Back to Journals » Clinical Ophthalmology » Volume 13
Evaluation of patients with dry eye for the presence of primary or secondary Sjӧgren’s syndrome
Authors Abd-Allah NM, Hassan AA, Omar G, Hamdy M , Abdelaziz STA , Abd El Hamid WM, Moussa RA
Received 28 May 2019
Accepted for publication 16 August 2019
Published 11 September 2019 Volume 2019:13 Pages 1787—1797
DOI https://doi.org/10.2147/OPTH.S217433
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Nashwa M Abd-Allah,1 Amal Aly Hassan,1 Gihan Omar,1 Mona Hamdy,1 Sahar Torky A Abdelaziz,2 Waleed Mahmoud Abd El Hamid,3 Rabab A Moussa4
1Rheumatology and Rehabilitation Department, Faculty of Medicine, Minia University, Minia, Egypt; 2Ophthalmology Department, Faculty of Medicine, Minia University, Minia, Egypt; 3Clinical Pathology Department, Faculty of Medicine, Minia University, Minia, Egypt; 4Pathology Department, Faculty of Medicine, Minia University, Minia, Egypt
Correspondence: Nashwa M Abd-Allah
Rheumatology and Rehabilitation Department, Faculty of Medicine, Minia University, New Minia City, first district, Youssef El-Sbaie Street, building (1), Minia 61511, Egypt
Tel +20 111 667 2116
Email [email protected]
Purpose: To assess the frequency of Sjӧgren’s syndrome (SS), either primary or secondary to rheumatic disease, in a cohort of patients with aqueous-deficient dry eye and to determine the most accurate objective test for diagnosis of SS.
Methods: A total of 111 patients with dry eye were recruited from Minia University’s Ophthalmology Outpatient Clinic (69 patients) and Rheumatology Outpatient Clinic (42 patients). The patients were screened for aqueous tear–deficient dry eye by abnormal test results of Schirmer test I (<10 mm) and tear-film break-up time (<10 seconds) in at least one eye. The diagnosis of SS was made according to the 2012 American College of Rheumatology criteria. A complete work up for SS was performed, including clinical examination, serological tests, ocular tests, and labial salivary–gland biopsy (LSGB).
Results: Of the 111 patients, 58 had aqueous-deficient dry eye: 23 in the ophthalmology clinic cohort (group I) and 35 in the rheumatology clinic cohort (group II). Three patients had pSS, and its frequency was 13% in group I and 5.2% among all studied patients. The ocular staining score is the most diagnostic ocular test (sensitivity 100% and specificity 90.9%). Anti-SSA/Ro antibody is the most accurate serological method (sensitivity 33.3% and specificity 100%). LSGB histopathology is the most diagnostic method for SS, with sensitivity, specificity, and positive and negative predictive values of 100%.
Conclusion: SS was detected with reasonable frequency among dry-eye patients, particularly pSS. Screening of dry eye for SS can select SS patients early in the disease course.
