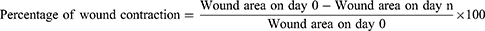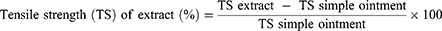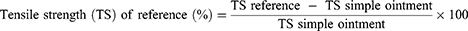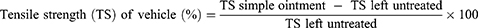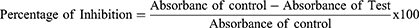Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Evaluation of the Antioxidant and Wound Healing Properties of 80% Methanol Extract and Solvent Fractions of the Leaves of Vernonia auriculifera Hiern. (Asteraceae)
Authors Ashenafi E , Abula T, Abay SM , Arayaselassie M, Sori M
Received 31 October 2022
Accepted for publication 24 January 2023
Published 28 January 2023 Volume 2023:16 Pages 279—299
DOI https://doi.org/10.2147/CCID.S393379
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Ephrem Ashenafi,1 Teferra Abula,1 Solomon Mequanente Abay,1 Mahlet Arayaselassie,2 Moti Sori2
1Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia; 2Department of Pathology, School of Medicine, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Ephrem Ashenafi, Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia, Email [email protected]
Background: The leaves of Vernonia auriculifera (Asteraceae) have traditionally been used to treat wounds in several regions of Ethiopia. The purpose of this study was to assess the wound healing properties of the leaf extract and solvent fractions of V. auriculifera in mice. The leaf extract and solvent fractions of V. auriculifera have also been evaluated for their anti-oxidant properties because of their impact on the wound healing process.
Material and Methods: Air-dried leaves were extracted using 80% methanol. They were also successively fractionated with n-hexane, ethyl acetate, and methanol. The residue was then macerated in water for 72 hr. Simple ointment bases were formulated according to British Pharmacopoeia. Thereafter, two types of ointment formulations, 2.5% w/w and 5% w/w, were formulated. Wound healing and acute dermal toxicity studies were performed on mice. To assess free radical scavenging activity, a 2,2-diphenyl-2-picrylhydrazyl free radical (DPPH) assay was performed.
Results: In both models, wounds treated with 2.5% and 5% (w/w) of the ME, the aqueous fraction (AQF), methanol fraction (MEF), and ethyl acetate fraction (EAF) ointments demonstrated significant wound healing activity, as shown by enhanced wound contraction, a shortened epithelialization time, increased hydroxyproline content, and enhanced tissue breaking strength. The extract and solvent fractions displayed free radical scavenging activity with IC50 values of 1.2 mg/mL, 1.46 mg/mL, 1.5 mg/mL, and 2.83 mg/mL for ME, AQF, MEF, and EAF, respectively, as compared to 1.42 mg/mL for ascorbic acid.
Conclusion: The result of this study indicates that 80% of methanol extract and solvent fractions are endowed with wound healing activity. Additionally, this study has also revealed that ME, AQF, MEF, and EAF have the capacity to scavenge free radicals. The study indicated that the wound healing effect could be attributed to the anti-inflammatory and antioxidant activities.
Keywords: Vernonia auriculifera, wound healing activity, excision model, incision model
A Letter to the Editor has been published for this article.
Introduction
A wound encompasses damage to anatomic structures and functions of the skin; consequently, it results in loss of epithelial continuity with or without loss of surrounding connective tissue.1 A wound can be classified in a variety of ways, but it is usually categorized as acute or chronic based on how much time it requires to heal. Acute wounds heal in a matter of weeks, whereas chronic wounds recur frequently, take longer to heal, or do not heal due to impaired wound-healing processes as a result of metabolic disturbances, infection, or underlying disease.2 In acute wounds, Staphylococcus aureus is the most common pathogen. Other than that, Streptococcus pyogenes (S. pyogenes), Pseudomonas aeruginosa (P. aeruginosa), Escherichia coli (E. coli), Acinetobacter spp., Klebsiella spp., Coliforms, and Enterococcus are common pathogens in acute wounds. On the other hand, the latent microorganisms of both aerobic and anaerobic bacteria are found in the majority of chronic wounds, with an increased predominance of anaerobic bacteria (eg, Pepto streptococcus species, Porphyromonas species, Prevotella, and Bacteroides species).3,4
Briefly, the wound healing process may be divided into 4 phases, ie, hemostasis, inflammation, proliferation, and remodeling.1 Many factors can interfere with the wound healing process, thus causing improper or impaired wound healing. The factors include infection, poor nutrition, diabetes, and other diseases, alcoholism, drugs, smoking, insufficient oxygenation, prolonged inflammation, depression, and others. Impaired wound healing cause severe morbidity, requiring patients to stay in the hospital for an extended period of time. The goal of wound treatment is to lessen the time it takes for a wound to heal and the risk of complications.14
Asteraceae, the biggest eudicotyledon family, is notable for its medicinal, cosmetic, and aromatic properties.5 Several Vernonia species are used in herbal medicine to treat a variety of diseases. Different species of Vernonia have also been investigated experimentally revealing many properties such as anti-plasmodial, analgesic, anti-inflammatory, antimicrobial, antidiabetic, antioxidant, and antitumor.5 In Uganda and Kenya, cold water infusion of V. auriculifera is taken orally to relieve fever associated with bacterial and viral infections.6 In Ethiopia, V. auriculifera, locally known as “Barewa”, is a known medicinal plant for wounds, fever reduction, hepatitis, headaches, venereal diseases, diabetes, and gastrointestinal problems.7
Wound is a major public health concern, in both developing countries as well as the developed world. Though it has a treatment strategy high cost, drug resistance, and other factors made the management of wounds inadequate. Wounds are the leading cause of disability and lost productivity worldwide. Aside from the incidence, chronic wounds are associated with causing a variety of health concerns.8 Chronic wounds, if not treated promptly, can cause significant functional disability, a decline in quality of life, and a significant financial burden for both individuals and the healthcare system.2
Although a recent experimental study reported wound healing activity of V. auriculifera, it used different solvents for fractionation and did not quantify the major phytoconstituents.28 Apart from that, it also assessed different histopathological parameters from the current study. On top of that, the study has not assessed the antioxidant activity. Accordingly, further studies that bridge these gaps are required. Hence, the present study was initiated to assess the efficacy of different fractions in both excision and incision models, to quantify major phytoconstituents thought to be responsible for the wound healing effect, and to assess the anti-oxidant activity. In this regard, the results of this study could serve as baseline information for the development of new wound healing drug and can give a clue for isolation and identification of active principle that can be used as a lead compound.
