Back to Journals » Clinical Ophthalmology » Volume 15
Evaluation of Same-Day versus Next-Day Implantation of Intracanalicular Dexamethasone for the Control of Postoperative Inflammation and Pain Following Cataract Surgery
Authors Saenz B , Ferguson TJ , Abraham N, Mueller BH, Parkhurst GD
Received 14 August 2021
Accepted for publication 12 November 2021
Published 7 December 2021 Volume 2021:15 Pages 4615—4620
DOI https://doi.org/10.2147/OPTH.S334297
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Scott Fraser
Bobby Saenz,1 Tanner J Ferguson,2 Noelle Abraham,1 Brett H Mueller,1 Gregory D Parkhurst1
1Parkhurst NuVision, San Antonio, TX, USA; 2Cole Eye Institute, Cleveland Clinic, Cleveland, OH, USA
Correspondence: Bobby Saenz
Parkhurst NuVision, 9725 Datapoint, Suite 106, San Antonio, TX, 78229, USA
Tel +1-210-585-2020
Email [email protected]
Purpose: To evaluate the safety and efficacy of a sustained-release intracanalicular dexamethasone insert for postoperative inflammation and pain implanted in a clinical setting preoperatively or on postoperative day 1.
Methods: Single-site, retrospective, contralateral eye study of patients undergoing cataract surgery. Included were subjects with a dexamethasone intracanalicular insert implanted in the clinic immediately prior to surgery in one eye (same-day) and on postoperative day 1 (POD1) in the contralateral eye. The primary outcome measure was the resolution of anterior chamber inflammation at 1 week postoperative. Secondary outcome measures included proportion of eyes requiring additional therapy for pain and inflammation through 1 month as well as the number of eyes with IOP spikes above baseline. Safety measures included adverse events through 1 month postoperative.
Results: Sixty-two eyes of 31 subjects were included in the case series. At 1 week postoperative, 52% of the eyes (n = 16) achieved complete resolution of inflammation in the same-day group and 58% (n = 18) met this endpoint at 1 week in the POD1 group. One subject in the same-day group required additional therapy for rebound inflammation and no eyes required additional therapy in the POD1 group. There were no reports of pain at 1 week or 1 month in either group. There were no implant-related adverse events in either group.
Conclusion: The favorable results of this study indicate that the sustained-release dexamethasone insert can be safely implanted in the clinic either preoperatively on the day of surgery or on postoperative day 1 for the control of pain and inflammation following cataract surgery.
Keywords: cataract surgery, dexamethasone, intracanalicular, ocular inflammation, ocular pain
Introduction
Topical corticosteroids are a mainstay of medication regimens following cataract surgery to mitigate postoperative inflammation and patient discomfort. Despite the continued innovation within the realm of cataract surgery translating to excellent outcomes and safety in cataract surgery, postoperative inflammation remains a common occurrence and must be appropriately recognized and treated to prevent further sequelae.1,2 If uncontrolled or not adequately treated, persistent ocular inflammation can be subjectively perceived by the patient as pain and photophobia and can clinically manifest with elevated intraocular pressure (IOP), synechiae formation and macular edema.3,4 Optimal control of postoperative inflammation is paramount for patient satisfaction and achieving the best surgical outcome.
Topical corticosteroids, when administered correctly, are an excellent option for management of postoperative inflammation and remain widely used by cataract surgeons and clinicians.5 However, adherence to drops, complex medication regimens and difficulties with instillation are well-recognized barriers to effective treatment with topical therapies across the field of ophthalmology.6–8 This is particularly true in the cataract surgery population, a cohort of patients that not uncommonly has minimal to no experience with regular eyedrop use.8 A prior study8 evaluating patients’ eyedrop instillation techniques noted greater than 90% of the patients incorrectly performed the technique. Furthermore, in monitoring subjects, this aforementioned study8 also reported a notable discrepancy between the patients’ perception of their ability to correctly instill the drop and the observed technique.
