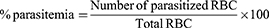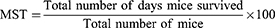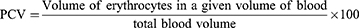Back to Journals » Journal of Experimental Pharmacology » Volume 15
Evaluation of the Anti-Malarial Activity of the Crude Root Extract and Solvent Fraction of Sesamum indicum (Fabaceae)
Authors Girmaw F , Ashagrie G
Received 10 February 2023
Accepted for publication 22 March 2023
Published 28 March 2023 Volume 2023:15 Pages 163—175
DOI https://doi.org/10.2147/JEP.S407557
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Fentaw Girmaw, Getachew Ashagrie
Department of Pharmacy, College of Health Science, Woldia University, Woldia, Ethiopia
Correspondence: Fentaw Girmaw, Department of Pharmacy, College of Medicine and Health Science, Woldia University, P.O. Box 400, Woldia, Ethiopia, Tel +251928383965, Fax +251335400606, Email [email protected]
Background: A major cumbersome factor in malaria control measure is the new coming antimalarial drug resistance strains. The increase of resistance to the available marketed antimalarial agents dictates the scientific community to search new alternative antimalarial agent from traditional plants. Therefore, our study assesses the antimalarial activity of the crude root extract and solvent fraction of Sesamum indicum in mice.
Methods: The roots of Sesamum indicum were extracted by 80% methanol and fractionated using three solvents with different polarities. The in vivo antimalarial activity was assessed at 200 mg/kg, 400 mg/kg, and 600 mg/kg of the root crude extract and solvent fraction using the 4-day suppressive test. Similarly, the n- butanol fraction extract, which showed better suppression potential in 4-day suppressive test from other fractions was also evaluated in the curative model to assess its curative potential. The % parasitemia suppression, mean survival time, body weight change, rectal temperature change, and packed cell volume change were also evaluated in both models.
Results: Our finding revealed that the crude extract and solvent fraction treated groups had a statistical significant parasitemia suppression and mean survival time improvement as compared to the negative control (p< 0.001) in both models in a dose-dependent fashion. The higher dose n-butanol fraction treated group (600mg/kg) showed the highest suppression effect and mean survival time prolongation in both tests from the other two fractions. However, the lowest suppressive effect was observed in 200 mg/kg aqueous fraction extract-treated groups in the 4-day suppressive test.
Conclusion: The crude root extract and solvent fractions of Sesamum indicum possessed a dose dependent antimalarial activity and a significant change in other parameters in both models that strengthen the traditional claim.
Keywords: antimalarial, parasitemia, Plasmodium, Sesamum indicum
A Letter to the Editor has been published for this article.
Background
Malaria is a well-known protozoan disease caused by plasmodium species such as P. falciparum, P. vivax, P. ovale, P. malariae and P. knowlesi.1,2 Globally, P. falciparum and P. vivax are the most common causes of malaria infection. P. falciparum is the most dominant malaria parasite in Africa and in Ethiopia. In Ethiopia above 99% of malaria cases are caused by P. falciparum and P. vivax.3 Malaria is highly prevalent in tropical and subtropical countries and predominantly affects pregnant mothers and children.2 Plasmodium berghei is among the rodent malaria parasites that are used in experimental in vivo studies of plant extracts that have antimalarial traditional claims. Moreover, P. berghei ANKA strain is an ideal parasite in order to test important cellular and molecular biology of malaria parasites.4–6
In Africa, especially in sub-Saharan countries, malaria causes a high economic burden targeting particularly children and pregnant mothers.7 Previous Ethiopian and sub-Saharan country reports showed that malaria is among the top causes for mortality and morbidity.8 Even if malaria is a treatable and preventable disease, designing appropriate measure to control and eliminate malaria have a paramount importance.8,9 The WHO African Region continues to carry the bulk of the malaria burden, which accounts 95% of all malaria cases (224 million cases) in 2021. Globally, four nations Nigeria (31%), the Democratic Republic of the Congo (13%), the United Republic of Tanzania (4%), and the Niger (4%), accounted for just over half of all malaria deaths.10 Cabo Verde, Eswatini, and Sao Tome and Principe reported zero malaria deaths in 2020, and Ethiopia and South Africa achieved a reduction in mortality rate of more than 40% or more. Cabo Verde, Ethiopia, the Gambia, Ghana, and Mauritania met the GTS 2020 target for a 40% reduction in malaria case incidence. Algeria was certified malaria-free by WHO in 2019.11
A major challenge in malaria control measure is the dramatic increase in resistance strains to antimalarial agents. Drug resistance to the available marketed antimalarial agents dictates the scientific community to search optional antimalarial agents that can be even active against resistant strains.12 Moreover, in developing countries 80% of population primary health-care need is dependent on traditional medicines.13