Materials
Plant Material
The leaves of V. auriculifera were collected from Hosanna town located in the Southern Nations, Nationalities, and People’s region, 230 km south of Addis Ababa (7°33’08.0“N 37°50’56.0”E) in August 2020. The town`s altitude ranges from 776 meters to 2220 meters above sea level and the mean annual temperature and mean annual rainfall is estimated to be 16.9°C and 1071mm, respectively. Mr. Melaku Wondafrash, a taxonomist, identified and authenticated the plant specimens, and voucher specimens (EA001) were placed in the National Herbarium, College of Natural and Computational Sciences, Addis Ababa University for future reference.
Experimental Animals
Healthy Swiss albino mice (8–12 weeks, weighing 25–33 grams) were acquired from the animal house of Addis Ababa University’s Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, College of Health Sciences, and EPHI’s animal unit, Addis Ababa, Ethiopia. They were kept in cages under standard conditions (22 ± 3°C, 40–70% relative humidity, and 12-hr light/dark cycles). They had free access to a pellet diet and water ad libitum. Before the trial, all animals were given a week to acclimatize to the working environment. All of the research was done in compliance with internationally accepted guidelines for the use, care, and handling of laboratory animals.9
Methods
Extraction of the Plant Material
Fresh leaves were collected, cleaned, and thoroughly washed with tap water before being dried in the open air at room temperature under a shade. Using a mortar and pestle, the dried leaves were coarsely ground. After that, the air-dried and ground plant components (733g) were extracted for 3 days at room temperature using a cold maceration process with 80% methanol. This process was repeated two more times with a fresh solvent added to the marc to maximize yield. The resulting combined liquid extract was then filtered with filter paper (Whatman no 1, Whatman Ltd., England). The filtrate was then concentrated in a rotary evaporator (Buchi Rotavapor R-200, Switzerland) under reduced pressure and at 40°C temperature. The concentrated extract was freeze-dried using a lyophilizer (Operon, Korea vacuum limited, Korea) to obtain a shiny dark green extract. The resulting extract was weighed and the yield was 10.47% (w/w). The extract was then transferred to a vial and kept in the refrigerator until further use.
Fractionation of the Plant Material
Solvent fractions of V. auriculifera leaves (250 g leaves) were obtained through sequential Soxhlet extraction with n-hexane, ethyl acetate, and methanol. The residue was then macerated in water for 72 hr. Then, each fraction, except for the water fraction, was dried under reduced pressure using a rotary evaporator. The water fraction, on the other hand, was freeze-dried with a lyophilizer. The resulting extract was weighed and the yield was hexane (2.3%), ethyl acetate (1.96%), methanol (11.2%), and aqueous (8.3%). The extract was then transferred to a vial and refrigerated until further use.
Ointment Formulation
To prepare the simple ointment, the measured quantities (Table 1) of hard paraffin and cetostearyl alcohol were mixed and melted in a water bath at 70°C. In another beaker, the mixture of wool fat and white soft paraffin was melted in a water bath at 70°C. Immediately after removal from the water bath, the former was added to the latter and stirred until cooled.10 By mixing 1g and 2g of the 80% methanolic extract with 39g and 38g simple ointment bases, 2.5% w/w and 5% w/w medicated ointments of the 80% methanolic extract were prepared. Similarly, the 2.5% w/w and 5% w/w ointments of each fraction were prepared by mixing 1g and 2g of the hexane (HF), ethyl acetate, methanol, and aqueous fraction each into a 39g and 38g of simple ointment base to get a 40g medicated ointment of each fraction.
 |
Table 1 Formula for the Preparation of Simple Ointment |
Acute Dermal Toxicity Test
In compliance with the Organization for Economic Cooperation and Development (OECD 402) guideline, adult albino female mice were used to carry out the acute dermal toxicity test for 80% methanol extract.11 The 80% methanol extract was applied to the exposed dorsal area uniformly. A porous gauze dressing and tape were used to keep the extract in touch with the skin for 24 hr. The starting dose was 200mg/kg and the dose was increased up to 2000mg/kg according to a flow chart presented by the guideline. Water was used to eliminate any remaining test chemicals at the end of the exposure period. The animals were monitored for the first thirty min after dosing, as well as on occasion throughout the first 24 hr, with special attention paid during the first two to six hr after the start of the exposure period, and daily afterward, for a period of 14 days.
Pilot Efficacy Study
Using an excision model, a pilot study was conducted to investigate the wound contraction effect of an 80% methanolic extract of V. auriculifera leaves. The study was also used to determine the concentration that was used in the main study.
Grouping and Dosing of Experimental Animals
For the excision model, mice were randomly assigned into four groups, with each group containing six mice. A simple ointment (SO) was applied to the first group and it served as a negative control. Nitrofurazone (0.2%) (NF) was used to treat Groups II (served as a positive control). Groups III and IV were given varying concentrations of 80% methanol extract and each solvent fraction (2.5% and 5%). In the excision model to study the wound healing effect of the 80% methanol extract twenty-four mice were used whereas to study the wound healing effect of the solvent fractions sixty mice were used. The incision model, on the other hand, included five groups of mice (each with six). The mice of Groups I–IV were treated similarly to the excision wound model, but mice in Group V were kept untreated (served as an untreated negative control). In the incision model to study the wound healing effect of the 80% methanol extract thirty mice were used; however, sixty-six mice were used to study the wound healing effect of the solvent fractions.
Wound Healing Studies
Excision Wound Model
The mice were anesthetized for wounding by administering ketamine (80mg/kg) and diazepam (5mg/kg) intraperitoneally. Following that, a circular wound (314.15 mm2) was carefully wounded using forceps and sterilized scissors after shaving the dorsothoracic area.12 Following recovery, each mouse was placed in a cage, and the day was marked as day 0. Starting on day one, the wound was treated with topical application of the ointments to the corresponding groups. The animals were observed for wound closure and measurement was taken every day until the wound healed completely. The wound-healing effect of the extract was calculated as follows.13,14
Where n = the days when the measurement was taken.