In recent years, considerable innovation has occurred across ophthalmology as it relates to drug delivery to overcome the aforementioned obstacles with topical medication.9–11 Recently, a sustained-release dexamethasone intracanalicular depot (Dextenza, Ocular Therapeutix, Bedford, MA) was approved by the FDA for postoperative ocular inflammation and pain.12 The insert, which is placed within the canaliculus and contains 0.4 mg of active dexamethasone, aims to provide a sustained and tapered release of drug to the ocular surface over the course of 30 days. Clinical trials leading to its approval reported favorable results regarding postoperative inflammation and subjective pain.11,12 Further, additional recently published work13,14 has highlighted the value of the insert beyond cataract surgery including a recent study in patients following photorefractive keratectomy (PRK) by Ibach et al that showed patients strongly preferred the insert compared to topical steroid drops.15
This present study aimed to evaluate the outcomes of cataract surgery patients implanted in an office setting with a dexamethasone intracanalicular insert preoperatively on the day of surgery or on postoperative day 1. To our knowledge, no prior studies have assessed outcomes with the dexamethasone intracanalicular insert placed preoperatively in the clinic either same day or on postoperative day 1. To collect data, a retrospective, consecutive case review was performed.
Methods
This was a single-site, retrospective review performed at a single site (San Antonio, TX). A de-identified data set was generated for analysis. This study was conducted in compliance with the Declaration of Helsinki and was reviewed and approved by the Advarra Institutional Board with a waiver of consent due to the retrospective nature of the study.
Inclusion/Exclusion
Data in the consecutive case series were collected for subjects 18 years or older with visually significant cataracts undergoing standard phacoemulsification without adjunctive use of a femtosecond laser from May to September 2020. All data was collected and evaluated from a single site, in which the optometrist implanted all inserts in the clinic setting. Included were subjects with a dexamethasone intracanalicular insert implanted immediately prior to surgery in one eye and on postoperative day 1 in the contralateral eye, constituting a contralateral eye study. Subjects with the insert placed in both eyes on postoperative day 1 were excluded. Subjects with the insert placed in both eyes on the day of surgery were excluded. The eyes implanted on the day of surgery will be denoted the same-day surgery group for this report; the eyes implanted with the insert on postoperative day 1 were postoperative day 1 group, or POD1. Postoperatively, both groups were uniformly started on a topical medication regimen that included besifloxacin 0.6% (Besivance, Bausch & Lomb, Bridgewater, NJ) four times daily for 9 days starting 2 days before surgery and bromfenac 0.07% (Prolensa, Bausch & Lomb, Bridgewater, NJ) once daily for 4 weeks starting the day of surgery. Exclusion criteria included eyes with a history of corneal pathology (eg, Fuchs’), history of uveitis, or a history of severe glaucoma.
Main Outcome Measures
Preoperative data, collected in the baseline visit that typically occurred 1–2 weeks prior to the surgery, was used to establish a baseline. Postoperatively, data was collected at 1 day, 1 week and 1 month. At each time point, anterior chamber inflammation, quantitatively assessed by cells and flare, was graded 0–4+ based on the Standardization of Uveitis Nomenclature (SUN) grading scheme on slit-lamp biomicroscopy. Intraocular pressure (IOP) via Goldmann Applanation Tonometry (GAT) was also assessed. Subjects were surveyed on their pain with dichotomous form of questioning (with or without pain).
The primary outcome measure was the resolution of anterior chamber (AC) inflammation at the 1-week visit. Secondary outcome measures included the proportion of eyes requiring additional therapy for pain and inflammation through 1 month postoperative. Other secondary measures included the number of patients with elevated IOP >10 mmHg above baseline as well as the incidence of adverse events through 1 month postoperative.
Study Device
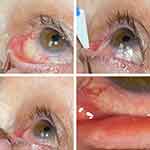
The sustained-release depot is a single-use insert containing 0.4 mg of active dexamethasone designed for intracanalicular use. The device was approved by the FDA for the treatment of ocular inflammation and pain associated with ocular surgery. The insert’s composition is polyethylene glycol (PEG) that utilizes hydrogel technology to deliver dexamethasone. The insert is also conjugated with fluorescein that enables visualization of the insert after it is inserted into the vertical canaliculus. With contact of fluid, the insert swells to securely conform to the canalicular location, allowing direct delivery of the preservative-free dexamethasone on to the ocular surface over the course of 30 days. Over time, with continuous hydrolysis, the insert gradually softens and degrades and is eventually cleared through the nasolacrimal drainage system without the need for removal. Figure 1 depicts the technique for implantation of the insert.
Statistical Analysis
Unpaired, parametric t-tests were performed at the 3 times points (1 day, 1 week and 1 month) postoperatively to compare the degree of inflammation between the two groups. The level of significance was taken to be 0.05. No formal power calculations were performed to assess sample size. All statistical analyses were performed using Prism 9 software (GraphPad Software, Inc.).
