Back to Journals » International Journal of Nanomedicine » Volume 9 » Supplement 2
Evaluation of silica nanoparticle toxicity after topical exposure for 90 days
Authors Ryu HJ, Seong N, So BJ, Seo H, Kim J, Hong J, Park M, Kim M, Kim Y, Cho K, Seo MY, Kim M, Meang E, Son SW
Received 20 November 2013
Accepted for publication 7 February 2014
Published 15 December 2014 Volume 2014:9(Supplement 2) Pages 127—136
DOI https://doi.org/10.2147/IJN.S57929
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Hwa Jung Ryu,1,* Nak-won Seong,2,* Byoung Joon So,1 Heung-sik Seo,2 Jun-ho Kim,2 Jeong-Sup Hong,2 Myeong-kyu Park,2 Min-Seok Kim,2 Yu-Ri Kim,3 Kyu-Bong Cho,4 Mu yeb Seo,2 Meyoung-Kon Kim,3 Eun Ho Maeng,2 Sang Wook Son1
1Department of Dermatology, Korea University College of Medicine, Seoul, South Korea; 2Korea Testing and Research Institute, Gyunggi-Do, South Korea; 3Department of Biochemistry and Molecular Biology, Korea University College of Medicine, Seoul, South Korea; 4Department of Clinical Laboratory Science, Shinheung College, Uijeongbu, South Korea
*These authors contributed equally to this work
Abstract: Silica is a very common material that can be found in both crystalline and amorphous forms. Well-known toxicities of the lung can occur after exposure to the crystalline form of silica. However, the toxicities of the amorphous form of silica have not been thoroughly studied. The majority of in vivo studies of amorphous silica nanoparticles (NPs) were performed using an inhalation exposure method. Since silica NPs can be commonly administered through the skin, a study of dermal silica toxicity was necessary to determine any harmful effects from dermal exposures. The present study focused on the results of systemic toxicity after applying 20 nm colloidal silica NPs on rat skin for 90 days, in accordance with the Organization for Economic Cooperation and Development test guideline 411 with a good laboratory practice system. Unlike the inhalation route or gastrointestinal route, the contact of silica NPs through skin did not result in any toxicity or any change in internal organs up to a dose of 2,000 mg/kg in rats.
Keywords: silica nanoparticles, toxicity, dermal route
Introduction
Silica or silicon dioxide (SiO2) occurs naturally in the form of quartz or sand. It exists in one amorphous form or multiple crystalline forms.1 The crystalline forms have been well investigated for their role in developing pulmonary silicosis, a lung pneumoconiosis characterized by alveolar proteinosis and diffuse fibrosis.2 Toxicities of the amorphous synthetic silica particles and nano-sized particles have only recently been studied. Amorphous SiO2 nanoparticles (NPs) are nano-sized structures of SiO2, and they are used in various areas, such as sunscreen lotions, drug delivery, cosmetics, food, and chemical industries.3–5 Due to their small size, NPs have the possibility of internalization through different routes of penetration to the body. Since skin is the largest organ of the body, the likelihood of absorption of NPs through the skin is high. Hence, the evaluation of their potential penetration through the skin and their toxicity are essential. SiO2 NPs, often called silica, may have quite different physicochemical characteristics due to their larger surface area than macro SiO2. Although NPs are made of nontoxic material, evidence of their potential interactions with several biological systems in various ways is accumulating, compared with the evidence of toxicity of the corresponding bulk material.6 Certain materials may become toxic when they are present in nanometer size, despite no toxicity in bulk form. SiO2 NPs revealed cytotoxic effects in a few in vitro studies.7–9 Nabeshi et al10 demonstrated that SiO2 NPs induced reactive oxygen species (ROS) generation and DNA damage in HaCaT cells, whereas their bulk-sized counterparts displayed much-reduced responses. It was suggested by other studies that SiO2 NPs caused cellular toxicity from initiating intracellular oxidative stress.9,11,12 A report by Berg et al13 indicated that all cells in the pulmonary system, lung epithelial cells, and pleural mesothelial cells exhibited susceptibility to SiO2-induced oxidative stress and damages. SiO2 NPs also revealed toxicity in human epithelial intestinal cells in vitro.14 Moreover, silica-coated nanomaterials were reported to have