Keywords: dry eye, Sjӧgren’s syndrome, rheumatic disease, ocular staining score, OSS, labial salivary–gland biopsy, LSGB
Introduction
Dry-eye syndrome (DES) is a common chronic multifactorial condition of the ocular surface characterized by failure to produce high-quality or sufficient tears to moisturize the eyes.1 DES can substantially affect vision and quality of life, as symptoms often interfere with daily activities.2 The International Dry Eye Workshop classified DES into aqueous-deficient and evaporative. Aqueous tear–deficient dry eye occurs when there is lacrimal acinar destruction or dysfunction with reduced lacrimal tear secretion and volume. In evaporative dry eye, there is excessive water loss from the exposed ocular surface with normal secretory function of lacrimal gland. Aqueous tear–deficient dry eye has two major subclasses: Sjӧgren’s syndrome (SS) dry eye and non-SS dry eye.3
DES is one of the key clinical features and possibly an early clinical presentation of SS. SS is an autoimmune disorder involving multiple organs. The key feature of SS is lymphocyte infiltration of exocrine glands leading to dry eye and dry mouth, which are crucial presentations of SS.4,5 SS can present as either primary (pSS) or secondary (sSS). pSS is defined as the presence of aqueous-deficient dry eye in combination with dry mouth, sicca symptoms, extraglandular manifestations, autoantibodies, and positive focus score on labial salivary–gland biopsy (LSGB). sSS has features of pSS associated with autoimmune rheumatic diseases, such as rheumatoid arthritis (RA), which is the most common and systemic lupus erythematosus.6
SS is underdiagnosed in clinical practice because of its diverse presentation, leading to a significant delay in diagnosis.7 The lack of specific diagnostic tests combined with the high frequency of sicca symptoms in older adults makes the diagnosis of SS even more complicated.8 The importance of making the diagnosis is cardinal, because patients with SS are likely to have reduced quality of life as a result of pain, fatigue, depressed mood, and cognitive symptoms. More importantly, systemic involvement is common in pSS and associated with disease morbidity and mortality.8,9
Many classification criteria have been used for the diagnosis. The American College of Rheumatology (ACR) criteria classification 2012 includes three objective measures to simplify the diagnosis and have high specificity.10 The aim of the current study was to assess the frequency of SS, either primary or secondary to rheumatic disease, in a cohort of patients with aqueous-deficient dry eye and to determine the most accurate objective test for diagnosis of SS.
Methods
Patients
A total of 111 patients with dry eye were recruited from Minia University's Ophthalmology Outpatient Clinic (69 patients) and Rheumatology Outpatient Clinic (42 patients). All patients were attending Minia University Hospital from October 2015 to May 2017. Patients were complaining of foreign-body sensation, burning, stinging, itching, dryness, soreness, heaviness of the lids, photophobia, or ocular fatigue. Other causes of dry eye, such as Stevens–Johnson syndrome, mucous-membrane pemphigoid, corneal surgery, sarcoidosis, trachoma, hepatitis C virus infection, and use of anticholinergic drugs, were excluded. The study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of the Faculty of Medicine, Minia University. Written informed consent was obtained from patients to be included in the study.
Diagnostic approach for dry-eye patients for SS
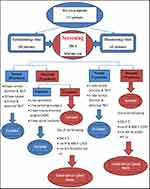
Patients screened as having aqueous tear –deficient dry eye by abnormal test results of Schirmer test I (<10 mm) and tear-film break-up time (TBUT) <10 seconds in at least one eye performed by an ophthalmologist. Patients with abnormal TBUT and Schirmer test I were selected for the presence of SS. Diagnosis of SS was made according to 2012 ACR classification criteria, which require at least two of three objective features: keratoconjunctivitis sicca with ocular staining score (OSS) ≥3, positive serum anti-SSA/Ro and/or anti-SSB/La or positive rheumatoid factor (RF) plus antinuclear antibody (ANA) titer ≥1:320, and LSGB exhibiting focal lymphocytic sialadenitis (FLS) with a focus score ≥1 focus/4 mm2.10 sSS was diagnosed by the previous criteria in addition to superimposed rheumatic disease evaluated by a rheumatologist. RA patients were diagnosed according to 2010 ACR–European League Against Rheumatism (EULAR) classification criteria for RA.11 Systemic lupus erythematosus (SLE) patients were diagnosed using 2012 Systemic Lupus International Collaborating Clinics classification criteria.12 Scleroderma patients were diagnosed by 2013 ACR–EULAR classification criteria for systemic sclerosis.13 A complete clinical evaluation for SS was performed, including age, sex, history of dry mouth and other sicca symptoms, salivary-gland enlargement, and symptoms/signs suggestive of disease-related extraglandular manifestations. The approach taken for dry-eye patients for diagnosis of SS is shown in Figure 1.