Results
Patient Demographics
Sixty-two eyes of 31 subjects were successfully implanted in the clinic with the dexamethasone insert either on the day of surgery or POD1. Data of this case series were available at 1 day, 1 week, and 1 month for all eyes. All subjects were evaluated by the same provider (B.S) at 1 day, 1 week and 1 month. The mean age of the subjects included in this series was 69.5 ± 9.0 years with 17/31 subjects being female. The baseline characteristics of the 31 subjects are summarized in Table 1.
 |
Table 1 Demographic and Clinical Characteristics |
Efficacy
In the same-day group, all eyes had presence of inflammation at the 1-day postoperative visit ranging from 1 to 2+ AC cell. By 1 week, 52% (n=16) of the eyes achieved complete resolution of inflammation; at 1 month, 100% of the eyes were absent of inflammation. One subject exhibited rebound inflammation 5 weeks after surgery that responded to a 3-week taper of topical loteprednol.
In the same-day group, 1 (3%) subject had evidence of IOP elevation >10 mmHg at the 1-week visit from 14 (baseline) mmHg to 26 mmHg. This subject was started on timolol once daily for 3 weeks and IOP was 16 mmHg at 1 month. The mean IOP at 1 week and 1 month was 15.9 ± 4.9 mmHg and 12.6 ± 3.5 mmHg, respectively. In this group, 100% of the subjects reported an absence of pain at the 1-week and 1-month time points.
In the POD1 group, all of the eyes included in the group had evidence of inflammation ranging from 1 to 2+ AC cells at 1 day postoperative. At 1 week, 58% (n=18) of eyes included in the series had complete resolution of inflammation. At 1 month, 97% (n=30) showed complete resolution of inflammation; there was 1 subject with evidence of 0.5+ AC cells at the 1-month visit. No eyes required additional or adjunct therapy for control of inflammation. There was no significant difference in the degree of inflammation between the POD1 and the same-day group at 1 day (p=0.17), 1 week (p=0.27) or 1 month (p=0.32). Table 2 compares the presence of anterior chamber inflammation at each time point.
 |
Table 2 Summary of the Presence of Anterior Chamber Inflammation at Each Time Point |
In the POD1 group, at 1 week, there were no instances of IOP elevation >10 mmHg above baseline. No eyes were treated with additional IOP-lowering medication. The mean IOP at 1 week and 1 month was 14.3 ± 4.4 mmHg and 13.1 ± 4.1 mmHg, respectively. In this cohort, at 1 week and 1 month, 100% of the subjects reported complete absence of pain.
There were no canalicular-related complications or adverse events related to implantation of the depot in either group. No patients in either the POD1 or same-day group required removal of the insert.
Discussion
This retrospective, contralateral eye study demonstrates that the 0.4 mg dexamethasone insert is a safe and effective option for control of postoperative inflammation following cataract surgery. In this series, in both the same-day surgery and POD1 group, greater than half the eyes in each group had complete resolution of AC inflammation by 1 week after surgery. By 1 month, there was only a single eye with evidence of inflammation between the two cohorts. In addition, there were no reports of pain at the 1-week or 1-month time point in either group, supporting the anti-inflammatory properties of the insert.
The results of this study indicate that the dexamethasone depot can be safely inserted preoperatively on the day of surgery or on postoperative day 1 for postoperative control of inflammation. Both groups demonstrated favorable results with regard to resolution of inflammation and there was no significant difference in clinical outcomes between the two eyes. Although the clinical trials and post-approval studies12,16,17 have primarily assessed the efficacy of the insert when inserted on the day of surgery following cataract surgery, the results of this present study offer meaningful implications for clinical practice. The favorable results with insertion either on the day of surgery or on postoperative day 1 promote the versatility of the device and indicate the insert can safely be inserted preoperatively on the day of surgery or on postoperative day 1. Additionally, to our knowledge, this study represents the first study to report outcomes assessing preoperative insertion of the dexamethasone insert.
Several prior studies11,12,16,18 have highlighted the safety and efficacy of the dexamethasone insert evaluated in this present study with regards to postoperative pain and inflammation. In a recent study by Tyson et al,16 a prospective, multicenter study compared the insert versus placebo and reported a greater proportion of patients in the dexamethasone insert arm met endpoints assessing the absence of pain and anterior chamber inflammation at key postoperative time points. In addition to excellent efficacy, the safety profile reported across these studies16 was also favorable with the dexamethasone arm demonstrating a similar safety profile in comparison to placebo. Furthermore, there were no intracanalicular complications, supporting the ease of insertion. Although limited by the lack of a control group, the findings of this present report corroborate those results described in the aforementioned RCTs in a real-world, clinical setting.