Traditional medicinal plants have been a good alternative as a source of drugs in the treatment of different human disease conditions like malaria.9 Worldwide millions of people’s income, health care and livelihood are dependent on medicinal traditional plants.14 Plants in the families of Aloaceae, Annonaceae, Asphodelaceae Euphorbiaceous, Fabaceae, Leguminosae, Meliaceae, Moraceae, Olacaceae and Rubiaceae are among the families of plants with better antimalarial activity.15
Sesamum indicum which is our study plant in the genus Sesamum and family Fabaceae is cultivated for its edible seeds and grow in pods.16 It is used for the treatment of different ailments like hypertension and diabetes mellitus. Sesamum seed is the oldest oilseed crop that had a nutrition value and were domesticated well over 3000 years ago. Sesamum also has many other species, most being native and wild to sub-Saharan Africa. It tolerates drought conditions well, with the highest oil contents from any other seed.17,18 According to research, sesame oil may be able to combat cancer. It has significant levels of linoleate in triglyceride form, which specifically slowed the formation of malignant melanoma.19 The use of sesame seeds in the treatment of respiratory conditions such as acute and chronic bronchitis, asthma, pneumonia, and airway spasms is beneficial. Magnesium, which improves respiratory function, is found in abundance in sesame seeds. Due to their high iron content, black sesame seeds are useful in the treatment of anemia.20 Calcium from sesame seeds is a useful option for preventing bone loss that can arise from menopause.21 Diarrhea and dysentery can both be cured with sesame seeds. Even today, it still has a long history of being a laxative. Along with treating headaches, dizziness, and impaired vision.22 In Ethiopia, by pounding the fresh root, mixed with boiled milk and drink a half cup of it in the morning and afternoon to treat malaria and snake bite in kunama society.23 As a result, this study was aimed to investigate the antimalarial activity of crude root extract and solvent fraction of Sesamum indicum in vivo using 4-day suppressive and Rane’s models in mice.
Methods
Plant Material
The fresh roots of Sesamum indicum were collected from Kafta Humera, northern Ethiopia, in October 2020. The plant was identified and authenticated as Sesamum indicum by a taxonomist and was deposited with voucher number FG002 in the National Herbarium, College of Natural and Computational Sciences, Addis Ababa University for future reference.
Experimental Animals and Parasites
Healthy Swiss albino mice with weight (20–35 g) or age (6–8 weeks) were used in our study. The mice were obtained from the animal house of the School of Pharmacy, Addis Ababa University. The mice were kept in 12-h light–dark cycle, acclimatized for 1 week and the laboratory diet with water ad libitum were accessed freely. International guidelines for the care and maintenance of experimental animals were implemented.24 Chloroquine sensitive Plasmodium berghei ANKA strain was obtained from the Ethiopian Public Health Institute (EPHI).
Plant Extraction and Solvent Fraction
After plant material collection, the root of Sesamum indicum was thoroughly washed and dried at room temperature (25–27oC) under shade with optimal ventilation for 1 month. Using a grinding mill, the dried root was pulverized to a coarse powder. About 800g of the air-dried powdered root of Sesamum indicum (100g in 600 mL of 80% methanol) was used for extraction by maceration technique. Later, from hydromethanolic crude extract 80gm was taken for successive fractionation using chloroform, n-butanol and distilled water. All the procedures required for extraction and fractionation were followed accordingly.
Acute Oral Toxicity Study
Acute oral toxicity study was employed according to OECD guidelines no. 425.25 First, 2000 mg/kg of the crude extract and solvent fractions were administered to mice after fasting for 4 h. Then, behavioral and physical changes were observed for 24 h. After this, another four mice were assigned to fast for 4 h and then the crude extract and solvent fractions were administered. The mice were then observed for the first 24 h. Finally, to check the development of toxicity, the mice were observed for 14 days.
Grouping and Dosing
The experimental animal was randomly divided into five groups (n=6). Group I (CON) was treated with 2% TW80; Group II, III, and IV was treated with 200 mg/kg, 400 mg/kg, and 600 mg/kg of the crude extract and solvent fraction, respectively, and Group V was treated with the positive control (25 mg/kg).26 The doses for the extract were chosen by conducting a pilot study, and the maximum volume (10 mL/1kg) was administered orally using oral gavage.27
Parasite Inoculation
The mice were infected with P. berghei ANKA strain, and the parasite was maintained by intraperitoneal serial passage of blood.28 Infected mice with 20–30% parasitemia level were used as a donor and blood was collected by a heparinized tube. The collected blood was then diluted with 0.9% normal saline, and each mouse was inoculated with 0.2mL of blood suspension intraperitoneally.