crossed the blood–brain barrier.15 SiO2 NPs are one of the most frequently applied NPs to skin, since SiO2 NPs are added to cosmetic products for a better glide for smoother cosmetic application without leaving a sticky residue. Moreover, SiO2 NPs are used as a coating material to make photo-unstable substances inert. Additionally, due to light reflectance and transparent properties, SiO2 NPs are also frequently used as ingredients in foundations and sunscreens along with titanium dioxide or zinc oxide NPs. Due to the large surface area of the skin, there has been great public concern regarding systemic absorption of these NPs. A few reports suggested dermal penetration of SiO2 NPs, where dermal exposure of SiO2 NPs for 3 days resulted in penetration into the skin barrier with localization of SiO2 NPs at lymph nodes.16 Next, dermal exposure of SiO2 NPs for over 28 days resulted in systemic absorption into the liver and brain.10 Rancan et al6 also demonstrated that SiO2 NPs less than 75 nm in size could be absorbed through the skin layer, whereas translocation of SiO2 NPs greater than 75 nm in size did not occur. The skin penetration and cellular localizations of well-dispersed amorphous silica NPs with a diameter of 70 nm was analyzed in mice by Hirai et al.16 They suggested that SiO2 NPs could penetrate the skin barrier and could be transported to the lymph nodes after topical exposures for prolonged durations. These findings suggested that additional studies would be needed to investigate the penetration of SiO2 NPs through the skin and the biological effects following dermal exposure to silica NPs. Although Nabeshi et al10 reported the induced ROS generation and DNA damage by SiO2 NPs in human keratinocytes in vitro, Hudson et al17 demonstrated no toxicity from the subcutaneous injection of SiO2 NPs in vivo.
Thus, due to vast cosmetic and medical applications with SiO2 NPs, leading to frequent dermal contacts, their penetration and toxicity should be investigated further.
Material and methods
Chemicals and animals
Colloidal SiO2 nanoparticles 20 nm in size (SiO2EN20(−)) were obtained from E & B Nanotech Co., Ltd. (Ansan, South Korea). To shift the strong negative charge of silica to a positive direction, it was treated with L-arginine, as reported.18 The zeta potential of SiO2 was −40 mV as prepared, and with L-arginine coating it was −10 mV to −20 mV.
One hundred Sprague Dawley rats were used in the study. These experimental animals, aged 6 weeks and with body weights of 150–210 g, were obtained from an in-house animal facility. The rats were housed in an animal room and maintained at 21.8°C±1°C, and 50.8%±10% relative humidity, with an alternating 12-hour:12-hour light–dark cycle. The rats were housed in stainless steel cages, and rodent food (Cargill Agri Purina, Inc., Seongnam, South Korea) and reverse osmosis water were provided ad libitum. All procedures using animals were reviewed and approved by the institutional animal ethics committee. The Sprague Dawley rats were randomly divided into four groups. Different dosages of SiO2EN20(−) were dermally administered to the rats in the experimental groups for 90 days. The groups and applied doses are listed in Table 1. Group 1 (normal control) animals were treated with distilled water for 90 days. Groups 2–4 were dermally treated with SiO2EN20(−) at doses of 500, 1,000, and 2,000 mg/kg, respectively. An additional five male and five female rats from group 1 and group 4 were brought in and observed during a 14-day recovery period.
Subchronic toxicity study
A repeated 90-day dermal toxicity assessment was conducted as per the Organization for Economic Cooperation and Development (OECD) 411 guideline, with modifications of dosage, biochemical parameters, and histopathologic evaluations. To evaluate the toxicity of SiO2 NPs from repeated dermal exposures, an area of 5 cm by 6 cm on the back of each rat was closely clipped free of hair once a week. The SiO2 NPs were applied to approximately 10% of the total body surface (4 cm by 5 cm in rats). Sterile gauze was soaked with the SiO2 NP solution, attached to the hairless segment of the back, and fixed with nonirritant tapes, Tegaderm (3M, Saint Paul, Minnesota, USA) and Coban (3M), for 6 hours. After 6 hours of application, the site was wiped with sterile water. This procedure was repeated every day for 90 days.