Epithelialization Period
The total number of days it needed for the dead tissue remains to peel off the wound surface without leaving a raw wound was regarded as the endpoint of complete epithelialization, and the days it took to get there were designated the epithelialization period.15
Incision Wound Model
The mice’s dorsal fur was shaved after they were anesthetized with ketamine (80mg/kg) and diazepam (5mg/kg) intraperitoneally, and a three-centimeter-long longitudinal paravertebral incision was made in the skin, and subcutaneous tissue. After that, the skin was stitched one centimeter apart. Beginning after 24 hr of injury (day 1), the animals were treated for nine days as specified in the grouping and dosing section. The sutures were removed on the eighth post-incision day, and tensile strength was measured on the tenth post-wounding day using the continuous water flow technique.16 The percent strength was calculated as follows.17
Histopathological Analysis
The histological examination was carried out to assess the progress of the healing process in the course of treatment in a new set of animals. The animals were sacrificed on day 7 post wounding using high dose anesthetics to evaluate inflammatory response and re-epithelialization. Tissue samples were immediately placed in 10% buffered formaldehyde to maintain their integrity and prevent cellular structure changes.18,19 Under a light microscope (100 x magnification), the specimens were examined for histopathological changes such as inflammatory cell infiltration, fibroblast proliferation, and re-epithelialization. The various animal groups were evaluated blindly, and the results were compared to those of the control groups. Using the method20 outlined elsewhere, a histopathological scoring was performed with minor modification, and each wound was given a semi-quantitative score with grades ranging from 1 to 3 for each of the parameters.20
Inflammation was designated as follows:
- None to minimal inflammation
- Modest inflammation
- Intense inflammation
Re-epithelialization was designated as follows:
- None to beginning re-epithelialization
- Partial re-epithelialization
- Re-epithelialized
Hydroxyproline Assay
Hydroxyproline (0.05 g) was dissolved in water and diluted to 400 mL by water. The solution was prepared up to 500 mL with water and concentrated HCl (20 mL). The hydroxyproline solution (100 μg/mL) was then diluted to yield 5, 10, and 15 μg/mL. Blank solutions as well as triplicate solutions of each concentration were prepared. Each test tube received one mL of 0.05 M CuSO4, followed by one mL of 2.5N NaOH, and the contents were mixed gently. The tubes were then placed in a water bath at 40°C for about 5 min, followed by the addition of 1mL of 6% H2O2. After that, the tubes were left in a bath for 10 min with occasional swirling and cooled with tap water, then 4mL of 3N H2SO4 and 2mL of 5% Dimethylaminobenzaldehyde (DMAB) solution were added followed by swirling after each addition. Tubes were covered with caps and placed in a water bath at 70°C for 16 min. The solutions were cooled, mixed, and their absorbance was measured in 1cm cells against a blank solution at 572 nm, and a calibration curve was established. The hydroxyproline assay was carried out by creating a circular wound with a diameter of 314 mm2, as described in the excision wound model on a new set of animals. The wounds were treated as described in the grouping and dosing section. On the 11th post-wounding day, the animals in each group were euthanized using high dose anesthetics, and the wound tissue was removed, weighed, and dried in an oven at 60°C for 12 hr, with the dry weights recorded. The tissue was then hydrolyzed for 24 hr at 110°C in sealed glass tubes with 6N HCl. The hydrolysate’s pH was adjusted to 7. A UV spectrophotometer (Jenway Model 6500, England) was used to measure the absorbance of one milliliter of supernatant solution from each acid hydrolysate that was treated in the same manner as the standard hydroxyproline. The amount of hydroxyproline in the samples was calculated using an equation derived from the standard hydroxyproline calibration curve.8
Antioxidant Activity Test
Free Radical Scavenging Activity
Using the approach outlined elsewhere,21 the ability of plant extracts to have free radical scavenging activity was assessed using a 2, 2-diphenyl-2-picrylhydrazyl free radical (DPPH) assay. In methanol, test samples were serially diluted at concentrations of 1, 0.5, 0.25, 0.125, and 0.065 mg/mL. Following the preparation of a standard stock solution of ascorbic acid (1 mg/mL in methanol), serial dilutions (0.5, 0.25, 0.125, and 0.0625 mg/mL) were prepared. Five milliliters of 0.004% DPPH solution (4mg/1000mL in methanol) were added to each 50L of test solution, ascorbic acid solution, and methanol in test tubes and allowed to react for 30 minutes at 37 o C in the dark. The absorbance was then measured in a UV spectrophotometer at 517 nm (Jenway Model 6500, England). All experiments were carried out in triplicate, and the results were averaged. The IC50 value, which was determined from the concentration Vs percent inhibition graph, was used to show DPPH scavenging activity. The IC50 value represents the sample concentration required to scavenge 50% of the DPPH free radicals. A lower IC50 value indicates that the antioxidant activity is higher. The free radical scavenging activity (percent antioxidant activity) was calculated using the equation below.
As a negative control, DPPH solution plus methanol was used, and ascorbic acid was used as a positive control. Similar procedures were used to determine the aqueous fraction’s free radical scavenging activity. However, due to the aqueous fraction’s insolubility in methanol, both the aqueous fraction (1mg/mL) and the standard (ascorbic acid) were dissolved in 50% methanol, and a calibration curve was created by preparing serial dilutions in methanol (50%) with concentrations of 1, 0.5, 0.25, 0.125, and 0.065 mg/mL.
Quantification of Phytochemical Constituents
Quantification of Total Flavonoid
The total flavonoid content of the extracts was estimated using an Aluminum Chloride complex forming assay. To get a calibration curve, serial dilutions of quercetin in methanol at concentrations of 1, 0.5, 0.25, 0.125, and 0.065 mg/mL were prepared. After that, one milliliter of quercetin was transferred into test tubes. NaNO2 (0.3 mL, 5%) was added and allowed to sit for 5 min. After that, another 0.3 mL of 10% AlCl3 was added to the solution and was left for 5 min. Following that, a 2 mL solution of 1M NaOH was added to the solution, then after, distilled water was added to get the total volume to 10 mL. Thereafter, the solution was allowed to sit at room temperature for 30 min. A UV-Vis spectrophotometer was used to measure the solution’s absorbance at 510 nm (Jenway Model 6500, England). The extract (1 mg/mL), fractions (1 mg/mL), and blank solutions were treated in the same manner. The total flavonoid content of the extracts was expressed as mg of quercetin equivalent per 1g of extract (QE). The experiment was repeated three times, and the average result was used.22 A similar procedure was used to determine the total flavonoid content of the aqueous fraction. But, due to the insolubility of the aqueous fraction in methanol, both the aqueous fraction (1mg/mL) and the standard (quercetin) were dissolved in 50% methanol and a calibration curve was established by preparing serial dilution of quercetin in methanol (50%) with concentration 1, 0.5, 0.25, 0.125 and 0.065 mg/mL.