Determination of Anti-Malarial Activity
The 4-Days Suppressive Test
The 4-day suppressive model was employed to evaluate the chemo-suppressive potential of crude and solvent fraction extract against parasite infected mice. All 30 mice were after infected on the first day were randomly distributed into five groups. After 2 h of inducing infection, treatment was started immediately at day 0 and the treatment was continued for three consecutive days. Then blood was collected from the tail of each mouse on day 4 of the experiment, and a thin smear was prepared on microscope slides. Finally, the level of parasitemia, weight, temperature, PCV change as well as were MST of each group were determined. Using the modified Peters and Robinson formula Percent parasitemia, percent parasitemia suppression and MST were calculated:29
Rane’s Model
This model is used to evaluate the curative potential of the extract according to the method described in Ryley and Peters.30 On day 1, mice were injected 1×107 P. berghei infected erythrocytes intraperitoneally. After 72 h, the mice were randomly divided into five groups and treated accordingly once daily for 3 days. Thin blood films were collected from the tail of each mouse daily to monitor levels of parasitemia, and other parameters were also determined similarly to the four-day suppressive model.
Determination of Body Weight, Rectal Temperature and Packed Cell Volume Changes
Body weight and rectal temperature were measured using a sensitive digital weighing balance and digital thermometer, respectively. The packed cell volume (PCV) was calculated after the blood was collected from the tail of each mouse, filling 3/4th of its volume in heparinized micro-hematocrit capillary tubes. The blood was then centrifuged using a microhematocrit centrifuge at 12, 000 rpm for 15 min. Finally, PCV was determined using the following formula:31
Phytochemical Screening
The crude extract and solvent fraction phytochemical screening were undertaken using standard tests.32
Statistical Analysis
SPSS Version 25 Software was employed to analyze the statistical data, and data were presented as mean ± standard error of the mean. The one-way ANOVA followed by Tuckey’s post hoc multiple-comparison test was used to determine the mean of all parameters in both models, and the two-way ANOVA was employed to analyze the level of parasitemia through the course of treatment in the curative test. P-values <0.05 were considered to be statistically significant.
Results
Determination of Anti-Malarial Activity
The Effect of Crude Extract in the 4-Day Suppressive Test
The crude root extract of Sesamum indicum in the 4-day suppressive test revealed the percentage parasitemia suppression was 63.17, 48.58, and 39.87 at 200 mg/kg, 400 mg/kg, and 600 mg/kg, respectively. The parasitemia suppression potential at all doses was lower than the positive control but higher than that of the negative control (p<0.001) as shown in Table 1. Similarly, the crude extract exhibited an increase in mean survival time at all doses as compared to negative control but lower than positive control. Both parasitemia suppression and survival time were significantly higher in 400mg/kg treated group as compared with the lower dose and middle dose (p<0.001) treated group but lower than the positive control. Likewise, the 200mg/kg crude extract treated group showed a significantly lower suppression potential (p<0.05) compared to the middle dose (400mg/kg) crude extract treated group. Regarding survival time, the lower dose treated group had a lower survival time improvement as compared to the middle dose (p<0.01) and higher dose (p<0.001) treated group. The middle dose treated group also had lower (p<0.05) potential to increase the survival time compared with the higher dose treated group (Table 1).
 |
Table 1 Parasitemia and Survival Time of Infected Mice Treated with Crude Root Extract of Sesamum indicum in the 4-Day Suppressive Test |
The crude extract significantly halted weight loss at all dose (p<0.001) compared to the negative control. When we compared the higher dose crude extract treated group with the chloroquine treated group, there was no significant difference in preventing weight loss. However, a statistically significant difference was detected in preventing weight loss between 200mg/kg and 400mg/kg treated group from the positive control (p<0.001) and 600mg/kg treated groups (p<0.01). Regarding the temperature change, at all doses the crude extract protected rectal temperature decline compared to the negative control (p<0.001). The lower dose treated group also had a significant difference (p<0.01) in rectal temperature stabilization as compared with the higher dose treated group. Similar to temperature and weight, the extract at all doses also significantly (p<0.001) prevented PCV drop compared to the negative control. However, in the lower dose-treated group attenuated PCV reduction was lower (p<0.01) than the higher dose treated group (Table 2).