Clinical observation
Body weight and food and water intake were measured weekly, and the animals were observed for signs of abnormalities and toxicity every day during the 90 days of treatment for all 100 rats, as well as for 20 rats in the recovery group during the 14-day recovery period.
Hematology and biochemical examination
Blood samples were taken from an abdominal artery after the animal was deeply anesthetized with isoflurane. The animals were fasted for 18 hours prior to the blood sampling. Hematological analysis was performed using an automatic hematological analyzer (ADVIA 120® Hematology System; Siemens, Erlangen, Germany). The blood parameters for each sample were measured as follows: white blood cell count, differential counts (neutrophils, lymphocytes, monocytes, eosinophils, basophils), red blood cell count, hemoglobin, hematocrit, mean cell volume, mean corpuscular hemoglobin, mean cell hemoglobin, mean cell hemoglobin concentration, and platelet count. The reticulocyte ratio was assessed for all animals using staining and microscopy techniques. Prothrombin time and activated partial thromboplastin time were performed with collected blood samples in a BD® Vacutainer with 9NC sodium citrate (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) after centrifugation at 3,000 rpm for 10 minutes, containing 3.2% sodium citrate as an anticoagulant. Biochemical analysis of serum samples was performed using automatic chemistry analyzers Hitachi 7060 (Hitachi Ltd., Tokyo, Japan) and EasyLyte (Medica Corporation®, Bedford, MA, USA). Biochemical parameters, total protein, albumin, albumin/globulin ratio, total bilirubin, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, creatinine, blood urea nitrogen, total cholesterol, triglycerides, blood glucose, calcium, inorganic phosphorus, creatine kinase (CK), sodium, potassium, and chlorine were measured.
Necropsy findings, organ weight measurement, and pathological examinations
At the end of the experiment, the animals were sacrificed. After exsanguination, the external surface, all orifices, the cranial cavity, and the thoracic and abdominal cavities and their contents were visually observed for any signs of gross abnormalities. Absolute organ weights, as well as relative organ weights (organ weight/body weight) were measured for each rat. For organs existing in multiples, the sum of those organs was measured. After collection, organs were preserved in 10% phosphate buffered formalin solution, and the testes were preserved in Davidson solution for the histopathological examination. The collected organs were skin, lymph nodes, salivary glands (submandibular), bone, bone marrow (sternum, femur), thymus, trachea, bronchial tubes, lungs, heart, thyroid gland, parathyroid glands, tongue, esophagus, stomach, small intestine, large intestine, liver, spleen, pancreas, kidneys, adrenal glands, bladder, seminal vesicles, prostate gland, testis, epididymis, ovaries, uterus, vagina, brain, pituitary gland, spinal cord, eyeballs, sciatic nerve, and skeletal muscle.
Statistical analysis
The data obtained regarding body weight, food and water consumption, hematology, biochemical examination, and organ weights were evaluated by one-way analysis of variance, after Levene’s test to examine the homogeneity of their variances. If homogeneous, the data were analyzed with the Scheffé multiple comparison test, and for the inhomogeneous cases, data were analyzed with Dunnett’s T3 multiple comparison test. The data were analyzed by SPSS (version 12.0; SPSS Inc., Chicago, IL, USA) and differences were considered to be significant when P-values were less than 0.05.
Results
After exposing rats to SiO2 NPs on the skin of the back intermittently for 90 days the following data were collected to evaluate the systemic toxicity.
Clinical observation
No animal death occurred during the experimental period. One female rat in the 500 mg/kg group developed alopecia on the back from 46 days to 49 days of SiO2EN20(−) administration, but she recovered by day 50. Other than this, there was no observable symptom. No observable difference in body weight and food intake was recorded between the experimental and control groups. On the other hand, significant decreases of water intake were noticed among male rats in the 500 mg/kg dosage groups in comparison to the control group at week 8. Decreased water intake was also observed among female rats in the 2,000 mg/kg dosage group at weeks 1 and 7, but there was no significant change in the recovery period in comparison to the control group.