Quantification of Total Phenols
The Folin-Ciocalteu method was used to calculate the total phenol content. To create a calibration curve, a series of dilutions of gallic acid in methanol were prepared at concentrations of 25, 12.5, 6.25, and 3.125/mL. One milliliter of gallic acid was then transferred to test tubes. Afterward, 5mL of distilled water and 0.5mL of 2N Folin–Ciocalteu’s reagent (1:20) were added to the test tubes. After 8 minutes, 2 mL of 7.5% Na2CO3 was added, and then distilled water was added until 10 mL was reached. After that, the solution was incubated at room temperature for 30 min. After that, the solution’s absorbance was measured at 765 nm using a UV-Vis spectrophotometer (Jenway Model 6500, England). The experiment was carried out in triplicate and the average result was taken. A Similar procedure was also carried out for the (1 mg/mL), fractions (1 mg/mL), and the blank solutions. The total phenolic content was expressed as mg of gallic acid equivalent per 1g of extracts (GAE).23 To quantify the total phenol content of the aqueous fraction similar procedures were followed. But, due to the insolubility of the aqueous fraction in methanol, both the aqueous fraction (1mg/mL) and the standard (gallic acid) were dissolved in distilled water and a calibration curve was established by preparing serial dilution of gallic acid in distilled water with concentration 50, 25, 12.5, 6.25 and 3.125 μ/mL.
Data Analysis
All results of the study are expressed as mean ± standard error of mean (SEM). The SPSS version 26 software was used to analyze the data. One-way analysis of variance (ANOVA) was used to analyze differences among groups. Subgroup analysis was done by Tukey’s multiple comparison tests. Statistical significance was defined as a P-value of less than 0.05.
Results
Acute Dermal Toxicity Test
After 24 hr of application of the extract, the site did not show any sign of inflammation, irritation, or redness. Moreover, no overt toxicity or fatality occurred throughout the 14 days observation period after topical application of the 80% methanol extract in increasing doses.
Excision Wound Model
In the excision wound model (Figure 1), topical administration of both 2.5% and 5% ME ointments significantly increased the wound contraction rate compared to negative controls (Table 2). The wound contraction of the mice treated with both 2.5% and 5% concentrations of the ME ointments had a comparable rate with that of the positive control (Nitrofurazone 0.2%) and on some days displayed a better contraction. These were seen on the 8th day the positive control having 48.55% contraction but the 2.5% ME and 5% ME having 55.61% and 60.79%. However, after the 8th day (Figure 2A–L), no significant difference was noted until the end of the observation period (Figure 3A–L).
 |
Table 2 Effect of Ointments Formulated from 80% Methanol Extract on Percentage Wound Contraction in Mice |
 |
Figure 1 Photograph of excision wound model on day 0. |
In the excision wound model, topical application of 2.5% and 5% of the AQF, MEF, and EAF ointments significantly increased wound contraction rate compared to negative controls, with a different onset of response (Table 3). The wound contraction of the mice treated with both 2.5% and 5% concentrations of the AQF, MEF, and EAF ointments had a comparable rate with that of the positive control (Nitrofurazone 0.2%) and on some days displayed a better contraction. These were seen on the 8th day the positive control having 49.25% contraction but the AQF 2.5% and AQF 5% having 57.12% and 56.02% contraction, respectively. Apart from the AQF, MEF had better contraction with 2.5% MEF and % MEF having 57.08% and 60.79%, respectively. In contrast, mice treated with both 2.5% and 5% concentrations of HF had insignificant wound healing activity compared to the negative control. Although the difference is statistically insignificant (HF being an exception), 5% MEF had better wound healing activity than the other treatment groups, as evidenced by a higher percentage of wound contraction and a shorter time for epithelialization. Furthermore, on days 8 and 12, the contraction produced by 5% MEF was significantly better (p < 0.05) than the positive control (Table 3). However, after the 12th day, no significant difference was observed until the completion of the observation period.
 |
Table 3 Effect of Ointments Formulated from Solvent Fraction on Percentage Wound Contraction in Mice |
Epithelialization Period
A Significantly (p < 0.05) shorter epithelialization period was observed in mice treated with the ME ointments as well as the standard treatment than the negative control (Table 4). Apart from that, no significant difference between the extracts and the standard, as well as between the different concentrations of the extract, was found.
 |
Table 4 Effect of Topical Application of the Ointment Formulated from 80% Methanol Extract of the Leaves of V. auriculifera on the Epithelialization Period |
Mice treated with the AQF, MEF, and EAF ointments, as well as the standard, displayed a significantly (p < 0.05) shorter epithelialization period than the negative control (Table 5). Furthermore, they also showed a significantly (p < 0.05) shorter epithelialization period compared to HF. Apart from that, no significant difference between the extracts and the standard, as well as between the different concentrations of the extracts, was found.
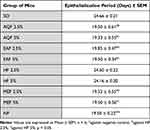 |
Table 5 Effect of Topical Application of the Ointment Formulated from Solvent Fractions of the Leaves of Vernonia auriculifera on the Epithelialization Period |
Incision Wound Model
Although all of the incised and sewed wounds (Figure 4) were completely healed, the measured tensile strength variable as shown in Table 6, mice treated with the 2.5% and 5% ointments of the 80% methanol extracts and the standard demonstrated significantly (p < 0.05) greater tensile strength than the controls (both treated and untreated negative controls).
 |
Table 6 Effect of Topical Application of the Ointment Formulated from 80% Methanol Extract of the Leaves of V. auriculifera on Tensile Strength of Incision Wound |
 |
Figure 4 Photograph of Incision wound on day 0. |
Animals treated with 2.5% and 5% concentrations of AQF, MEF, and EAF ointments, as well as the standard, displayed significantly (p < 0.05) greater tensile strength than the controls (both treated as well as untreated negative controls); as shown in Table 7. Tensile strength exhibited by both 2.5% and 5% concentrations of HF was somewhat lower than that of the other groups even though it was not statistically significant. When compared to untreated controls, the mean tensile strength in the negative control group treated with a simple ointment base increased by roughly 9.89%. Although the tensile strength appears to be higher, it did not achieve statistical significance.