 |
Table 2 Effect of Crude Root Extract of Sesamum indicum on Body Weight, Rectal Temperature and Packed Cell Volume of Infected Mice in the 4-Day Suppressive Test |
The Effect of the Solvent Fraction in 4-Day Suppressive Test
In this test, all the solvent fractions had statistically significant (p<0.001) higher parasitemia suppression as compared to the negative control in a dose-dependent manner but lower than the standard drug treated group. The highest parasitemia suppression (47.87%) was observed at 600mg/kg n-butanol fraction extract treated group and the lowest parasitemia suppression (12.70%) were detected at 200mg/kg aqueous fraction treated group. Furthermore, all the solvent fraction treated groups had significantly (p<0.001) lower parasitemia suppression compared with the standard drug treated groups. Likewise, all the solvent fraction treated groups showed statistically significant (p<0.001) difference in prolonging the mean survival time compared to the negative and positive controls. Survival time increment were in dose-dependent fashion from all solvent fractions with higher survival time was 15.44 days in those 600mg/kg BF treated group and lower survival time was 9.15 days in the 200mg/kg AF treated group. In addition, the 200mg/kg treated groups of all three fractions significantly (p<0.05) prolonged survival time lower than the 600mg/kg treated groups (Table 3).
 |
Table 3 Parasitemia, Suppression and Mean Survival Time of Infected Mice Treated with Solvent Fractions of the Root of Sesamum indicum in the 4-Day Suppressive Test |
In the 4-day suppressive test, all three fractions at all doses revealed a statistically significant (p<0.001) preventive effect of body weight loss, rectal temperature drop and packed cell volume decline in a dose-dependent fashion compared with the negative control. The preventive effect of the solvent fractions to the above mentioned variables is statistically lower than standard drug treated groups (p<0.001). When comparing the same solvent fraction at the three doses (200,400,600mg/kg), all the lower doses had significant (p<0.05 for AF and CF; p<0.01 for BF) protective effect of body weight loss but lower than the higher dose treated groups in all the three fractions. In case of the protection of rectal temperature decline and PCV drop, only the lower dose of butanol fraction treated group showed statistically significant (p<0.05) difference from the higher dose treated group (Table 4).
 |
Table 4 Body Weight, Rectal Temperature and Packed Cell Volume of Infected Mice Treated with Solvent Fractions of the Root of Sesamum indicum in the 4-Day Suppressive Test |
The Effect of n-Butanol Fraction in the Rane’s Test
The percentage parasitemia suppression potential of this fraction in curative test at 200 mg/kg, 400 mg/kg, and 600 mg/kg was 47.14%, 50.28%, and 57.22% (p<0.001), respectively, compared to the negative control but lower when compared to the standard drug treated group. Regarding the mean survival time, there was a statistically significant (p<0.001) prolongation at all doses compared with the negative control in a dose-dependent manner but lower than the positive control. Moreover, there was also a statistical significant difference in parasitemia suppression between the lower and middle dose treated group (p<0.001 for 200mg/kg; p<0.01 for 400mg/kg) compared with 600mg/kg treated group. Likewise, the mean survival time improvement was lower in the lower and middle dose treated group (p<0.05 for 200mg/kg and 400mg/kg) as compared with 600mg/kg treated group (Table 5).
 |
Table 5 Parasitemia, Suppression and Survival Time of Infected Mice Treated with n-Butanol Fraction of the Root of Sesamum indicum in the Curative Test |
In the curative test of n-butanol fraction, there were a dose-dependent prevention effect of weight loss, temperature and PCV decline at all doses significantly (p<0.001) compared with negative control. However, the protective effect of those variables was lower (p<0.001) than the positive control. Additionally, there was also a statistically significant difference between the lower dose and higher treated group in preventing weight loss (p<0.01) and attenuating PCV decline (p<0.001) as shown in Table 6. The degree of parasitemia development in n-butanol fraction treated groups was slow as compared to the negative control dose dependently. In the chloroquine treated group the parasitemia level became zero after day 6 of treatment, which was the standard drug (Figure 1).
 |
Table 6 The Effect of n-Butanol Fraction of Sesamum indicum on Body Weight, Rectal Temperature and Packed Cell Volume of Infected Mice in the Curative Test |
 |
Figure 1 Parasitemia development throughout the 5-day treatment with n-butanol fraction of the root of Sesamum indicum in Rane’s model. |
Phytochemical Screening
This screening revealed the presence of secondary metabolites, including alkaloids, flavonoids, glycosides, polyphenols, saponins, steroids, tannins, and terpenoids in the crude extract. Saponins are absent in all solvent fractions, and alkaloid is also absent in the aqueous fraction (Table 7).
 |
Table 7 Phytochemical Screening of the Root Crude Extract and Solvent Fraction of Sesamum indicum |
Discussion
In this study, the crude extract and solvent fraction of Sesamum indicum was evaluated for its antimalarial activity in the 4-day suppressive test (a standard test for antimalarial screening) against early infection. Similarly, the n-butanol fraction, which was the most active fraction in 4-day suppressive test was also further investigated for its curative potential in established infection using Rane’s models. The P. berghei ANKA strain was used to induce malaria parasite,33 and chloroquine is presented as the standard drug (positive control) in experimental mice.34 Percentage parasitemia suppression and survival time are the dominant reliable parameters in in vivo antimalarial activity models.35 According to OECD guideline no. 425, the acute oral toxicity study showed that the plant extract did not produce mortality and no behavioral changes were observed in mice at 2000 mg/kg.