Ocular staining score
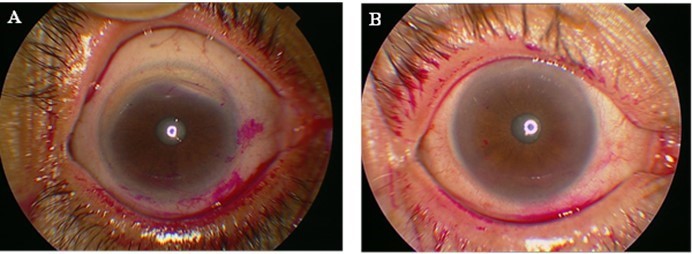
Tests were done to assess the extent of ocular surface damage using rose bengal dye. Scoring was done according to the van Bijsterveld scoring (vBS) system. A score ≥3 on vBS is considered abnormal and sufficient to cause the same score on the Sjögren’s International Collaborative Clinical Alliance (SICCA) OSS after taking the opinion of a ophthalmologist. Different OSS scores are shown in Figure 2.
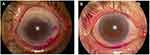 |
Figure 2 Ocular staining score (OSS). (A) OSS with rose bengal dye giving a score >3. (B) OSS with rose bengal dye giving a score <3. |
Serological tests
Serological tests included ANAs determined by indirect immunofluorescence assay on HEp2 cells (titer ≥1/320 considered positive) and RF detected by latex-agglutination test supplied by Spinreact. Antibodies to the extractable nuclear antigens, Ro/SSA and La/SSB, were detected by ELISA (Alegria assay supplied by Orgentec Diagnostika).
Labial salivary–gland biopsy
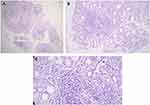
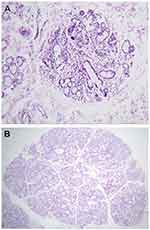
LSGB was performed in selected patients who had one of OSS ≥3, positive RF and ANA titer ≥1/320, and positive anti-Ro and/or anti-La. LSGBs were performed after local-anesthetic infiltration to harvest five to ten glands and then fixed in neutral-buffered formalin. Three to five formalin-fixed LSGPs were processed by the pathology department (paraffin embedding, sectioning, and H&E staining). Histological sections were analyzed under light microscopy (Olympus CX 23L) according to criteria of: number of salivary-gland lobules; percentage of adipose tissue on the salivary glands (<10% or >10%); absence or presence of ductal dilatation, acinar atrophy, and sclerosis of glandular connective tissue; acinar:ductal ratio (normal ratio — similar to normal glands, reduced ratio — with increased ductal component); the presence of lymphocytic foci (one focus is composed of at least 50 grouped lymphocytes); and ratio number of lobules:number of foci. A positive diagnostic biopsy for SS has been defined as FLS with a focus score ≥1 per 4 mm2.14 FLS is identified by the presence of one or more dense aggregates of ≥50 lymphocytes (usually several hundred or more lymphocytes), usually located in perivascular or periductal locations, adjacent to normal- appearing mucous acini in gland lobes or lobules lacking duct dilation or interstitial fibrosis, and containing no more than a minority proportion of plasma cells. This diagnosis is assigned when these foci are the only inflammation present in a specimen or the most prominent feature. Focus-score in the setting of FLS is then assigned by assessing the glandular area and calculating the number of lymphocytic foci present per 4 mm2 of glandular area by an equation (focus score = number of foci ×4/total glandular area). The total area of H&E-stained thin sections was determined by using the ocular micrometer scale (reticule). A focus score ≥1 is diagnostic for SS (Figure 3). Other histopathological features were considered:nonspecific chronic sialadenitis, granulomatous inflammation, lymphoma, or within normal limits (Figure 4).
Statistical analysis
Data collected were coded, tabulated, and statistically analyzed using SPSS 25. Descriptive statistics are given for parametric quantitative data as means ± SD and for nonparametric quantitative data as medians and IQR, while categorical data are presented as numbers and percentages. Distribution of the data was assessed by Shapiro–Wilk test.
Analyses were done between the two groups for parametric quantitative data using independent-sample t-tests and for nonparametric quantitative data using Mann–Whitney U tests. Analyses were done between the two groups for qualitative data using χ2 tests (expected number per cell >5) and Fisher’s exact tests (expected number per cell <5). Correlations between different variables were done using Spearman’s ρ correlation coefficient. Sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were assessed for prediction of SS. The level of significance was P<0.05.
