Back to Journals » OncoTargets and Therapy » Volume 11
Evaluation of supportive and barrier-protective skin care products in the daily prevention and treatment of cutaneous toxicity during systemic chemotherapy
Authors Lüftner D, Dell’Acqua V , Selle F, Khalil A, Leonardi MC , De La Torre Tomás A , Shenouda G, Romero Fernandez J, Orecchia R, Moyal D, Seité S
Received 28 October 2017
Accepted for publication 26 April 2018
Published 17 September 2018 Volume 2018:11 Pages 5865—5872
DOI https://doi.org/10.2147/OTT.S155438
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Video abstract presented by George Shenouda.
Views: 2358
Diana Lüftner,1 Veronica Dell’Acqua,2 Frédéric Selle,3 Ahmed Khalil,3 Maria Cristina Leonardi,2 Alejandro De La Torre Tomás,4 George Shenouda,5 Jesus Romero Fernandez,4 Roberto Orecchia,2,6 Dominique Moyal,7 Sophie Seité7
1Department of Hematology, Oncology and Tumor Immunology, Charité University Hospital Berlin, Campus Benjamin Franklin, Berlin, Germany; 2Department of Radiotherapy, IEO, European Institute of Oncology IRCCS, Milan, Italy; 3Tenon Hospital, Paris, France; 4Puerta de Hierro Hospital, Madrid, Spain; 5McGill Hospital, Montreal, Canada; 6Milan University, Milan, Italy; 7La Roche-Posay Dermatological Laboratory, Levallois-Perret, France
Introduction: The purpose of this multicenter, prospective, observational, open-label study was to evaluate the use and tolerability of dermo-cosmetic products in preventing skin reactions associated with cancer treatments.
Patients and methods: A 12-product kit was supplied to patients before chemotherapy began and was to be used throughout the treatment phase. Cutaneous adverse events were evaluated at each treatment session. Physicians evaluated skin reactions (edema, erythema, dryness, desquamation, pigmentation disorders, and cracks) and gave their opinion on the skin benefit for patients at the end of the study. Patients also evaluated the product benefit using the Patient Benefit Index (PBI) questionnaire. Results were analyzed by subgroups of casual and regular users, based on number and frequency of products used.
Results: A total of 147 patients were enrolled in cancer services in Germany, France, Italy, Spain, and Canada. Mean age was 59 years with 71% being female. Product tolerance on whole body was rated good to excellent for at least 89% of the patients for each product. Aggravated skin reactions during the study were reported more frequently by casual users than regular users (39.5% versus 22%; p=0.029). Similarly, casual users reported more erythema aggravation (p=0.02) and desquamation (p=0.03) than regular users. PBI >1 was reported for 95.5% of patients and regular users had significantly higher scores than casual users (p=0.049).
Discussion: Overall, the 12-product kit was very well tolerated, with regular users reporting benefits more frequently than casual users. Results support international recommendations to use appropriate skin care products to minimize the impact of cutaneous reactions associated with chemotherapy.