In the 4-day suppressive test, the crude extract and solvent fraction prevented parasitemia development and improved survival time in a dose-dependent manner. Since the percentage parasitemia suppression was greater than 30%36 at all doses, the plant extracts were assumed to be effective in mitigating33 early infection. This might lead to a total reduction in the pathogenic effect of the infection.37 From all experimental doses, 600mg/kg crude extract treated group produced maximum parasitemia suppression and 200mg/kg aqueous fraction produces a minimum parasitemia suppression. Similarly, when we also observed the mean survival time, the highest dose of the crude extract resulted in a longest survival time compared to the solvent fraction treated group at all doses as well as 200mg/kg and 400mg/kg crude extract treated groups. This result supports the assumption that secondary metabolites with antimalarial activity might be high in high-dose crude extracts. This was due to a combination of polar and nonpolar components having antimalarial activity. The suppression effect of the infection might be due to additive and/or synergistic potential of these compounds in the crude extract.
The n-butanol fraction showed a greater parasitemia suppression effect and prolonging survival time as compared to the other two solvent fractions in the 4-day suppressive test. In addition, the potential protective effect in weight loss, temperature and PCV drop was also best. This indicated that compounds having antimalarial activity were extracted more in the n-butanol fraction. The critical issue here is that the highest suppression potential of n-butanol extract might be due to additive and synergistic effects of secondary metabolites. This result is comparable with other reports.31,38 Therefore, further investigation of the n-butanol fraction in curative test was acceptable.
The curative activity of the n-butanol fraction evaluated in Rane’s test revealed that there was a gradual parasitemia development prevention. Throughout the course of treatment from day 3 to day 7, there were substantial increment of the inhibitory effect of this fraction to parasite development. This showed that there is a dose-dependent suppressive potential of n-butanol fraction in early and established infection as shown in Figure 1 using a two-way ANOVA. However, unlike standard drug, the level of parasitemia in this fraction did not reach zero even in the last date of treatment at all experimental doses. This is due to the fact that the specific active agent for the antimalarial activity was already isolated in chloroquine (standard drug) but not in the n- butanol fraction. This is consistent with other research on Hypoestes forskalei, which demonstrated a significant increase in the n-butanol fraction’s ability to limit the development of the parasite in Rane’s test.39
When we compared the results of the two models, the curative test demonstrated more parasitemia suppression in the n-butanol fraction than the 4-day chemo suppressive test. This could be as a result of the n-butanol fraction’s superior ability to mitigate against established infection compared to its schizontocidal effect against early infection. This is consistent with a study done on Olea europaea leaves crude extract and solvent fractions.31
Clinical features of malaria-infected mice like PCV decline, body weight loss and temperature reduction are linked with levels of parasitemia.40,41 Body weight loss is mainly associated with the loss of appetite, metabolism disturbance and hypoglycemic effect of the malaria parasite. Plant extracts that contain antimalarial active compounds are assumed to prevent the reduction of these parameters in addition to parasitemia suppression and survival time improvement. The crude extract of Sesamum indicum prevented weight loss, temperature decline, and PCV reduction in infected mice dose dependently. This finding is in agreement with other studies.42 The preventive effect of weight loss might be due to the presence of appetite-stimulant and nutrient contents of the plant material. Another option for the protective effect on weight loss, temperature drop and PCV decline might be associated with a reduction in the level of parasitemia by secondary bioactive metabolites. This is in agreement with the previous study on other plants.31
