Back to Journals » Psychology Research and Behavior Management » Volume 10
Evaluation of sleep profile in schizophrenia patients treated with extended-release paliperidone: an open-label prospective study in Southeast Asia
Authors Kongsakon R, Thavichachart N, Chung KF, Lim L, Azucena B, Rondain E, Go B, Costales F, Nerapusee O
Received 12 January 2017
Accepted for publication 30 June 2017
Published 27 October 2017 Volume 2017:10 Pages 323—327
DOI https://doi.org/10.2147/PRBM.S132272
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Igor Elman
Ronnachai Kongsakon,1 Nuntika Thavichachart,2 Ka Fai Chung,3 Leslie Lim,4 Beverly Azucena,5 Elizabeth Rondain,6 Benson Go,7 Fe Costales,8 Osot Nerapusee9
1Department of Psychiatry, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 2Department of Medicine, Division of Psychiatry, King Chulalongkorn Memorial Hospital, Chulalongkorn University, Bangkok, Thailand; 3Department of Psychiatry, University of Hong Kong, Hong Kong, People’s Republic of China; 4Department of Psychiatry, Singapore General Hospital, Singapore; 5National Center for Mental Health, Mandaluyong, Philippines; 6Makati Medical Center, Makati, Philippines; 7Northern Mindanao Medical Center, Cagayan De Oro, Misamis Oriental, Philippines; 8Perpetual Succour Hospital, Cebu, Philippines; 9Medical Affairs, Janssen‑Cilag, Bangkok, Thailand
Objective: To evaluate the effect of 6 months of treatment with paliperidone extended-release (ER) tablets on the sleep profile of patients with schizophrenia.
Methods: A total of 984 patients meeting the The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for schizophrenia who switched their antipsychotic to paliperidone ER were recruited from 61 sites in five countries in Southeast Asia. We recorded patient demographics and assessed sleep quality and daytime drowsiness using visual analog scales.
Results: Approximately 70% of patients completed the 6-month study. After the use of paliperidone ER, patients reported significantly better sleep quality (76.44 vs 65.48; p<0.001) and less daytime drowsiness compared with their baseline value (23.18 vs 34.22; p<0.001). Factors predicting sleep profile improvement were completion of the study and higher baseline Positive and Negative Syndrome Scale scores.
Conclusion: Paliperidone ER can help schizophrenia patients to improve sleep quality and reduce daytime drowsiness; this was seen especially in the patients who completed the 6-month treatment period and had higher baseline Positive and Negative Syndrome Scale scores.
Keywords: paliperidone ER, sleep profile, schizophrenia, CGI-S, PANSS
Introduction
One major concern in management of schizophrenia is sleeping domain. Because sleep difficulty is commonly reported in around 30%–80% of schizophrenia patients, and might due to the disease itself and from the effect of antipsychotic medication, reduction in quality of sleep might worsen daily activity function and reduce patients’ quality of life.1 In schizophrenia patients, their sleep pattern compared to healthy population is significantly different including the variation in sleep latency and density, which has various stages such as stage two sleep, slow-wave sleep, and rapid eye movement (REM).
First-generation antipsychotics show inconsistent effects in measures of sleep continuity and structure. Treatment of schizophrenia with first-generation antipsychotics might be associated with increases in total sleep time, sleep efficiency, and, primarily, an increase in REM latency; however, the influence on specific sleep stages is more variable. Accordingly, withdrawal of such treatment is followed by a change in sleep structure mainly in the opposite direction, with a deterioration of sleep quality. Also, there are studies suggesting that first-generation antipsychotics have shown rather inconsistent effects on sleep in healthy subjects, but seem to exert effects indirectly on sleep in patients with schizophrenia on high-potency drugs, and this is by suppressing stressful psychotic symptoms.2
In contrast, the available data regarding the use of second-generation antipsychotics (clozapine, olanzapine, risperidone, and paliperidone) demonstrate a relatively consistent effect on measures of sleep continuity in patients and in healthy subjects, with an increase in total sleep time and sleep efficiency, which results in improvement of sleep quality. Additionally, clozapine and olanzapine demonstrate comparable influences on other sleep variables, such as slow-wave sleep or REM density, in controls and schizophrenic patients.3
Paliperidone extended-release (paliperidone ER) formulation is recommended for use as once-daily dosing for the treatment of schizophrenia patients. Paliperidone ER has shown efficacy and safety data at the recommended dose between 3 and 12 mg per day in three clinical studies. The results from these studies suggest that paliperidone significantly improves personal and social function scores compared to placebo, which might be due to the improvement of sleep function.4–6 The efficacy data of paliperidone in schizophrenia symptom control is established in both acute and maintenance setting.7,8 For the sleeping domain, available evidence suggests that paliperidone ER might increase the sleep quality and reduce daytime drowsiness.9 However, there is still limited evidence to prove the effect of paliperidone ER on sleeping domain, and especially the available data are very scarce in Asian population. Therefore, the main objective of this study was to evaluate the sleep profiles after the use of paliperidone ER in Southeast Asian patients with schizophrenia.