Adherence to topical medication regimens is a complex issue impacted by a multitude of factors.8,19 Factors such as inexperience, age, finance and complexity of medication regimens are all important considerations when starting a patient on a topical medication regimen. Postoperative medication regimens following cataract surgery, in particular corticosteroids, can add a layer of complexity for the patient owing to the tapering schedule and difference in dosing schedules between the eyes. Moreover, many patients who undergo cataract surgery are on existing medications and having to wait >5 minutes prior to drop instillation for each medication which creates a sizable burden. These challenges have prompted the development of nontopical medication options such as the sustained-release intracanalicular insert evaluated in this present study.
There are considerations unique to corticosteroids as it relates to nontopical and/or sustained drug-delivery medication options. The sustained-release insert described herein offers attributes that overcome the potential side effects and challenges of topical corticosteroid use.20,21 Topical agents, which are instilled intermittently, inevitably lead to variability in drug concentration owing to the poor bioavailability of the agent. The insert’s intracanalicular location, with proximity to the ocular surface, purports to offer a superior bioavailability profile with improved consistency of drug concentration in contrast to topical administration. The tapered delivery of drug from the insert over the course of 30 days minimizes the risk for rebound inflammation, which can occur with abrupt discontinuation of topical steroids. In addition, the preservative-free composition of the insert circumvents issues with preservatives and ocular surface toxicity, a well-recognized problem with long-term use of topical drops.22
This study is not without limitations. The sample size was modest in size and was performed at a single site. There was no control arm of patients using topical medications for comparison. The subjective method of pain assessment employed in this study is not standardized and is an acknowledged drawback of this study. Despite these limitations, the results of this contralateral eye study are compelling as the results are sourced from a real-world clinical population without strict inclusion criteria. The real-world study design provides results that are possibly generalizable to other practicing clinicians and supports the use of the insert in an office setting for control of postoperative inflammation.
Conclusion
The challenges of topical medication use are widespread and well recognized across ophthalmology. The results of the present study favor the use of the dexamethasone insert as a means to optimize medication delivery for patients without compromising safety or efficacy. The favorable outcomes in this report demonstrate that the sustained-release dexamethasone insert placed in the clinic either preoperatively on the day of surgery or postoperative day 1 is a safe and effective option for control of postoperative inflammation and pain following cataract surgery.
Disclosure
Dr Bobby Saenz reports grants from Ocular Therapeutix, during the conduct of the study; personal fees from Ocular Therapeutix, outside the submitted work. Dr Tanner J Ferguson reports personal fees from Equinox and Glaukos, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Juthani VV, Clearfield E, Chuck RS. Non-steroidal anti-inflammatory drugs versus corticosteroids for controlling inflammation after uncomplicated cataract surgery. Cochrane Database Syst Rev. 2017;7. doi:10.1002/14651858.CD010516.pub2
2. Cooke DL, Cooke TL. Comparison of 9 intraocular lens power calculation formulas. J Cataract Refract Surg. 2016;42(8):1157–1164. doi:10.1016/j.jcrs.2016.06.029
3. Chang DT, Herceg MC, Bilonick RA, Camejo L, Schuman JS, Noecker RJ. Intracameral dexamethasone reduces inflammation on the first postoperative day after cataract surgery in eyes with and without glaucoma. Clin Ophthalmol. 2009;3:345. doi:10.2147/OPTH.S5730
4. Porela-Tiihonen S, Kokki H, Kaarniranta K, Kokki M. Recovery after cataract surgery. Acta Ophthalmol. 2016;94(Suppl 2):1–34. doi:10.1111/aos.13055