Likewise, in both models, the solvent fractions also attenuate the weight loss, temperature drop and hemolysis (anemia), which was substantially higher compared to the negative control. However, the protective effect was lower than in chloroquine treated groups. This might be due to the presence of secondary bioactive metabolites like alkaloids, polyphenols, flavonoids, glycosides, steroids, tannins and terpenoids that had the potential to stabilize these parameters.43,44 In addition, parasite suppression also probably controls pathological process, immune system and metabolic process, which later prevent abnormal weight, temperature and PCV changes. The extracts prevented RBC from oxidative stress mediated damage due to the presence of antioxidant polyphenols, tannins, and flavonoids. This is in line with similar studies conducted in other plant materials.43,45
Preliminary phytochemical screenings revealed that the crude extract of Sesamum indicum had various secondary metabolites such as alkaloids, flavonoids, glycosides, polyphenols, saponins, steroids, tannins and terpenoids. However, all three solvent fraction lacked saponins. In addition, the aqueous fraction also lacked alkaloids. The probable mechanism of secondary metabolites for their antimalarial activity is related with the stimulation of the host defense mechanisms (immunomodulation), free radical scavenging, and anti-oxidation, DNA intercalation, inhibition of growth and protein synthesis on the parasites.31,46 By preventing haem polymerization and creating a toxin for the parasite as a result, polyphenols may help with antimalarial activity.47 Similarly, it was discovered that steroids exercise their antiplasmodial function by changing the membrane of infected RBCs, which subsequently prevents the parasite from receiving vital nutrients through the RBCs.48 In addition, flavonoids attempt their antimalarial activity by preventing the entry of myoinositol and L-glutamine, which are necessary for the growth of parasites, into infected RBCs.49 Other secondary bioactive metabolites may also exert their antiplasmodial activity by directly impacting the pathogen or by indirectly stimulating natural and adaptive defense mechanisms of the host.50 As a result, the mechanism of action of the antimalarial activity of the crude extract and solvent fraction Sesamum indicum might be due to the combined or individual mechanism stated above.
Conclusion
This study generally revealed that the crude extract and solvent fraction of Sesamum indicum produce significant parasitemia suppression, survival time improvement and stabilize other parameters. Regarding the solvent fraction, the n-butanol fraction induces maximum suppression and a long survival time followed by the chloroform fraction in both tests. The secondary metabolites might be mainly responsible for the antimalarial activity of the plant material. Therefore, further study should be done to identify, isolate the active component and elucidate the structural activity relationship of this plant material.
Abbreviations
MST, Mean survival time; PCV, Packed cell volume; SEM, Standard error of the mean; OECD, Organization for Economic Cooperation and Development.
Data Sharing Statement
All data are available on the hand of the corresponding author.
Ethical Approval
The experimental protocol for the use of animals was in accordance with internationally accepted OECD guidelines, no 425 for the welfare of laboratory animals. Ethics approval was obtained from the Research and Ethics Committee of the School of Pharmacy of Addis Ababa University.
Acknowledgments
We thank Addis Ababa University (AAU) School of Pharmacy for their use of laboratory space and equipment.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The research was mainly self-sponsored.
Disclosure
The authors declared no conflicts of interest in this work.
References
1. De Ridder S, Van der Kooy F, Verpoorte R. Artemisia annua as a self-reliant treatment for malaria in developing countries. J Ethnopharmacol. 2008;120(3):302–314. doi:10.1016/j.jep.2008.09.017
2. Srivastava A, Creek DJ, Evans KJ, et al. Host reticulocytes provide metabolic reservoirs that can be exploited by malaria parasites. PLoS Pathog. 2015;11(6):e1004882. doi:10.1371/journal.ppat.1004882
3. Woyessa A, Deressa W, Ali A, Lindtjørn B. Prevalence of malaria infection in Butajira area, south-central Ethiopia. Malar J. 2012;11:1–8. doi:10.1186/1475-2875-11-84
4. Blagborough AM, Delves MJ, Ramakrishnan C, Lal K, Butcher G, Sinden RE. Assessing transmission blockade in Plasmodium Spp. Methods Protocols. 2013:9;577–600.
5. Waako PJ, Gumede B, Smith P, Folb PI. The in vitro and in vivo antimalarial activity of Cardiospermum halicacabum L. and Momordica foetida Schumch. Et Thonn. J Ethnopharmacol. 2005;99(1):137–143. doi:10.1016/j.jep.2005.02.017
6. Cloete TE. Resistance mechanisms of bacteria to antimicrobial compounds. Int Biodeterior Biodegradation. 2003;51(4):277–282. doi:10.1016/S0964-8305(03)00042-8
7. Idowu OA, Soniran OT, Ajana O, Aworinde DO. Ethnobotanical survey of antimalarial plants used in Ogun State, Southwest Nigeria. Af J pharmacy pharmacol. 2010;4(2):055–60.