Methods
Population
The study was conducted during June 2007 to April 2009. We recruited 984 patients with schizophrenia from five countries in Southeast Asia (Thailand, Singapore, Malaysia, Hong Kong, and the Philippines). The diagnosis of schizophrenia was based on The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria. Our focus population includes patients who have had previously unsuccessful treatment with oral atypical antipsychotic medication, either because of unsatisfactory efficacy or tolerability, or due to other reasons such as lack of compliance or loss to follow-up. Inclusion criteria also included age (at least 18 years old), provision of signed informed consent, and good physical health based on vital screening and baseline physical examination. Exclusion criteria included patients who had received other long-acting antipsychotics during the 3 months preceding the study or those with known hypersensitivity to risperidone or paliperidone. This study was reviewed and approved by the responsible ethics committee within each country*.
*Ethics committees in each country are as follows:
- Hong Kong: Institutional Review Board of the University of Hong Kong
- Malaysia: Medical Research and Ethics Committee Ministry of Health Malaysia and University Malaya Medical Centre Medical Ethics Committee
- Philippines: St. Luke’s Institutional Ethics Review Board
- Singapore: Parkway Independent Ethics Committee, National Healthcare Group Domain Specific Review Board and Singapore General Hospital Institutional Review Board
- Thailand: Joint Research Ethics Committees, Bangkok, Thailand
Assessment
Patients enrolled in the study received paliperidone ER for 6 months. Throughout the study, flexible dosing of 3–12 mg per day was given, depending on efficacy and tolerability. Assessments of efficacy and safety were carried out at baseline and throughout the study period. To examine efficacy, we used Positive and Negative Syndrome Scale (PANSS; total and subscale scores) and Clinical Global Impression severity score, personal and social performance scale, and patient satisfaction score. For tolerability, safety assessments were monitored throughout the 6-month study period and comprised adverse events reporting, pregnancy testing, extrapyramidal symptom scoring, physical examination, and vital signs. Concomitant medication including the use of benzodiazepines and other sleeping pills was recorded throughout the study period. Missing data were handled using the last-observation-carried-forward method.
For assessment of the drug’s effects on sleep, we used patient-completed visual analog scales of sleep quality and daytime drowsiness, scored from 0 to 100 (higher scores indicating better sleep quality in sleep quality scale and higher score indicating more daytime drowsiness in daytime drowsiness scale). We collected these data at three periods of the study: baseline, after 1 month of treatment, and at the end of the study.
Statistical analysis
All subjects who received paliperidone ER at least once were included in the analysis. Descriptive statistics were used to analyze demographic data and baseline characteristics. For sleep profile (sleep quality and daytime drowsiness scales), we used paired t-tests to compare sleeping score at the end of study period to baseline. We also performed regression analysis using the Enter method to evaluate the important factors associated with sleep profile. Statistical tests for differences between the endpoint and baseline values were interpreted at the 5% significance level (two-tailed) without multiplicity adjustment.
Results
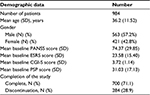
We recruited 984 patients with schizophrenia, with a mean age of 36.2 years. Of these, 70% completed the full 6 months of paliperidone ER treatment as specified in the study protocol. Patient characteristics including gender and baseline scores are summarized in Table 1.
Compared with baseline, patients receiving paliperidone treatment showed significant improvements in their sleep quality (76.44 vs 65.48; p<0.001; Table 2) and a significant reduction in daytime drowsiness (23.18 vs 34.22; p<0.001).
  | Table 2 Sleep profile Notes: Statistical analysis using paired t-test. |
We also conducted linear regression analysis to explore factors associated with sleep quality. Multivariate analysis indicated that mean baseline PANSS score and completion of the study are the important predictive factors for improvement of sleep quality (Table 3).
The daytime drowsiness scale showed a similar (inverse) pattern to the sleep quality scale, with multivariate analysis indicating that mean baseline PANSS score and completion of the study predict reductions in daytime drowsiness (Table 4).
Discussion
The present study demonstrates that paliperidone ER improves sleep profile in people with schizophrenia. Patients receiving paliperidone ER showed improvements in sleep quality and daytime drowsiness compared to baseline values (before the switch to paliperidone ER).
Our results are consistent with those of Luthringer et al,9 which suggested that paliperidone ER has a positive effect on sleep architecture, continuity, and patient-rated sleep quality, without producing or worsening daytime sleepiness. Therefore, the improvement of schizophrenia symptoms by this antipsychotic might be associated with improvements in sleep quality and a resulting decrease in daytime drowsiness.