Hematology and biochemical examination
The results of hematology and biochemical examination are shown in Tables 2, 3 and 4. Change in hematologic parameters was not observed during the application period, but significant decreases of lymphocytes (P<0.05), increases of large unstained cells (P<0.01), and decreases in the number of neutrophils (P<0.01) were noticed among female rats in the 2,000 mg/kg group during the recovery period. During the administration period, CK was significantly decreased in female rats dosed at 2,000 mg/kg (P<0.05), and significantly increased CK was noted among female rats dosed at 500 mg/kg (P<0.05). During the recovery period, decreased levels of alanine aminotransferase were recorded among male rats in the 2,000 mg/kg dosage group (P<0.05) in comparison to the vehicle control group. For the female rats, significantly decreased alkaline phosphatase (P<0.05) and increased total cholesterol (P<0.05) were measured in the 2,000 mg/kg dosage group in comparison to the control group.
Necropsy findings, organ weight measurement, and pathological examinations
Necropsy findings are summarized in Table 5. A left kidney fossa with diameter of 1 mm was observed in one male rat of the control group. One case of light brown discoloration was observed in the control group on the dorsal side of the bifurcation of the right lateral lobe and right medial lobe of the liver. A dark black spot was observed in the left lateral lobe of the liver in one male rat in the 500 mg/kg group. In female rats of the 500 mg/kg group, an area of light yellow necrosis was seen on the dorsal side of the right lateral lobe, and an area of yellow discoloration on the bifurcation of the right lateral lobe of the liver was noticed in another rat within the same group. A cyst of 1 mm was seen on the right kidney of a female in the 1,000 mg/kg dosage group. One male rat in the 2,000 mg/kg dosage group had an inflated lung, filled with foamy liquefied material. Weights of various organs among male and female rats were not significantly different in comparison to the control group.
 
|
Table 5 Necropsy findings of each group |
When the experimental group, recovery group, and high-dosage group were compared during the pathological examination, no toxicological effect was observed in relation to silica NPs among them. Trivial multiple hemorrhages were noticed in the liver of one male case from the 500 mg/kg dosage group. In addition, trivial multiple brown pigmented macrophage infiltrations were seen in the liver of one male rat from the recovery group of 2,000 mg/kg dosage. Moderate lobar necrosis and tension lipidosis were observed in one female case from the 500 mg/kg group. A cortical cyst with inflammatory cell infiltration in the kidney was seen in one female rat from the 1,000 mg/kg dosage group.
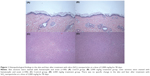
Figure 1 shows biopsy findings of the skin and liver after treatment with SiO2 NPs at a dose of 2,000 mg/kg for 90 days. For the biopsy findings of the skin, compared with the control group (Figure 1A), no abnormal change is shown in the SiO2 2,000 mg/kg group (Figure 1B). Biopsy findings of the liver after treatment with SiO2 NPs at a dose of 2,000 mg/kg for 90 days (Figure 1D) also show no change compared with the control group (Figure 1C).
Discussion
The present study investigated the localizations and toxic effects of SiO2 NPs after dermal administrations for 90 days. The general condition of the rats was recorded, including detailed evaluating parameters like body weight change, oral intake, hematologic and biochemical changes, and observation of organs. According to Hirai et al16 SiO2 NPs could be dispersed throughout the body through the lymphatic transport system; hence, they noted that the biological effects from dermal exposures of NPs should include an assessment of the whole body. Therefore, we investigated every possible organ after exposures of SiO2 NPs through skin, according to OECD recommendations with good laboratory practice.
From this study, no target organ was identified with signs of SiO2 NP toxicity. The pathologic findings of some organs did not have dose-related causes and seemed to be coincidental. Although some hematologic and biochemical parameters have shown a few changes, they did not have the corresponding changes in clinical observation or pathologic findings and are regarded as not related to SiO2 NP application. Therefore, the “no observed adverse effect level” of SiO2 NPs (20 nm, negative charge) would be less than 2,000 mg/kg body weight in both sexes of rats. To our knowledge, this is the first subchronic toxicity test of dermally applied SiO2 NPs. Nabeshi et al10 suggested that SiO2 NPs may penetrate the skin barrier and cause systemic exposure in mice after observing terminal deoxynucleotidyl transferase-mediated X-dUTP (terminal deoxynucleotidyltransferase-mediated addition of labeled [X] deoxyuridine triphosphate nucleotides) nick-end labeling (TUNEL), stating that TUNEL-positive cells were found after a 28-day application of 70 nm SiO2 NPs. They assumed that SiO2 NPs could move from the skin to the blood stream. Contrary to their report, the results of the present study revealed no localization of SiO2 NPs, and induced toxicity by SiO2 NPs was not found in any organ of the rats after 90 days of dermal application on the back. Although, a TUNEL assay was not done in the present study, it would be great to do so in further study, for comparison.