8. Karunamoorthi K, Girmay A, Fekadu S. Larvicidal efficacy of Ethiopian ethnomedicinal plant Juniperus procera essential oil against Afrotropical malaria vector Anopheles arabiensis (Diptera: Culicidae). Asian Pac J Trop Biomed. 2014;1;4:S99–106. doi:10.12980/APJTB.4.2014C687
9. World Health Organization. World Malaria Report 2019. Geneva: World Health Organization; 2019.
10. World Health Organization. World Malaria Report 2022; 2021.
11. World Health Organization. World Malaria Report 2021; 2021.
12. Rosenthal PJ. Proteases of malaria parasites: new targets for chemotherapy. Emerg Infect Dis. 1998;4(1):49. doi:10.3201/eid0401.980107
13. WHO. The African Health Monitor: African Traditional Medicine Day. Geneva: World Health Organization; 2010.
14. Uprety Y, Asselin H, Dhakal A, Julien N. Traditional use of medicinal plants in the boreal forest of Canada: review and perspectives. J Ethnobiol Ethnomed. 2012;8(1):1–4. doi:10.1186/1746-4269-8-7
15. Chinsembu KC. Plants as antimalarial agents in Sub-Saharan Africa. Acta Trop. 2015;1(152):32–48. doi:10.1016/j.actatropica.2015.08.009
16. Bedigian D. Systematics and evolution in Sesamum L. (Pedaliaceae), part 1: evidence regarding the origin of sesame and its closest relatives. Webbia. 2015;70(1):1–42. doi:10.1080/00837792.2014.968457
17. Bajaj YP. Medicinal and aromatic plants I. Springer Sci Business Media. 2012;1:32.
18. Langham DR. Issues in new crops and new uses. Phenol sesame. 2007;144–182.
19. Smith DE, Salerno JW. Selective growth inhibition of a human malignant melanoma cell line by sesame oil in vitro. Prostaglandins Leukot Essent Fatty Acids. 1992;46(2):145–150. doi:10.1016/0952-3278(92)90221-4
20. Ensingmer AH, Ensminger ME, Konlande JE, Robson JR. Choline in food for health. A nutrition encyclopedia.
21. Hyun TH, Barrett-Connor E, Milne DB. Zinc intakes and plasma concentrations in men with osteoporosis: the Rancho Bernardo Study. Am J Clin Nutr. 2004;80(3):715–721. doi:10.1093/ajcn/80.3.715
22. Collinge W. The American Holistic Health Association complete guide to alternative medicine. Hachette UK. 2009:2;19.
23. Gidey M, Beyene T, Signorini MA, Bruschi P, Yirga G. Traditional medicinal plants used by Kunama ethnic group in Northern Ethiopia. J Med Plants Res. 2015;9(15):494–509. doi:10.5897/JMPR2014.5681
24. Habinshuti J, Akenga T. In vitro anti-malarial activity evaluation of extracts from plants often used in the East African Region. Eur J Med Plants. 2018;22(4):1–11. doi:10.9734/EJMP/2018/40227
25. OECD. Guideline for the testing of chemicals: acute oral toxicity; up-and down procedure (UDP). OECD, No. 2008:3;425.
26. Mothana RA, Al-Musayeib NM, Al-Ajmi MF, Cos P, Maes L. Evaluation of the In Vitro Antiplasmodial, Antileishmanial, and Antitrypanosomal Activity of Medicinal Plants Used in Saudi and Yemeni Traditional Medicine. Evid Based Complement Alternative Med. 2014;2014:1–7. doi:10.1155/2014/905639
27. Asrade S, Mengesha Y, Moges G, Gelayee DA. In vivo antiplasmodial activity evaluation of the leaves of Balanites rotundifolia (Van Tiegh.) Blatter (Balanitaceae) against Plasmodium berghei. J Exp Pharmacol. 2017;26:59–66. doi:10.2147/JEP.S130491
28. Fidock DA, Rosenthal PJ, Croft SL, Brun R, Nwaka S. Antimalarial drug discovery: efficacy models for compound screening. Nat Rev Drug Discov. 2004;3(6):509–520. doi:10.1038/nrd1416
29. Peters W, Robinson BL. The chemotherapy of rodent malaria. XLVII. Studies on pyronaridine and other Mannich base antimalarials. Ann Trop Med Parasitol. 1992;86(5):455–465. doi:10.1080/00034983.1992.11812694
30. Ryley JF, Peters W. The antimalarial activity of some quinolone esters. Ann Trop Med Parasitol. 1970;64(2):209–222. doi:10.1080/00034983.1970.11686683
31. Misganaw D, Engidawork E, Nedi T. Evaluation of the anti-malarial activity of crude extract and solvent fractions of the leaves of Olea europaea (Oleaceae) in mice. BMC Complement Altern Med. 2019;19(1):1–2. doi:10.1186/s12906-019-2567-8
32. Debella A. Manual for phytochemical screening of medicinal plants, Department of Drug Research, Ethiopian Nutrition and Research Institute. Addis Ababa, Ethiopia. Int J Med. 2002;45–71.