The effect of paliperidone on sleep might reflect its pharmacodynamics. Paliperidone ER has a very high affinity for serotonin and dopamine receptors, which underlies its efficacy in the treatment of schizophrenia. Conversely, it has very little effect on muscarinic acetylcholine receptors, which might be the reason for the lower rates of sleep disturbance observed with paliperidone ER compared with other antipsychotics with a high affinity for cholinergic receptors.10
People who have higher PANSS scores at baseline seem to have a better quality of sleep and greater reduction of daytime drowsiness after treatment with paliperidone ER. This finding is consistent with previous results that showed that schizophrenia patients with higher PANSS scores have a greater response to antipsychotics than those with lower PANSS scores.11
Another observation from the present study was that patients within the completion group had significantly better quality of sleep, which reflects the association between compliance in taking antipsychotic medication and schizophrenia treatment outcomes.12 Together, these results highlight the importance of improving patient compliance in the overall outcome of schizophrenia treatment, including sleep profile.
Our study has some limitations. As we used subjective measures of sleep quality and daytime drowsiness, ie, patients’ self-administered questionnaires, this might lead to reporting bias that can reduce the validity and the reliability of the study result. Further studies using objective outcomes including sleeping indicated parameters or sleeping architecture graph might give a more validated result. Also, as the concomitant medication and concomitant disease might have confounding effects on the result, the results might need to be adjusted. However, we could not measure and evaluate the effect of concomitant medication and concomitant disease in this study.
A recent systematic review and meta-analysis revealed that patients with schizophrenia have a greater degree of sleep dysfunction than healthy subjects.13 Therefore, medications that improve sleep could be beneficial for patients with schizophrenia.
Conclusion
Paliperidone ER can help schizophrenia patients to improve sleep quality and reduce daytime drowsiness, and this was seen especially in the patients who completed the 6-month treatment period and had higher baseline PANSS score.
Disclosure
Osot Nerapusee is an employee of Janssen-Cilag. The authors report no other conflicts of interest in this work.
References
Miller DD. Atypical antipsychotics: sleep, sedation and efficacy. J Clin Psychiatry. 2004;6:3–7. | ||
Monti JM, Monti D. Sleep disturbance in schizophrenia. Int Rev Psychiatry. 2005;17(4):247–253. | ||
Cohrs S. Sleep disturbance in patients with schizophrenia: Impact and effect of antipsychotic. CNS Drug. 2006;22(11):939–962. | ||
Marder SR, Kramer M, Ford L, et al. Efficacy and safety of paliperidone extended release tablets: results of a 6-week, randomized, placebo-controlled study. Biol Psychiatry. 2007;62:1363–13670. | ||
Davidson M, Emsley R, Kramer M, et al. Efficacy, safety and effect on functioning of paliperidone extended-release tablets in the treatment of acute schizophrenia: an international 6-week placebo-controlled study. Schizophrenia Res. 2007;93:117–130. | ||
Kane J, Canas F, Kramer M, et al. Treatment of schizophrenia with paliperidone extended-release tablets: a 6-week placebo-controlled trial. Schizophrenia Res. 2007;90:147–161. | ||
Meltzer H, Kramer M, Gassmann-Mayer C, et al. Efficacy and tolerability of oral paliperidone extended-release tablets in the treatment of acute schizophrenia: pooled data from three 6-week placebo-controlled studies. Int J Neuropsychopharmacol. 2006;9:S225. | ||
Kramer M, Kushner S, Vijapurkar U. Delaying symptom recurrence in patients with schizophrenia with paliperidone extended-release tablet: an international, randomized, double-blind, placebo-controlled study. Presented at the Collegium Internationale Neuro-Psychopharmacologicum; July 9–13; 2006; Chicago, IL. | ||
Luthringer R, Staner L, Noel N, et al. A double-blind, placebo-controlled, randomized study evaluating effect of paliperidone extended-release tablets on sleep architecture in patients with schizophrenia. Int Clin Psychopharmacol. 2007;22:299–308. | ||
Janicak P, Winans E, Paliperidone ER. A review of the clinical trial data. Neuropsychiatric Disease Treatment. 2007;3(6):869–883. | ||
Thavichachart N, Kongsakon R, Lo WT, et al. The psychopathological characteristics of treatment discontinuation group in 6-month treatment with paliperidone ER. Int J Clin Pract. 2012;66(10):969–975. | ||
Birnbaum M, Sharif Z. Medication adherence in schizophrenia: patient perspectives and the clinical utility of paliperidone ER. Patient Prefer Adherence. 2008;2:233–240. | ||
Chan MS, Chung KF, Yung KP, Yeung WF. Sleep in schizophrenia: A systematic review and meta-analysis of polysomnographic findings in case-control studies. Sleep Med Rev. 2017;32:69–84. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.