 |
Table 7 Effect of Topical Application of the Ointment Formulated from Solvent Fractions of the Leaves of V. auriculifera on Tensile Strength of Incision Wound |
Histopathological Analysis
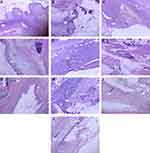
In hematoxylin and eosin-stained sections; On the 7th day mice treated with 2.5% ME has shown inflammation that was not intense as the negative control group, as demonstrated in Figure 5C. The mice that were treated with 5% ME had re-epithelialized wounds with no inflammation, as shown in Figure 5D. In the MEF group, mice that were treated with 2.5% concentration showed beginning epithelialization with modest inflammation, as demonstrated in Figure 5G. But those treated with 5% concentration of MEF showed re-epithelialized wounds with mild inflammation as well as active fibroblast proliferation, as demonstrated in Figure 5H. Mice treated with both 2.5% and 5% concentration of AQF has shown better epithelialization than the control group. The group that was treated with 2.5% AQF has shown partial epithelialization with mild inflammation, as demonstrated in Figure 5I. But the group that was treated with 5% AQF had dermal inflammation, re-epithelialized wound, and fibroblast proliferation, as demonstrated in Figure 5J. The mice that were treated with 2.5% EAF showed re-epithelialization with intense inflammation, as demonstrated in Figure 5E. Those treated with 5% EAF had shown re-epithelialization, as demonstrated in Figure 5F. The group that was treated with nitrofurazone showed a re-epithelialized wound with modest inflammation, as demonstrated in Figure 5A. Overall, after treatment with the 80% methanolic extract as well as the solvent fractions, there was a significant proliferation of epithelial cells mixed with fibroblasts. However, in the negative control group, the healing process was marked by a wide ulcer base that is intensely inflamed with no epithelialization, as demonstrated in Figure 5B. In general, mice in the 5% ME, EAF, and MEF groups exhibited better epithelialization and minimal inflammatory cell infiltration when compared to the other treatment groups, as demonstrated in Table 8. Altogether, the histopathological studies indicated that the 80% methanolic extract, as well as solvent fractions, increased re-epithelialization when compared to the negative control group. The inflammatory cell infiltration was also markedly lower as compared to the negative control group.
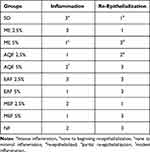 |
Table 8 Histopathological Grading Score for Different Histomorphological Changes |
Estimation of Hydroxyproline Content
Following 10 days of treatment, the hydroxyproline content of wound tissues was estimated, and the results are presented in Table 9 and Figure 6. In comparison to the negative control, hydroxyproline levels were significantly higher in animals treated with the NF, ME, AQF, EAF, and MEF.
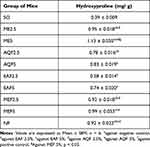 |
Table 9 Estimated Level of Hydroxyproline in Excision Wounds |
 |
Figure 6 Calibration curve for concentration of hydroxyproline (μg/mL). |
Antioxidant Activity
Free Radical Scavenging Activity
Regression equations were found to be y = 18.014x + 24.33, R² = 0.946 for ascorbic acid, y = 25.345x + 19.335, R² = 0.8818 for the 80% methanolic extract, y = 10.955x + 33.923, R² = 0.8956 for the aqueous fraction, y = 19.506x + 20.66, R² = 0.9438 for the methanol fraction, and y = 11.793x + 16.544, R² = 0.9707 for the ethyl acetate fraction. From these equations, the IC50 values were calculated to be 1.42 mg/mL, 1.2 mg/mL, 1.46 mg/mL, 1.5 mg/mL, and 2.83 mg/mL, respectively (Figure 7).
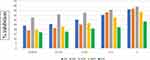 |
Figure 7 Free radical scavenging activity (%) of 80% methanol leaf extract and solvent fractions of V. auriculifera. |
Total Phenols and Flavonoid Content Analysis
The total phenol content quantified from MEF was (17.29 mg of GAE/g extract), from EAF (3.02 mg of GAE/g extract) and AQF (8.878 of GAE/g extract) as shown in Table 10 and Figure 8A. Likewise, the total flavonoid content found from MEF was (2.97 mg QE/g extract), from EAF (0.726 mg of QE/g extract), and AQF (1.022 mg QE/g extract) as indicated in Table 10 and Figure 8B.
 |
Table 10 Total Phenolic and Flavonoid Content of MEF, EAF & AQF of V. auriculifera |
 |
Figure 8 (A) Calibration curve for concentration of gallic acid (μg/mL). (B) Calibration curve for concentration of quercetin (mg/mL). |
Discussion
The present study attempted to assess the anti-oxidant and wound-healing effects of 80% methanolic extract as well as solvent fractions of the leaves of V. auriculifera.
The wound-healing effect of V. auriculifera has been documented in different ethnobotanical studies.24,25 Previously, wound healing activity of 5 and 10% w/w ointments of 80% methanolic extract, chloroform, ethyl acetate, and the aqueous faction of V. auriculifera leaves were reported28 which are comparable to the findings of the current study. Extracts of the root, stem, and leaf of V. auriculifera have been shown to have antibacterial action against several bacterial strains, in addition to wound healing activities.6,7 An infection can delay the progress of wound healing through numerous mechanisms. Reduced blood flow, disrupted leukocyte activity, and debridement phases, as well as the production of proteolytic enzymes, are all part of this process. Furthermore, wound infections can result in a sustained increase in pro-inflammatory cytokines like IL-1 and TNF-α, as well as the formation of free radicals at or near the wound site, obstructing wound healing. As a result, infection is a major wound complication, and antibacterial medicines are vital in the wound-healing process.26,48
To evaluate wound healing, neither a single model nor an in vitro study is reliable. To more accurately evaluate wound healing, it is necessary to employ two or more different in vivo models.8 As a result, the wound healing potential of the 80% methanolic extract and solvent fractions of V. auriculifera leaves were assessed in this study using two different wound models.
The ointments prepared from the 80% methanol extract exhibited enhanced wound contraction in both a previous study conducted elsewhere28 as well as in the current study. The ability of plant extracts to stimulate the proliferation of epithelial cells may be the cause of this increased wound contraction by ointments formulated from 80% methanol extract as well as solvent fractions.27 The leaves of V. auriculifera are known to possess an anti-microbial activity and this effect can also be involved in the wound healing effect of the leaf extracts as compounds with anti-microbial activity are thought to enhance wound healing. The leaves of V. auriculifera have also been reported to contain tannins7,28 and tannins may perhaps improve wound healing by boosting the formation of blood vessels and fibroblasts, eliminating free radicals, and enhancing the area’s ability to contract.29 Additionally, β-sitosterol which has been reported elsewhere6 to be present in the leaves of V. auriculifera tends to promote wound healing by stimulating neovascularization.30 Furthermore, their activity seems to be potentiated when they are combined with flavonoids.31 And the leaves of V. auriculifera have been shown to have flavonoids, particularly the methanol fraction which might explain the methanol fraction`s superior activity on wound healing. The flavonoids by themselves also tend to enhance wound healing.31 The centripetal movement of the wound edge and adjacent undamaged skin toward the center, known as wound contraction, is a hallmark of healing full-thickness cutaneous wounds.32 A wound after contraction can become 40–80% smaller.33 Activated fibroblasts are found in granulation tissue and they tend to acquire α-smooth muscle actin expression and become myofibroblasts. The component of the ECM that will eventually replace the transitory matrix is synthesized and deposited by these cells. Myofibroblasts have contractile capabilities as a result of the expression of α-Smooth Muscle actin in microfilament bundles or stress fibers, making them essential for the contraction and maturation of granulation tissue.34,35 When compared to control groups, the wound contraction rate and epithelialization period demonstrated by the groups treated with the 80% methanolic extract as well as the solvent fractions indicate that the wound has rapidly and significantly contracted. This could be attributable to the wound site’s rapid proliferation and transformation of fibroblasts into myofibroblasts.