33. Waako PJ, Gumede B, Smith P, Folb PI. The in vitro and in vivo antimalarial activity of Cardiospermum halicacabum L. and Momordica foetida Schumch. Et Thonn. J Ethnopharmacol. 2005;99(1):137–143.
34. Pierrot C, Adam E, Lafitte S, et al. Age-related susceptibility and resistance to Plasmodium berghei in mice and rats. Exp Parasitol. 2003;104(1–2):81–85. doi:10.1016/S0014-4894(03)00134-6
35. Peter IAV. The current global malaria situation. Malaria parasite biology, pathogenesis, and protection. ASM Press, WDC. 1998;1:1–22 p.
36. Krettli A, Adebayo J, Krettli L. Testing of Natural Products and Synthetic Molecules Aiming at New Antimalarials. Curr Drug Targets. 2009;10(3):261–270. doi:10.2174/138945009787581203
37. Basir R, Rahiman SF, Hasballah K, et al. Plasmodium berghei ANKA infection in ICR mice as a model of cerebral malaria. Iran J Parasitol. 2012;7(4):62.
38. Alemu BK, Misganaw D. Antimalarial activity of Fagaropsis angolensis (Rutaceae) crude extracts and solvent fractions of its stem bark against Plasmodium berghei in mice. J Exp Pharmacol. 2021;5:683–693. doi:10.2147/JEP.S289478
39. Misganaw D, Amare GG, Mengistu G. Chemo suppressive and curative potential of Hypoestes forskalei against Plasmodium berghei: evidence for in vivo antimalarial activity. J Exp Pharmacol. 2020;7:313–323. doi:10.2147/JEP.S262026
40. Langhorne J, Quin SJ, Sanni LA. Mouse models of blood-stage malaria infections: immune responses and cytokines involved in protection and pathology. Chem Immunol. 2002;80(80):204–228. doi:10.1159/000058845
41. Cross CE, Langhorne J. Plasmodium chabaudi chabaudi (AS): inflammatory cytokines and pathology in an erythrocytic-stage infection in mice. Exp Parasitol. 1998;90(3):220–229. doi:10.1006/expr.1998.4335
42. Fentahun S, Makonnen E, Awas T, Giday M. In vivo antimalarial activity of crude extracts and solvent fractions of leaves of Strychnos mitis in Plasmodium berghei infected mice. BMC Complement Altern Med. 2017;17:1–2. doi:10.1186/s12906-016-1529-7
43. Asres K, Bucar F, Knauder E, Yardley V, Kendrick H, Croft SL. In vitro antiprotozoal activity of extract and compounds from the stem bark of Combretum molle. Phytotherapy Res. 2001;15(7):613–617. doi:10.1002/ptr.897
44. Girmaw F, Engidawork E. In Vivo Anti-Malarial Activity of the Aqueous Root Extract of Euclea divinorum Hiern (Ebenaceae) against Plasmodium berghei ANKA. Evid Based Complement Alternative Med. 2022;2022:30. doi:10.1155/2022/2640648
45. Okokon JE, Nwafor PA. Antiplasmodial activity of root extract and fractions of Croton zambesicus. J Ethnopharmacol. 2009;121(1):74–78. doi:10.1016/j.jep.2008.09.034
46. Muluye AB, Desta AG, Abate SK, Dano GT. Anti-malarial activity of the root extract of Euphorbia abyssinica (Euphorbiaceae) against Plasmodium berghei infection in mice. Malar J. 2019;18(1):1–8. doi:10.1186/s12936-019-2887-7
47. Taramelli D, Monti D, Basilico N, Parapini S, Omodeo-Sale F, Olliaro P. A fine balance between oxidised and reduced haem controls the survival of intraerythrocytic plasmodia. Parassitologia. 1999;41(1–3):205–208.
48. Elford BC, Cowan G, Ferguson D. Parasite-regulated membrane transport processes and metabolic control in malaria-infected erythrocytes. Biochem J. 1995;308(Pt 2):361. doi:10.1042/bj3080361
49. Saxena M, Saxena J, Nema R, Singh D, Gupta A. Phytochemistry of medicinal plants. J Pharmacogn Phytochem. 2013;1(6):22.
50. Masihi KN. Fighting infection using immunomodulatory agents. Expert Opin Biol Ther. 2001;1(4):641–653. doi:10.1517/14712598.1.4.641
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.