Results
A total of 58 of 111 patients had aqueous-deficient dry eye were included in the current study: 23 (39.7%) in the ophthalmology clinic cohort (group I) and 35 (60.3%) in the rheumatology clinic cohort (group II). There were 52 females (89.7%) and six males (10.3%). Their age ranged from 25 to 75 years with a mean of 44.76±11.7 years. In group II, 30 (85.7%) patients had RA, four (11.4%) had SLE, and one (2.9%) had scleroderma. Patient characteristics are summarized in Table 1. OSSs showed severe ocular surface damage (OSS ≥3) in four (17.4%) patients in group I and four (11.4%) patients in group II. Patients in group II had more positive RF compared to group I (P=0.002). ANA was positive in one (4.3%) patient in group I with a titer of 1/40 and one (2.9%) patient in group II with a titer of 1/20. Only one (4.3%) patient from group I had antibodies to SSA/Ro, and none was positive for anti-SSB/La. LSGBs were necessary in seven (12.1%) patients to complete diagnosis of SS according to ACR 2012 criteria. Among the patients in group I, two had positive LSGBs (focus score ≥1), while no patients in group II had a positive biopsy. Figure 5 summarizes the diagnostic flow of the study in SS patients according to ACR criteria.
 |
Table 1 Characteristics of dry-eye patients studied |
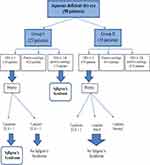 |
Figure 5 Diagnostic flow of study in Sjӧgren’s syndrome patients. Abbreviations: OSS, ocular staining score; FLS, focal lymphocytic sialadenitis; NSCS, nonspecific chronic sialadenitis. |
Frequency of Sjӧgren’s syndrome in dry-eye patients
After application of ACR criteria for SS, three patients had pSS, and its frequency was 13% in the ophthalmology clinic cohort. sSS was not found in our study among the rheumatology clinic cohort. The frequency of SS in all studied dry-eye patients was 5.2%. All SS patients had OSS ≥3, two were positive on biopsy, and one had anti-SSA/Ro. This patient with positive serology developed extraglandular manifestations, including fatigue, constitutional symptoms, arthralgia, and arthritis. RF and ANA were not detected in SS patients.
Comparison between Sjӧgren’s and non-Sjӧgren’s patients
Table 2 compares objective tests in patients with SS and non-SS. SS patients had more severe ocular dryness on Schirmer test (P=0.007) and OSS (P=0.002). SS patients more frequently displayed LSGBs compatible with SS (FLS ≥1, P=0.048). Comparison of autoantibodies between SS and non-SS patients did not show statistical significance.
 |
Table 2 Comparisons of ocular tests, autoantibodies, and LSGB with Sjӧgren’s syndrome |
Diagnostic values of ocular tests and different ACR parameters
Tables 3 and 4 show the results of such-and-such analysis. Schirmer's and the OSS were the most sensitive tests and produced similar sensitivity of 100%, but the OSS was more specific for diagnosis of SS, with specificity of 90.9% compared to 83.6% for the Schirmer test. In addition, OSS had the highest positive and negative predictive values (37.5% and 100%, respectively). TBUT had lower sensitivity (33.3%) and specificity (56.4%). Schirmer test and OSS were significantly positively correlated with SS (P<0.001 for each). Anti-SSA/Ro antibody produced the highest sensitivity (33.3%), specificity (100%), positive predictive value (100%), and negative predictive value (96.5%) for SS diagnosis compared to RF and ANA. Anti-SSA/Ro was significantly positively correlated with SS (P<0.001). LSGB exhibiting FLS focus score ≥1 was the most accurate for SS diagnosis, with sensitivity, specificity, and positive and negative predictive values of 100% and showing a significant positive correlation with SS (P<0.001).