The process of cellular detachment, proliferation, and differentiation is known as epithelialization. The marginal basal cells travel as a monolayer toward the wound’s center and display contact guidance. As long as the area to be covered is not excessively vast, epithelial cells fill up the region of the wound that is left following wound contraction. These cells travel until they come into touch with cells migrating in the other direction, at which point contact inhibition stops the migration.36 TGF-α and EGF which are produced by activated wound macrophages, keratinocytes, and platelets stimulate this process.37 Depending on the size of the wound and the condition of the granulation tissue, epithelialization might take anywhere from days to weeks.36 The time required for epithelialization was significantly reduced from 24 days (control) to 19 and 18 days for 2.5% and 5% of ME, respectively. The epithelialization period was also significantly shorter for MEF, EAF, and AQF. Hence, the shorter epithelialization time of the ME, as well as MEF, EAF, and AQF may well be attributable to enhanced wound contraction, migration, and proliferation of epithelial cells. Early epithelialization and enhanced wound contraction in treated wounds could be attributed to the increased keratinocytes proliferation and their transformation to the wound site. The lower level of inflammatory cell infiltration that was seen in the treatment group could also be due to the anti-bacterial and anti-inflammatory properties of the leaf extract and solvent fractions. Apart from that, vascularization was also greatly enhanced by the extracts. Thus, V. auriculifera affects different features of wound healing including inflammatory cell infiltration, epithelialization, vascularization, and wound contraction.
Reactive oxygen species (ROS) are derivatives of stress stimuli (both biotic and abiotic) and intrinsic oxygen metabolism. Their generation causes cancer, chronic inflammation, and aging plays a role in diabetes, HIV infection, and other diseases by inactivating enzymes and damaging essential cellular organelles and membranes.38 ROS destroys lipids, DNA, and extracellular structure proteins. Additionally, they enhance signaling pathways, extending the inflammatory stage of wound healing. As a result, they delay the wound-healing process.39 Since phenolic compounds and flavonoids donate hydrogen to free radicals; they can be regarded as antioxidant substances by acting as reducing agents and by free-radical scavenging.40 DPPH free radical scavenging assay was used to assess the antioxidant activity of the extract and solvent fractions. Free radical scavenging activity was seen in the extract and solvent fractions with IC50 values of 1.2 mg/mL, 1.46 mg/mL, 1.5 mg/mL, and 2.83 mg/mL for ME, AQF, MEF, and EAF, respectively, as compared to 1.42 for ascorbic acid. Hence, the antioxidant activity of the extracts and the solvent fractions could be attributed to the presence of phenols and flavonoids.
Flavonoids are abundant in plants and have been found to have a wide range of biological activities including antioxidant, anti-inflammatory, antibacterial, antiviral, and antifungal properties.40–42 Because of their widespread occurrence, multiplicity, and natural origin, they make suitable chemical scaffolds for novel drugs.43 Accordingly, one of the most crucial parameters for evaluating a plant’s wound-healing activity is its quantitative determination of flavonoids. In this regard, a quantitative colorimetric assay was conducted to quantify flavonoids and showed the presence of 2.97mg QE/g of extract of flavonoid content in MEF of V. auriculifera 1.022 mg QE/g of extract of flavonoid content in AQF of V. auriculifera and 0.726 mg QE/g of extract of flavonoid content in EAF of V. auriculifera. So, the extract’s wound-healing activities could be attributed to the synergistic effect of flavonoids with sitosterol as well as other phytochemical constituents like triterpenoids which possess anti-microbial activity. Flavonoids, due to the high reactivity of their hydroxyl group, tend to stabilize reactive oxygen species by interacting with the reactive part of the radical.44 As a result, they contribute significantly to wound healing by protecting and preventing free radical oxidative damage.45 Flavonoids have also been reported to mitigate lipid peroxidation by averting or delaying the onset of cell necrosis, as well as improve vascularity and thus collagen fiber strength by either improving circulation or preventing cell damage by boosting DNA synthesis.46 This effect of flavonoids might be behind the increased tensile strength that was elicited by the extracts as compared to the negative control. Phenolic compounds are a major class of active compounds found in herbals. They are well known for their antibacterial activity, which is attained by disrupting the bacterium’s cell wall, interfering with the ATP pool, and altering its membrane potential, resulting in the bacterium’s death.40 And antibacterial compounds can accelerate wound healing and prevent complications. Therefore, the anti-bacterial activity of these compounds could have additional effects on the management of wound-healing. Accordingly, the quantification of phenol is an important parameter for evaluating a plant’s wound healing activity. Consequently, a quantitative colorimetric assay was conducted to quantify phenolic compounds and showed the presence of 17.29 mg of GAE/g of extract of phenol content in MEF of V. auriculifera, 8.878mg of GAE/g of extract of phenol content in AQF of V. auriculifera and 3.02 mg GAE/g of extract of phenol content in EAF of V. auriculifera. Hence, the anti-microbial effect of phenols could have supplemented the extract’s wound-healing activity.