Keywords: dermocosmetic, dermatological toxicity, skin side effect, skin reaction, palliative care
Introduction
Chemotherapy and combined radio- and chemotherapy protocols have improved the prognosis and long-term survival for many malignancies. This means that millions of people are living with the diagnosis of cancer. However, chemotherapy and combination therapy with other medical and radiation therapies are associated with an array of adverse cutaneous reactions; this is true whether therapy is classic mono- or polychemotherapy, hormonal agents, or newer targeted therapies. These cutaneous toxicities are not only frequent but also highly visible on the face, arms, and/or upper torso. Thus, patients and treating physicians increasingly need to manage the acute cutaneous reactions associated with chemotherapy.1,2
Skin toxicities associated with classic cytotoxic drugs and hormonal therapy include dry skin, as well as hair and nail alterations. Antimetabolites such as capecitabine and 5-fluorouracil can cause hand–foot syndrome which is characterized by erythema, swelling, blisters, ulceration, and desquamation on the palms and soles, associated with paresthesia, pain, and pruritus.3 Alkylating agents are associated with alopecia, hyperpigmentation, and recall radiation dermatitis.4 Anti-spindle or anti-mitotic agents including vinca alkaloids and taxanes cause nail changes, maculopapular rash, erythema, and alopecia.4 In addition, DNA modifiers such as bleomycin and topoisomerase inhibitors can cause erythema, paronychia, alopecia, hyperpigmentation, and sclerosis.4
Inhibiting EGFR by using monoclonal antibodies and tyrosine kinase inhibitors commonly causes acneiform eruption, papulopustular rash, and xerosis. Acneiform eruption is experienced by 67%–86% of patients using EGFR inhibitors.5 Other common toxicities include paronychia (inflammation of the lateral nail wall), abnormal hair growth (including scalp, face, eye lashes), maculopapular rash, fissures, telangiectasia, mucositis, hand–foot syndrome, and postinflammatory hyperpigmentation.3,6–8 These side effects are unsurprising as EGFRs are expressed in the skin (keratinocytes, follicular epithelium, sweat glands, and sebaceous glands), and EGFR signaling is involved in the homeostasis of hair follicles.9 PD-1 immune checkpoint inhibitors are associated with rash, pruritus, and vitiligo.10–12
Frequently, dysfunction of both interfollicular keratinocytes and epidermal stem cell proliferation and differentiation is observed. This leads to clinical symptoms such as dry and scaly skin, observed often with most classes of chemotherapeutic treatments. The resulting inflammation and pruritus prompt scratching.13
Side effects of the skin, hair, and nails are usually not life threatening, but can impair patient quality of life, and in severe cases can lead to decreased compliance or even interruption of cancer treatment.14,15
For these reasons, research into supportive skin care to prevent and manage skin side effects of chemotherapy is becoming increasingly important. Emollients can prevent and treat eczema and xerosis by improving epidermal barrier integrity, while sunscreens can prevent hyperpigmentation and telangiectasias.9 Recent guidelines recommend gentle cleansing with an acidic product, hydration of the face and body with an emollient, and use of broad-spectrum sunscreen on exposed skin.16,17
This study investigates the use of a 12-product nonpharmaceutical skin care kit as a way to prevent and manage skin reactions to maintain quality of life in cancer patients undergoing chemotherapy (with or without radiotherapy).
Patients and methods
Study design
This prospective, observational open-label study without registration number was performed from July 18, 2013 until February 09, 2015 in 5 centers: Paris (France), Milan (Italy), Berlin (Germany), Madrid (Spain), and Montreal (Canada).
Patients
The study included patients before starting anticancer chemotherapy which could be combined with targeted agents, radiotherapy, or sequential treatments, and for which the investigating doctor expected dermatological side effects.
All skin phototypes from I to VI18 were considered. Patients were excluded if they had metastatic cancer, primary skin cancer, any preexisting skin disorders that could interfere with the results of the study (like atopic dermatitis, contact dermatitis, psoriasis, rosacea, severe photosensitivity, scleroderma, xerosis), and any known allergy to cosmetic ingredients or past history of allergy to cosmetic products.
Products
Once enrolled, each patient received a kit containing 12 commercially available, nonpharmaceutical skin care products and an information brochure explaining the indications, properties, and use of each product based on previously published guidelines.17 The products in the kit were specifically formulated with gentle ingredients that respect skin physiology and tested for use on sensitive skin. It included a thermal spring water spray, a body balm emollient, a body cleansing oil, an extra gentle shampoo, a wound healing balm, a repairing hand cream, a repairing foot cream, a face moisturizer for intolerant skin, a face cleanser for intolerant skin, and body and face SPF50+ sunscreens. Forty-seven female patients also received a silicium colorless nail polish. Tolerance of products was evaluated by patients for the whole body according to 4 categories (excellent, good, medium, and bad) including the irradiated area for patients with concomitant radiotherapy. At the end of the study, the investigating physician rated their opinion regarding the skin benefit of the product kit (very good, quite good, good, or neither good nor bad).