Several variables could influence the toxicity of SiO2 NPs, such as surface charge, size of the particles, and porosity of the SiO2 NPs.19,20 The human skin equivalent model, EpiDerm™ (MatTek Corporation, Ashland, MA, USA), was used to investigate the effect of the size and surface charge of SiO2 NPs on cutaneous toxicity.20 It was concluded that the smaller-size (20 nm) SiO2 NPs were more toxic than particles of a larger size (100 nm). The surface charge of NPs also seemed to influence their toxicity, where the negatively charged SiO2 NPs were more toxic than weakly negatively charged SiO2 NPs. In contrast, Rancan et al6 showed that positively charged SiO2 NPs had enhanced cellular uptake in vitro. However, weakly negatively charged SiO2 NPs tended to aggregate much more, which led to lower internalization ratios, especially by primary skin cells. Consequently, negatively charged SiO2 NPs may cause more toxicity. Lee et al19 demonstrated that colloidal SiO2 NPs, but not mesoporous SiO2, acted as an immunogenic sensitizer and induced contact hypersensitivity. Several other studies revealed that NPs could cause toxicity by ROS formation,10,21 where SiO2 NPs could also induce intracellular ROS generation. Eom and Choi22 demonstrated that SiO2 NPs exerted their toxic effects through oxidative stress, as they caused significant increases in cellular H2O2 concentrations. Interestingly, human bronchial epithelial cells, Beas-2B, were used in their study and heme oxygenase-1 induction by SiO2 NPs through the nuclear factor-E2-related factor-2 (Nrf-2-ERK) mitogen-activated protein kinase pathway was observed, rather than NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) activation, which was known to be the major stress response transcription factor in response to a wide variety of environmental stressors.22 In a study on the effects of SiO2 NPs on neuronal cells23 it was suggested that SiO2 NPs caused neuronal cell death and oxidative stress by elevating intracellular ROS, triggering DNA damage in a dose-dependent manner.
Since prolonged exposures of NPs are a great health concern, the evaluation of the systemic effects and presence of toxicity after 90 days of intermittent dermal exposure of SiO2 NPs on the skin of the back was performed. The present study did not show any evidence of internal organ injury or change in observed parameters up to a dose of 2,000 mg/kg/day in rats when SiO2 NPs were applied on the skin of the back for 90 days. Both the characteristics of NPs and their routes of administration are important. Unlike the inhalation and gastrointestinal routes, dermal contacts with SiO2 NPs are relatively safe. However, there are many differences between humans and rats in the degree of permeation and absorption of SiO2 NPs due to difference in hair follicles, lipid composition, and skin barrier function. Therefore, further studies using different-sized particles and surface charges should be warranted in humans due to continued concerns.
Conclusion
SiO2 NPs administered through the dermal route for 90 days by topical application on the skin of the back was safe, without any internal organ damage, up to a dose of 2,000 mg/kg in rats.
Acknowledgments
This research was supported by a grant (10182MFDS991) from the Ministry of Food and Drug Safety between 2010 and 2013, and Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT and Future Planning (NRF-2013R1A2A2A01068137).
Disclosure
The authors report no conflicts of interest in this work.