The level of hydroxyproline was measured as a biochemical marker of collagen turnover in the present study. The ointments prepared from 80% methanolic extract as well as solvent fractions of V. auriculifera significantly increased (P < 0.05) hydroxyproline levels in wound tissue, revealing an increased collagen content leading to rapid wound healing. The higher collagen level and stability of the collagen fibers might also be responsible for the enhanced tensile strength of the treated wounds. Increased hydroxyproline content in the 80% methanolic extract, solvent fractions, and standard treated groups may be associated with fibroblast proliferation and migration, as well as collagen deposition.8 Accordingly, the observed reduction in epithelialization time and increase in wound contraction following the application of the ointments formulated from the extract and solvent fractions could be related to the plant’s ability to promote collagen synthesis, as wound contraction and collagen synthesis start virtually simultaneously.47
Lastly, this study has greatly verified the wound-healing activity of V. auriculifera through biophysical, biochemical, and histological evaluations. It has also quantified major phytoconstituents thought to be behind the observed wound-healing effect. On top of that, the study has assessed the antioxidant activity. The findings of this not only verify the plant’s traditional claim but also provide clues for further inquiry into its active ingredients for the development of safe and effective wound healing medications.
Conclusion
The finding of this study revealed that 80% methanol extract as well as the solvent fractions of V. auriculifera have wound healing activity (hexane fraction being an exception) compared with negative control which validates the folkloric uses. This study’s findings also confirmed that the 80% methanol extract, as well as the solvent fractions, are endowed with free radical scavenging ability. According to our findings, the wound healing effect could be attributed to antioxidant and anti-inflammatory activity. The quantification study suggests that phenols and flavonoids are present, which might be behind the observed activity. Generally, the findings of this study not only verify the plant’s traditional claim but also provide clues for further inquiry into its active ingredients for the development of safe and effective wound-healing medications.
Data Sharing Statement
On reasonable request, the data used to support the findings of this study will be made available by the corresponding author.
Ethics Approval
The study protocol was approved on August 17, 2020, G.C by the ethical review committee of Addis Ababa University’s College of Health Sciences, School of Pharmacy with a reference number (ERB/SOP/177/12/2020).
Disclosure
The authors declare that there are no conflicts of interest with this work.
References
1. Miladiyah I, Prabowo BR. Ethanolic extract of Anredera cordifolia (Ten) Steenis leaves improved wound healing in Guinea pigs. Universa Medicina. 2012;31(1):4–11. doi:10.18051/UnivMed.2012.v31.4-11
2. Tessema Z, Makonnen E, Debella A, Molla Y. Evaluation of in vivo wound healing and anti-inflammatory activity of crude extract of the fruits of Brucea antidysentrica in mice. Wound Med. 2018;21:16–21. doi:10.1016/j.wndm.2018.05.005
3. Ayele TT, Regasa MB, Delesa DA. Evaluation of antimicrobial activity of some traditional medicinal plants and herbs from Nekemte district against wound causing bacterial pathogens. Sci Technol Arts J. 2015;4(2):199–203. doi:10.4314/star.v4i2.24
4. Bowler PG, Davies BJ. The microbiology of infected and noninfected leg ulcers. Int J Dermatol. 1999;38(8):573–578. doi:10.1046/j.1365-4362.1999.00738.x
5. Antonio CNS, Elnatan BDS, Raquel ODSF. A review on antimicrobial potential of species of the genus Vernonia (Asteraceae). J Med Plant Res. 2015;9(31):838–850. doi:10.5897/JMPR2015.5868
6. Joyce JK, Neil AK, Hafizah C. Triterpenoids from Vernonia auriculifera Hiern exhibit antimicrobial activity. Afr J Pharm Pharmacol. 2011;5(8):1150–1156. doi:10.5897/AJPP11.183
7. Albejo B, Endale M, Kibret B, Anza M. Phytochemical investigation and antimicrobial activity of leaves extract of Vernonia auriculifera Hiern. J Pharm Pharmacogn Res. 2015;3(6):141–147.
8. Mulisa E, Asres K, Engidawork E. Evaluation of wound healing and anti-inflammatory activity of the rhizomes of Rumex abyssinicus J. (Polygonaceae) in mice. BMC Complement Altern Med. 2015;15(1):1–10. doi:10.1186/s12906-015-0878-y
9. National Research Council. Guide For the Care and Use of Laboratory Animals.
10. British Pharmacopoeia. Department of Health and Social Security Scottish Home and Health Department. Office of the British Pharmacopoeia Commission, UK; 1988:713.
11. OECD. Test No. 402: Acute Dermal Toxicity, OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publishing Paris; 2017.
12. Udupa S, Udupa A, Kulkarni D. Anti-inflammatory and wound healing properties of Aloe vera. Fitoterapia. 1994;65(2):141–145.
13. Sharma GN, Dubey SK, Sati N, Sanadya J. Evaluation of wound healing activity of aegle marmelos seed. Pharmacologyonline. 2011;2:171–178.
14. Mekonnen A, Sidamo T, Asres K, Engidawork E. In vivo wound healing activity and phytochemical screening of the crude extract and various fractions of Kalanchoe petitiana A. Rich (Crassulaceae) leaves in mice. J Ethnopharmacol. 2013;145(2):638–646. doi:10.1016/j.jep.2012.12.002
15. Pawar RS, Chaurasiya PK, Rajak H, Singour PK, Toppo FA, Jain A. Wound healing activity of Sida cordifolia Linn. in rats. Indian J Pharmacol. 2013;45(5):474. doi:10.4103/0253-7613.117759
16. Wang J-P, Ruan J-L, Cai Y-L, et al. In vitro and in vivo evaluation of the wound healing properties of Siegesbeckia pubescens. J Ethnopharmacol. 2011;134(3):1033–1038. doi:10.1016/j.jep.2011.02.010
17. Süntar I, Akkol EK, Keleş H, Oktem A, Başer KHC, Yeşilada E. A novel wound healing ointment: a formulation of Hypericum perforatum oil and sage and oregano essential oils based on traditional Turkish knowledge. J Ethnopharmacol. 2011;134(1):89–96. doi:10.1016/j.jep.2010.11.061
18. Masson‐Meyers DS, Andrade TA, Caetano GF, et al. Experimental models and methods for cutaneous wound healing assessment. Int J Exp Pathol. 2020;101(1–2):21–37. doi:10.1111/iep.12346
19. Birch M, Tomlinson A, Ferguson MW. Animal models for adult dermal wound healing. In: Fibrosis Research. Springer; 2005:223–235. doi:10.1385/1-59259-940-0:223
20. Tkalčević VI, Čužić S, Parnham MJ, Pašalić I, Brajša K. Differential evaluation of excisional non-occluded wound healing in db/db mice. Toxicol Pathol. 2009;37(2):183–192. doi:10.1177/0192623308329280
21. Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199–1200. doi:10.1038/1811199a0
22. Nigatu H, Belay A, Ayalew H, et al. In vitro antileishmanial activity of some Ethiopian medicinal plants. J Exp Pharmacol. 2021;13:15. doi:10.2147/JEP.S285079
23. María R, Shirley M, Xavier C, et al. Preliminary phytochemical screening, total phenolic content and antibacterial activity of thirteen native species from Guayas province Ecuador. J King Saud Univ Sci. 2018;30(4):500–505. doi:10.1016/j.jksus.2017.03.009
24. Bitew H, Gebregergs H, Tuem KB, Yeshak MY. Ethiopian medicinal plants traditionally used for wound treatment: a systematic review. Ethiop J Health Dev. 2019;33:2.