Evaluations
Throughout the course of the chemotherapy, and particularly at the initial visit and at the last visit (9±3 weeks later), related skin reactions (including edema, erythema, dryness, desquamation, pigmentation disorders, and cracks with a 4 grade-scale: absent, low [grade 1], moderate [grade 2], and severe [grade 3]) and cutaneous clinical signs (pruritus, skin pain, sensitivity, tingling, and burning sensations with a 10 cm-VAS, 0 for absence to 10 for maximal sensation) were assessed by the oncologists. Cutaneous comfort and tolerance of the products supplied were also evaluated. National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI CTCAE)-defined skin toxicities were recorded. Patient satisfaction was assessed using the Patient Benefit Index (PBI)19 as well as patient morale (via the well-being questionnaire).20 If a patient mentioned an adverse event during a treatment follow-up visit, an intermediate evaluation was performed. Noncutaneous adverse events were reported in this study only if the investigator judged it clinically significant.
Statistics
Analyses were performed with SAS software (version 9.4, SAS Institute Inc., Cary, NC, USA). Qualitative variables were described as absolute number and percentage, and quantitative variables as absolute number, mean, standard deviation, median, minimum, maximum, as well as number of missing data. The significance threshold was 5% except for normality tested at the threshold of 1% (Shapiro–Wilk test).
Different user groups were compared at baseline by the χ2 test for categorical variables, the parametric statistical analysis t-test for continuous variables, and the nonparametric Mann–Whitney test when the assumption of normality was questionable.
The primary criterion, aggravation of the cutaneous reactions, was defined by an increased clinical reactions score (sum of the grade × surface of each sign: erythema, dryness, edema, desquamation, pigmentation disorders, and cracks) between baseline and the end of study. These groups were compared by a χ2 test. A multivariate analysis for binary data (SAS proc glimmix) took into account the relevant variables, including the inhomogeneity between casual and regular users at baseline.
For other criteria, groups were compared by analysis of covariance using the baseline as covariable for quantitative data (SAS proc mixed) and by a binary or multinomial mixed model (SAS proc glimmix) for qualitative data.
Ethics and legal statement
Patients received an information leaflet explaining the study aims, duration, methods, constraints, and foreseeable risks associated with the product use. Every patient gave written informed consent according to the Declaration of Helsinki. All written medical data remained anonymous, and each patient was identified by a unique alphanumeric code comprising a 2-letter country code and a 3-digit number. The investigating physicians and all trial staff were to keep all trial information strictly confidential.
The electronic file compiling the data recorded in the study was submitted to the French Commission in charge of Personal Data Protection in September 2012 and was approved for data treatment. The study protocol and corresponding appendices were also transmitted to a French Research Ethics Committee (CPP, Poitiers, France) for information purposes in December 2012. The CPP confirmed the nonnecessity to get an approval for this study. However, each investigating center was responsible for submitting the protocol to its hospital ethics committee and obtaining the corresponding approval, whenever applicable. A copy of each written approval was sent to the monitoring center of this study.
Results
Patient characteristics
One hundred and fifty-five patients were included, and 8 of them were excluded from the analysis because of a lack of data after the baseline. The 147 patients who completed the study were recruited from 5 centers: Paris, France (31% of patients); Milan, Italy (28%); Berlin, Germany (23%); Madrid, Spain (12%); and Montreal, Canada (6%). Mean age (±SD) was 59±11 years (range: 28–84) and 71% of patients were female. Sixty-one percent had fair skin (skin types I–III), and 39% had dark skin (skin types IV–VI). Thirty-nine percent were treated for female cancer (ie, ovary or breast), 31% for digestive system cancer (ie, colon or rectum), 20% for a head and neck cancer, and 10% for other cancer types (including leukemia or pancreatic carcinoma). Sixteen percent were initiating chemotherapy alone (3% neoadjuvant chemotherapy, 31% adjuvant chemotherapy), 35% concomitant radiochemotherapy, and 15% sequential radiochemotherapy. Twenty-seven percent started a monochemotherapy and 73% a polychemotherapy. Types of chemotherapy included alkylating agents (80% of patients), antimetabolites (44%), anti-spindle agents (30%), DNA modifier (21%), and other molecules (12%). The mean number of planned chemotherapy cycles was 5.3±3.3 (range: 1–16) and mean cycle duration was 19.2±8.0 days (range: 5–51). Out of the female population, 14% had hormonal agents included in their treatment but only 2 women were taking those already at inclusion.
