11. Barrecheguren M, Román-Rodríguez M, Miravitlles M. Is a previous diagnosis of asthma a reliable criterion for asthma–COPD overlap syndrome in a patient with COPD? Int J Chronic Obstructive Pulm Dis. 2015;10:1745–1752.
12. Global Initiative for Asthma, Global Initiative for Chronic Obstructive Lung Disease. Diagnosis of disease of chronic airflow limitation: asthma, COPD, and Asthma–COPD Overlap Syndrome (ACOS). Available from: http://www.ginasthma.org/local/uploads/files/ACOS_2015.pdf.
13. Donohue JF, Herje N, Crater G, Rickard K. Characterization of airway inflammation in patients with COPD using fractional exhaled nitric oxide levels: a pilot study. Int J Chronic Obstructive Pulm Dis. 2014;9:745–751. doi:10.2147/COPD
14. Tamada T, Sugiura H, Takahashi T, et al. Biomarker-based detection of asthma-COPD overlap syndrome in COPD populations. Int J Chronic Obstructive Pulm Dis. 2015;10:2169–2176. doi:10.2147/COPD.S88274
15. Takayama Y, Ohnishi H, Ogasawara F, et al. Clinical utility of fractional exhaled nitric oxide and blood eosinophils counts in the diagnosis of asthma–COPD overlap. Int J Chronic Obstructive Pulm Dis. 2018;13:2525–2532. doi:10.2147/COPD
16. McDonald CF. Eosinophil Biology in COPD. N Engl J Med. 2017;377:1680–1682. doi:10.1056/NEJMe1710326
17. Vedel-Krogh S, Nielsen SF, Lange P, Vestbo J, Nordestgaard BG. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease — the copenhagen general population study. Am J Respir Crit Care Med. 2016;193:965–974. doi:10.1164/rccm.201509-1869OC
18. Matthes S, Stadler J, Barton J, et al. Asthma features in severe COPD: identifying treatable traits. Respir Med. 2018;145:89–94. doi:10.1016/j.rmed.2018.10.027
19. Pavord ID. Biologics and chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141:1983–1991. doi:10.1016/j.jaci.2018.04.020
20. Alving K, Malinovschi A. Basic aspects of exhaled nitric oxide. Eur Respir Soc Monogr. 2010;49:1–31.
21. Stirling RG, van Rensen EL, Barnes PJ, Chung KF. Interleukin-5 induces CD34(+) eosinophil progenitor mobilization and eosinophil CCR3 expression in asthma. Am J Respir Crit Care Med. 2001;164:1403–1409. doi:10.1164/ajrccm.164.8.2010002
22. Bafadhel M, Pavord ID, Russell REK. Eosinophils in COPD: just another biomarker? Lancet Respir Med. 2017;5:747–759. doi:10.1016/S2213-2600(17)30217-5
23. Pavord ID, Chanez P, Criner GJ, Kerstjens HAM, Korn S. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med. 2017;377:1613–1629. doi:10.1056/NEJMoa1708208
24. Maltby S, Gibson PG, Powell H, McDonald VM. Omalizumab treatment response in a population with severe allergic asthma and overlapping. COPD Chest. 2017;151:78–89. doi:10.1016/j.chest.2016.09.035
25. Chinese Thoracic Society. The Chinese national guidelines of pulmonary function test.Chin J Tuberc Respir Dis. 2014;37:566–571. Available from http://zhjhhhxzz.yiigle.com/CN112147201408/860037.htm.
26. American Thoracic Society,European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi:10.1164/rccm.200406-710ST
27. Couillard S, Larivée P, Courteau J, Vanasse A. Eosinophils in COPD exacerbations are associated with increased readmissions. Chest. 2017;151:366–373. doi:10.1016/j.chest.2016.10.003
28. Sin DD. Asthma-copd overlap syndrome: what we know and what we don’t. Tuberc Respir Dis. 2017;80:11–20. doi:10.4046/trd.2017.80.1.11
29. George L, Brightling CE. Eosinophilic airway inflammation: role in asthma and chronic obstructive pulmonary disease. Ther Adv Chronic Dis. 2016;7:34–51. doi:10.1177/2040622315609251
30. Carr TF, Zeki AA, Kraft M. Eosinophilic and Noneosinophilic Asthma. Am J Respir Crit Care Med. 2018;197:22–37. doi:10.1164/rccm.201611-2232PP
31. Río Ramírez MT, Juretschke Moragues MA, Fernández González R, et al. Value of exhaled nitric oxide (FeNO) and eosinophilia during the exacerbations of chronic obstructive pulmonary disease requiring hospital admission. Int J Chronic Obstructive Pulm Dis. 2018;15:369–376. doi:10.1080/15412555.2018.1482532
32. Kharitonov SA, Yates DH, Barnes PJ. Inhaled glucocorticoids decrease nitric oxide in exhaled air of asthmatic patients. Am J Respir Crit Care Med. 1996;153:454–457. doi:10.1164/ajrccm.153.1.8542158
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.