25. Maryo M, Nemomissa S, Bekele T. An ethnobotanical study of medicinal plants of the Kembatta ethnic group in Enset-based agricultural landscape of Kembatta Tembaro (KT) Zone, Southern Ethiopia. Asian J Plant Sci Res. 2015;5(7):42–61.
26. Fahimi S, Abdollahi M, Mortazavi SA, Hajimehdipoor H, Abdolghaffari AH, Rezvanfar MA. Wound healing activity of a traditionally used poly herbal product in a burn wound model in rats. Iran Red Crescent Med J. 2015;17(9). doi:10.5812/ircmj.19960
27. Getie M, Gebre-Mariam T, Rietz R, et al. Evaluation of the anti-microbial and anti-inflammatory activities of the medicinal plants Dodonaea viscosa, Rumex nervosus and Rumex abyssinicus. Fitoterapia. 2003;74(1–2):139–143. doi:10.1016/S0367-326X(02)00315-5
28. Lambebo MK, Kifle ZD, Gurji TB, Yesuf JS. Evaluation of wound healing activity of methanolic crude extract and solvent fractions of the leaves of Vernonia auriculifera hiern (Asteraceae) in mice. J Exp Pharmacol. 2021;13:677. doi:10.2147/JEP.S308303
29. Hosseinkhani A, Falahatzadeh M, Raoofi E, Zarshenas MM. An evidence-based review on wound healing herbal remedies from reports of traditional Persian medicine. J Evid Based Complement Altern Med. 2017;22(2):334–343. doi:10.1177/2156587216654773
30. Moon E-J, Lee YM, Lee O-H, et al. A novel angiogenic factor derived from Aloe vera gel: β-sitosterol, a plant sterol. Angiogenesis. 1999;3(2):117–123. doi:10.1023/A:1009058232389
31. Abbas MM, Al-Rawi N, Abbas MA, Al-Khateeb I. Naringenin potentiated β-sitosterol healing effect on the scratch wound assay. Res Pharm Sci. 2019;14(6):566. doi:10.4103/1735-5362.272565
32. Tranquillo RT, Murray J. Mechanistic model of wound contraction. J Surg Res. 1993;55(2):233–247. doi:10.1006/jsre.1993.1135
33. Yang L, Witten TM, Pidaparti RM. A biomechanical model of wound contraction and scar formation. J Theor Biol. 2013;332:228–248. doi:10.1016/j.jtbi.2013.03.013
34. Thangavel P, Vilvanathan SP, Kuttalam I, Lonchin S. Topical administration of pullulan gel accelerates skin tissue regeneration by enhancing collagen synthesis and wound contraction in rats. Int J Biol Macromol. 2020;149:395–403. doi:10.1016/j.ijbiomac.2020.01.187
35. Darby I, Laverdet B, Bonté F, Desmoulière A. Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol. 2014;7:301–311. doi:10.2147/CCID.S50046
36. Thiruvoth F, Mohapatra D, Sivakumar D. Current concepts in the physiology of adult wound healing. Plast Aesthetic Res. 2015;2:250. doi:10.4103/2347-9264.158851
37. Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9(1):283–289. doi:10.2741/1184
38. Kayani WK, Dilshad E, Ahmed T, Ismail H, Mirza B. Evaluation of Ajuga bracteosa for antioxidant, anti-inflammatory, analgesic, antidepressant and anticoagulant activities. BMC Complement Altern Med. 2016;16(1):1–13. doi:10.1186/s12906-016-1363-y
39. Upadhyay A, Chattopadhyay P, Goyary D, Mitra Mazumder P, Veer V. Ixora coccinea enhances cutaneous wound healing by upregulating the expression of collagen and basic fibroblast growth factor. Int Sch Res Notices. 2014;2014. doi:10.1155/2014/751824
40. Oliveira RN, Mancini MC, Oliveira F, et al. FTIR analysis and quantification of phenols and flavonoids of five commercially available plants extracts used in wound healing. Matéria. 2016;21:767–779. doi:10.1590/S1517-707620160003.0072
41. Mohammed MS, Osman WJ, Garelnabi EA, et al. Secondary metabolites as anti-inflammatory agents. J Phytopharmacol. 2014;3(4):275–285. doi:10.31254/phyto.2014.3409
42. Govindappa M, Naga SS, Poojashri M, Rappa C. Antimicrobial, antioxidant and in vitro anti-inflammatory activity of ethanol extract and active phytochemical screening of Wedelia trilobata (L). Hitchc. J Pharmacogn Phytotherapy. 2011;3(3):43–51.
43. Umesh C, Jamsheer A, Prasad M. The role of flavonoids in drug discovery—review on potential applications. RJLBPCS. 2018;4(1):70–77. doi:10.26479/2018.0401.06
44. Nijveldt RJ, Van Nood E, Van Hoorn DE, Boelens PG, Van Norren K, Van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74(4):418–425. doi:10.1093/ajcn/74.4.418
45. Agyare C, Dwobeng AS, Agyepong N, et al. Antimicrobial, antioxidant, and wound healing properties of Kigelia africana (Lam).Beneth. and Strophanthus hispidus DC. Adv Pharmacol Sci. 2013;2013. doi:10.1155/2013/692613
46. Ukwueze S, Duru O, Shorinwa O. Evaluation of the cutaneous wound healing activity of solvent fractions of Chromolaena odorata Linn. Indo Am J Pharm Res. 2013;3(4):3316–3323.
47. Iyyam Pillai S, Palsamy P, Subramanian S, Kandaswamy M. Wound healing properties of Indian propolis studied on excision wound-induced rats. Pharm Biol. 2010;48(11):1198–1206. doi:10.3109/13880200903578754
48. Abdel-Motaal FF, Maher ZM, Ibrahim SF, El-Mleeh A, Behery M, Metwally AA. Comparative studies on the antioxidant, antifungal, and wound healing activities of solenostemma arghel ethyl acetate and methanolic extracts. Appl Sci. 2022;12(9):4121. doi:10.3390/app12094121
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.