For patients receiving concomitant radio- and chemotherapy (n=51), the average area treated by radiotherapy was 12.2±7.7 palms (range: 2.5–36) and the total average dose of Gray received during treatment was 59±12 Gy (range: 10–70).
Throughout the study, 16% of patients had therapeutic regimen changes or dose adaptations.
Product use
Figure 1 shows the frequency of each product use during the study. The 5 most frequently used products were the cleansing oil, the hand cream, the body balm, the shampoo, and the repairing foot cream. Seventy-three percent of patients used 8 products or more during the study and 44% used 7 products or more, often, or every day. Three categories users were defined by the number and the frequency of products used (never, sometimes, often, and every day) as follows: casual users (0–6 products used often or every day), regular users (7 or more products used often or every day), and not evaluable (because of missing data). Seventy-nine patients were classified as casual users (54%), 63 as regular users (43%), and 5 patients (3%) were not evaluable. Table 1 presents patient demographics for casual and regular users.
  | Figure 1 Frequencies of use of the different products of the kit. |
Product tolerance on the whole body was rated good to excellent by more than 89% of the patients (Figure 2). For the 37 patients with concomitant radiotherapy, the tolerance of the 5 products used on the irradiated zone was rated good to excellent by more than 94% of the patients.
  | Figure 2 Evaluation of products’ tolerance on the whole body. |
Skin reactions, clinical signs, and skin toxicities
At baseline, 40 patients (27%) declared at least 1 skin reaction (16 patients had 1 symptom, 13 patients had 2 symptoms, 8 patients 3 symptoms, and 3 patients 4 symptoms). The most common skin reaction was dryness (26% of patients), followed by erythema (11%), edema (8%), desquamation (8%), pigmentation disorders (4%), and cracks (2%). Skin reactions were most commonly grade 1 (low), with lower incidence of grade 2 (moderate) reactions.
The patients were followed for 9 weeks on average. Aggravation of skin reactions (increased grade) during the study, the primary criterion, was reported by 44 patients (31%). Interestingly, aggravation was reported by a significantly lower percentage of regular users (22%) versus casual users (39.5%) (p=0.0295) (Figure 3). A similar trend for lower rates of aggravation in regular users was observed for erythema (p=0.0159) and desquamation (p=0.0275) (Figure 4).
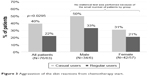  | Figure 3 Aggravation of the skin reactions from chemotherapy start. |
  | Figure 4 Evolution of the skin erythema (left) and skin desquamation (right) during the study. |
A multivariate analysis including confounding variables (variables showing differences between casual and regular users at baseline; country, gender, radiotherapy, presence of clinical sign(s) at baseline, chemotherapy by spindle poison or by antimetabolite) indicates that concomitant radiotherapy is highly linked to this aggravation. Seventy-eight percent of patients with concomitant radiotherapy showed an aggravation versus 7% of patients without. Of patients who underwent concomitant radiotherapy, aggravation was more frequent for casual (82%) versus regular users (69%) (p=0.47).
Clinical skin symptoms (pruritus, skin pain, sensitivity, and tingling and burning sensations) were increased compared to the baseline. However, the global scores for clinical symptoms were comparable between casual and regular users at baseline (2.6±4.6 and 3.05±4.8, respectively) and at the final visit (4.4±7.5 and 4.5±7.8, respectively).
During the study, 31 patients (21%) had a NCI CTCAE-defined skin toxicity: 15 patients (48.4%) had radiodermatitis, 10 (32.3%) had erythema, 2 (6.5%) had acneiform rash, and edema, pruritus or rash was reported for 1 patient each. The frequency of skin reactions was comparable between casual and regular users (p=0.30). At onset, skin toxicity was grade 1 (low) for 52% of patients and grade 2 (moderate) for 48%. Mean onset to skin reaction from chemotherapy initiation was 23.4±11.6 days (22.9±12.9 days for casual users, 25.6±7.3 days for regular users) with no significant difference.