References
Speck-Hernandez CA, Montoya-Ortiz G. Silicon, a possible link between environmental exposure and autoimmune diseases: the case of rheumatoid arthritis. Arthritis. 2012;2012:604187. | ||
Hamilton RF Jr, Thakur SA, Holian A. Silica binding and toxicity in alveolar macrophages. Free Radic Biol Med. 2008;44(7):1246–1258. | ||
Rancan F, Nazemi B, Rautenberg S. Ultraviolet radiation and nanoparticle induced intracellular free radicals generation measured in human keratinocytes by electron paramagnetic resonance spectroscopy. Skin Res Technol. Epub 2013 Sep 3. | ||
Tago T, Tashiro S, Hashimoto Y, Wakabayashi K, Kishida M. Synthesis and optical properties of SiO2-coated CeO2 nanoparticles. J Nanopart Res. 2003;5(1–2):55–60. | ||
Izak-Nau E, Voetz M, Eiden S, Duschi A, Puntes VF. Altered characteristics of silica nanoparticles in bovine and human serum: the importance of nanomaterial characterization prior to its toxicological evaluation. Part Fibre Toxicol. 2013;10(1):56. | ||
Rancan F, Gao Q, Graf C, et al. Skin penetration and cellular uptake of amorphous silica nanoparticles with variable size, surface functionalization, and colloidal stability. ACS Nano. 2012;6(8):6829–6842. | ||
Chang JS, Chang KL, Hwang DF, Kong ZL. In vitro cytotoxicity of silica nanoparticles at high concentrations strongly depends on the metabolic activity type of the cell line. Environ Sci Technol. 2007;41(6):2064–2068. | ||
Jin Y, Kannan S, Wu M, Zhao JX. Toxicity of luminescent silica nanoparticles to living cells. Chem Res Toxicol. 2007;20(8):1126–1133. | ||
Lin W, Huang YW, Zhou XD, Ma Y. In vitro toxicity of silica nanoparticles in human lung cancer cells. Toxicol Appl Pharmacol. 2006;217(3):252–259. | ||
Nabeshi H, Yoshikawa T, Matsuyama K, et al. Systemic distribution, nuclear entry and cytotoxicity of amorphous nanosilica following topical application. Biomaterials. 2011;32(11):2713–2724. | ||
Park EJ, Park K. Oxidative stress and pro-inflammatory responses induced by silica nanoparticles in vivo and in vitro. Toxicol Lett. 2009;184(1):18–25. | ||
Sun L, Li Y, Liu X, et al. Cytotoxicity and mitochondrial damage caused by silica nanoparticles. Toxicol In Vitro. 2011;25(8):1619–1629. | ||
Berg JM, Romoser AA, Figueroa DE, Spencer West C, Sayes CM. Comparative cytological responses of lung epithelial and pleural mesothelial cells following in vitro exposure to nanoscale SiO2. Toxicol In Vitro. 2013;27(1):24–33. | ||
Sergent JA, Paget V, Chevillard S. Toxicity and genotoxicity of nano-SiO2 on human epithelial intestinal HT-29 cell line. Ann Occup Hyg. 2012;56(5):622–630. | ||
Kim JS, Yoon TJ, Yu KN, et al. Toxicity and tissue distribution of magnetic nanoparticles in mice. Toxicol Sci. 2006;89(1):338–347. | ||
Hirai T, Yoshikawa T, Nabeshi H, et al. Dermal absorption of amorphous nanosilica particles after topical exposure for three days. Pharmazie. 2012;67(8):742–743. | ||
Hudson SP, Padera RF, Langer R, Kohane DS. The biocompatibility of mesoporous silicates. Biomaterials. 2008;29(30):4045–4055. | ||
Kim KM, Kim HM, Choi MH. Colloidal properties of surface coated colloidal silica nanoparticles in aqueous and physiological solutions. Sci Adv Mat. 2014;6:1573–1581. | ||
Lee S, Yun HS, Kim SH. The comparative effects of mesoporous silica nanoparticles and colloidal silica on inflammation and apoptosis. Biomaterials. 2011;32(35):9434–9443. | ||
Park YH, Bae HC, Jang Y, et al. Effect of the size and surface charge of silica nanoparticles on cutaneous toxicity. Mol Cell Toxicol. 2013;9:67–74. | ||
Napierska D, Thomassen LC, Lison D, Martens JA, Hoet PH. The nanosilica hazard: another variable entity. Part Fibre Toxicol. 2010;7(1):39. | ||
Eom HJ, Choi J. Oxidative stress of silica nanoparticles in human bronchial epithelial cell, Beas-2B. Toxicol In Vitro. 2009;23(7):1326–1332. | ||
Kim YJ, Yu M, Park HO, Yang SI. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by silica nanomaterials in human neuronal cell line. Mol Cell Toxicol. 2010;6(4):336–343. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.