5. Zafar S, Wang P, Schein OD, Srikumaran D, Makary M, Woreta FA. Prescribing patterns and costs associated with postoperative eye drop use in Medicare beneficiaries undergoing cataract surgery. Ophthalmology. 2020;127(5):573–581. doi:10.1016/j.ophtha.2019.11.005
6. Robin AL, Novack GD, Covert DW, Crockett RS, Marcic TS. Adherence in glaucoma: objective measurements of once-daily and adjunctive medication use. Am J Ophthalmol. 2007;144(4):533–540. doi:10.1016/j.ajo.2007.06.012
7. Gupta R, Patil B, Shah BM, Bali SJ, Mishra SK, Dada T. Evaluating eye drop instillation technique in glaucoma patients. J Glaucoma. 2012;21(3):189–192. doi:10.1097/IJG.0b013e31820bd2e1
8. An JA, Kasner O, Samek DA, Lévesque V. Evaluation of eyedrop administration by inexperienced patients after cataract surgery. J Cataract Refract Surg. 2014;40(11):1857–1861. doi:10.1016/j.jcrs.2014.02.037
9. Lewis RA, Christie WC, Day DG, et al. Bimatoprost sustained-release implants for glaucoma therapy: 6-month results from a Phase I/II clinical trial. Am J Ophthalmol. 2017;175:137–147. doi:10.1016/j.ajo.2016.11.020
10. Medeiros FA, Walters TR, Kolko M, et al. Phase 3, randomized, 20-month study of bimatoprost implant in open-angle glaucoma and ocular hypertension (ARTEMIS 1). Ophthalmology. 2020;127(12):1627–1641. doi:10.1016/j.ophtha.2020.06.018
11. Walters T, Bafna S, Vold S, et al. Efficacy and safety of sustained release dexamethasone for the treatment of ocular pain and inflammation after cataract surgery: results from two phase 3 studies. J Clin Exp Ophthalmol. 2016;7(4). doi:10.4172/2155-9570.1000572
12. Walters T, Endl M, Elmer TR, Levenson J, Majmudar P, Masket S. Sustained-release dexamethasone for the treatment of ocular inflammation and pain after cataract surgery. J Cataract Refract Surg. 2015;41(10):2049–2059. doi:10.1016/j.jcrs.2015.11.005
13. Suñer IJ, Peden MC. Dexamethasone sustained-release intracanalicular insert for control of postoperative inflammation after pars plana vitrectomy. Clin Ophthalmol. 2021;15:3859. doi:10.2147/OPTH.S330255
14. Trivedi RH, Wilson ME. A sustained-release intracanalicular dexamethasone insert (Dextenza) for pediatric cataract surgery. J AAPOS. 2021;25(1):43–45. doi:10.1016/j.jaapos.2020.10.001
15. Ibach MJ, Shafer BM, Wallin DD, Puls-Boever KR, Thompson VM, Berdahl JP. The effectiveness and safety of Dextenza 0.4 mg for the treatment of postoperative inflammation and pain in patients after photorefractive keratectomy: the RESTORE trial. J Refract Surg. 2021;37(9):590–594. doi:10.3928/1081597X-20210610-05
16. Tyson SL, Bafna S, Gira JP, et al. Multicenter randomized phase 3 study of a sustained-release intracanalicular dexamethasone insert for treatment of ocular inflammation and pain after cataract surgery. J Cataract Refract Surg. 2019;45(2):204–212. doi:10.1016/j.jcrs.2018.09.023
17. Greenwood MD, Gorham RA, Boever KR. A randomized fellow-eye clinical trial to evaluate patient preference for dexamethasone intracanalicular insert or topical prednisolone acetate for control of postoperative symptoms following bilateral femtosecond laser in site keratomileusis (LASIK). Clin Ophthalmol. 2020;14:2223. doi:10.2147/OPTH.S265311
18. Foster B. Same-day versus next-day dexamethasone intracanalicular insert administration for inflammation and pain control following cataract surgery: a retrospective analysis. Clin Ophthalmol. 2021;15:4091–4096. doi:10.2147/OPTH.S335764
19. Newman-Casey PA, Robin AL, Blachley T, et al. The most common barriers to glaucoma medication adherence: a cross-sectional survey. Ophthalmology. 2015;122(7):1308–1316. doi:10.1016/j.ophtha.2015.03.026
20. Lee A, Blair HA. Dexamethasone intracanalicular insert: a review in treating post-surgical ocular pain and inflammation. Drugs. 2020;1–8. doi:10.1007/s40265-019-01241-7
21. Blizzard C, Desai A, Driscoll A. Pharmacokinetic studies of sustained-release depot of dexamethasone in beagle dogs. J Ocul Pharmacol Ther. 2016;32(9):595–600. doi:10.1089/jop.2016.0025
22. Aguayo Bonniard A, Yeung JY, Chan CC, Birt CM. Ocular surface toxicity from glaucoma topical medications and associated preservatives such as benzalkonium chloride (BAK). Expert Opin Drug Metab Toxicol. 2016;12(11):1279–1289. doi:10.1080/17425255.2016.1209481
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.