Physician and patient opinion
At the end of the study, the investigating physicians rated their opinion regarding the skin benefit of the 12-product kit as being good. There was a higher percentage of good opinion reports for regular than casual users (100% versus 92%; p=0.0094).
The patient-assessed global morale score was numerically, although nonsignificantly (p=0.08) higher for regular than casual users at baseline (61±16 and 62±18, respectively) and at the final visit (59±16 and 63±16, respectively). PBI >1 indicating relevant benefit of the kit was reported for 100% of regular users versus 92% of casual users. Global PBI score was significantly higher for regular (3.23±0.7) than casual users (2.8±1.1; p=0.0495).
Discussion
To our knowledge, this is the first study to investigate the benefit of using a nonpharmaceutical skin care kit containing body and face cleansers, body, face, hand and foot moisturizers, a shampoo, a thermal spring water spray, a wound healing product, and a body and face sunscreen for preventing skin side effects induced during chemotherapy alone or with radiotherapy. This differs from previous research which studied 1 product or skin washing only.17 The most frequently used products in this case were the cleansing oil, the hand cream, the body balm, the shampoo, and the repairing foot cream, which appear the essential products in most cases. In this study, the subjects using the kit were patients with various malignancies who received chemotherapy alone or concomitant or sequential radiotherapy and chemotherapy. Study results provide additional support for the use of appropriate skin care protocol in the management of cutaneous reactions due to chemotherapy associated or not with radiotherapy. We found that 31% of patients had aggravated clinical signs, erythema, dryness, desquamation, edema, pigmentation disorders, and cracks during the study, but if patients received a concomitant radiotherapy the percentage reporting aggravation increased to 78% (versus 7% without concomitant radiotherapy). However, in a similar study, performed with breast cancer patients, 85% of patients had at least 1 skin toxicity appearing within 5 days from treatment initiation.21
These data indicate that radiotherapy induced more clinical skin signs (in 77.6% of treated patients) than chemotherapy alone or in sequential treatment with chemotherapy (in only 6.7% of treated patients). This is also supported by the fact that during this study, radiation dermatitis was a frequent skin toxicity. Therefore the use of appropriate skin care products to minimize the impact of secondary cutaneous reactions is more important in the case of radiotherapy (alone or associated with chemotherapy).
Nevertheless, as previously demonstrated for breast cancer patients receiving radiotherapy only, regular users of well-tolerated skin care products presented with skin toxicities later than low users. Interestingly, significantly fewer regular users had aggravated skin symptoms (22%) compared with casual users (40%).
This indicates that following a skin care routine with appropriate products could reduce the aggravation of skin reactions and could also treat some of them (disappearance of skin signs in 9% of patients). This further supports the current thinking that therapy-related cutaneous adverse events are linked to skin barrier dysfunction.17 In addition, these results were associated with a better overall opinion of investigating physicians and a higher relevant benefit for the patients. All the skin care products used during this study were well tolerated.
Limitations
Further controlled research is needed to confirm the efficacy of the individual products. Although the study was prospective, the design was limited by the absence of a control group. Also, it would have been valuable to have had more detailed recordings of the dermatologist’s clinical evaluations.
Acknowledgment
This study was funded by La Roche-Posay Laboratoire Dermatologique, France. The authors acknowledge Gene Colon, Amanda Whereat, Speak the Speech Consulting, and Fiona Dunlevy for assistance in writing this manuscript, as well as Nedjwa Abbadi MD, Morgane Bernard, and Christelle Guyomard from IRIS, the monitoring center in this study. We also thank, M Fortuné and G Le Dantec for technical assistance, and SYLIA-STAT for statistical analyses.
Disclosure
S Seité and D Moyal are employees of La Roche-Posay Laboratoire Dermatologique, France. The authors report no other conflicts of interest in this work.
References
Balagula Y, Rosen ST, Lacouture ME. The emergence of supportive oncodermatology: the study of dermatologic adverse events to cancer therapies. J Am Acad Dermatol. 2011;65(3):624–635. | ||
Skolarus TA, Holmes-Rovner M, Northouse LL, et al. Primary care perspectives on prostate cancer survivorship: implications for improving quality of care. Urol Oncol. 2013;31(6):727–732. | ||
Fabbrocini G, Cameli N, Romano MC, et al. Chemotherapy and skin reactions. J Exp Clin Cancer Res. 2012;31:50. | ||
Heidary N, Naik H, Burgin S. Chemotherapeutic agents and the skin: an update. J Am Acad Dermatol. 2008;58(4):545–570. | ||
Roe E, Garcia Muret MP, Marcuello E, Capdevila J, Pallares C, Alomar A. Description and management of cutaneous side effects during cetuximab or erlotinib treatments: a prospective study of 30 patients. J Am Acad Dermatol. 2006;55(3):429–437. | ||
Segaert S, Tabernero J, Chosidow O, et al. The management of skin reactions in cancer patients receiving epidermal growth factor receptor targeted therapies. J Dtsch Dermatol Ges. 2005;3(8):599–606. | ||
Segaert S, Chiritescu G, Lemmens L, Dumon K, Van Cutsem E, Tejpar S. Skin toxicities of targeted therapies. Eur J Cancer. 2009;45(Suppl 1):295–308. | ||
Lupu I, Voiculescu VM, Bacalbasa N, Prie BE, Cojocaru I, Giurcaneanu C. Cutaneous adverse reactions specific to epidermal growth factor receptor inhibitors. J Med Life. 2015;8(Spec Issue):57–61. | ||
Galimont-Collen AF, Vos LE, Lavrijsen AP, Ouwerkerk J, Gelderblom H. Classification and management of skin, hair, nail and mucosal side-effects of epidermal growth factor receptor (EGFR) inhibitors. Eur J Cancer. 2007;43(5):845–851. | ||
Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12–25. | ||
Abdel-Rahman O, Fouad M. Correlation of cetuximab-induced skin rash and outcomes of solid tumor patients treated with cetuximab: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2015;93(2):127–135. | ||
Goldinger SM, Stieger P, Meier B, et al. Cytotoxic cutaneous adverse drug reactions during anti-PD-1 therapy. Clin Cancer Res. 2016;22(16):4023–4029. | ||
Agha R, Kinahan K, Bennett CL, Lacouture ME. Dermatologic challenges in cancer patients and survivors. Oncology (Williston Park). 2007;21(12):1462–1472; discussion 1473, 1476, 1481 passim. | ||
Joshi SS, Ortiz S, Witherspoon JN, et al. Effects of epidermal growth factor receptor inhibitor-induced dermatologic toxicities on quality of life. Cancer. 2010;116(16):3916–3923. | ||
Jatoi A, Rowland K, Sloan JA, et al. Tetracycline to prevent epidermal growth factor receptor inhibitor-induced skin rashes: results of a placebo-controlled trial from the North Central Cancer Treatment Group (N03CB). Cancer. 2008;113(4):847–853. | ||
Perez-Soler R, Saltz L. Cutaneous adverse effects with HER1/EGFR-targeted agents: is there a silver lining? J Clin Oncol. 2005;23(22):5235–5246. | ||
Bensadoun RJ, Humbert P, Krutmann J, et al. Daily baseline skin care in the prevention, treatment, and supportive care of skin toxicity in oncology patients: recommendations from a multinational expert panel. Cancer Manag Res. 2013;5:401–408. | ||
Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124(6):869–871. | ||
Blome C, Augustin M, Behechtnejad J, Rustenbach SJ. Dimensions of patient needs in dermatology: subscales of the patient benefit index. Arch Dermatol Res. 2011;303(1):11–17. | ||
Pouwer F, Snoek FJ, van der Ploeg HM, Ader HJ, Heine RJ. The well-being questionnaire: evidence for a three-factor structure with 12 items (W-BQ12). Psychol Med. 2000;30(2):455–462. | ||
Berger A, Regueiro C, Hijal T, et al. Interest of Supportive and Barrier Protective Skin Care Products in the Daily Prevention and Treatment of Cutaneous Toxicity During Radiotherapy for Breast Cancer. Breast Cancer (Auckl). 2018;12. eCollection 2018. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