 |
Table 3 Sensitivity, specificity, positive predictive value, negative predictive value and accuracy of ocular tests, autoantibodies and LSGB for diagnosis of Sjӧgren’s syndrome |
 |
Table 4 Correlations between ocular tests, autoantibodies, LSGB, and Sjӧgren’s syndrome |
Discussion
Diagnosis of SS is difficult and complex. There is no single diagnostic test with satisfactory validity. In clinical settings, it is not common for ophthalmologists to screen for SS among dry-eye patients, because extraglandular involvement is not very frequent and ophthalmologists are not familiar with systemic extraglandular manifestations. Different classification criteria have been set for diagnosis; however, the ACR 2012 classification includes three objective measures that can simplify the diagnosis.10
In the present study, three (5.2%) of 58 dry-eye patients had SS. They were pSS, resulting in a frequency of 11.4% in the ophthalmology clinic cohort. sSS was not detected in the studied patients. Other studies have reported different frequencies of SS among dry-eye patients. Akpek et al reported that of 220 patients with dry eye, 24 (10.9%) patients had pSS.6 Zhang et al studied the prevalence of SS in 327 patients with aqueous-deficient dry eye. They reported that 21 (6.4%) had pSS and 17 (5.2%) had sSS.15 Lee et al observed that SS was present in 58 (28%) of all dryeye patients and 39 (19%) patients showed pSS.16 Kan et al found that 14 of 45 dry-eye patients (31.1%) had pSS.17 Henrich et al found that the frequency of pSS in 228 dry-eye patients was 11%.18 Yen et al found incidence of developing SS of 4.8% for DES patients.19 These differences in frequency may be due to different study populations and use of different criteria for SS diagnosis.
The OSS (a parameter of the ACR) showed the best ratio of specificity (90.9%) to sensitivity (100%) for SS diagnosis, followed by Schirmer test (specificity 83.6% and sensitivity 100%). Both tests were significantly positively correlated with SS (P<0.001). TBUT had poor diagnostic value (33.3% sensitivity and 56.4% specificity). In agreement with other results, Versura et al proved that OSS (lissamine green staining) had the best diagnostic performance for SS (sensitivity 0.63 and specificity 0.89) and TBUT had poor diagnostic performance (sensitivity 0.92 and specificity 0.17). Conversely, they found that the Schirmer test showed poor diagnostic performance (sensitivity 0.42 and specificity 0.76).20 Contrary to our results, Akpek et al found the severity of DES measured by Schirmer test, TBUT, and OSS did not correlate with a diagnosis of SS.6
It has been shown that autoantibodies may be present before the onset of SS symptoms, as found in Jonsson et al and Theander et al;21,22 however, our study reported low frequency of autoantibodies in studied patients. This could be explained with different reasons. First, autoantibody positivity is associated with more active and systemic disease.23 Second, the presence of SSA/Ro antibodies is associated with a higher risk of extraglandular manifestations.6 Last, follow-up of pSS patients while seronegative has shown they remain polysymptomatic. Further, 39% of these seronegative patients were given revised diagnoses over the followup, which included RA, SLE, mixed connective-tissue disease, and scleroderma.24 The diagnostic value of autoantibodies in this study showed that anti-SSA/Ro antibody was the most accurate serological test, producing sensitivity of 33.3%, specificity 100%, PPV 100%, and NPV 96.5%. The correlation of SS with autoantibodies showed a significant positive correlation with anti-SSA/Ro (P<0.001), but not with other antibodies. Similarly, Theander et al found that anti-SSA/Ro was 51% sensitive and 98% specific for pSS diagnosis. However, the predictive value for developing pSS was highest for anti-SSB/La.22 In disagreement with our results, Solomon et al reported the average sensitivity of ANA in SS was 48% and specificity 52%.25 ACR classification by Shiboski et al showed that combined positive RF and ANA titer of ≥1/320 had reasonable specificity, but low sensitivity for SS diagnosis. They also found that anti-Ro and/or anti-La had the highest sensitivity and specificity.10 Akpek et al found that SS was significantly correlated with autoantibodies, not only with anti-Ro but also anti-La, ANA and RF.6 In Zhang et al, pSS was significantly more likely to occur in patients with positive ANA and RF.15
In the present study, positive LSGB allowed for a definitive diagnosis of SS, resulting in sensitivity, specificity, PPV, and NPV of 100%. There was also a significant positive correlation between LSGB and SS (P<0.001). As in previous studies, LSGB was considered the most accurate for diagnosis of SS. Guellec et al reported the sensitivity of LSGB ranged from 63.5% to 93.7% and specificity from 61.2% to 100%.26 Giovelli et al found that LSGB had high diagnostic value, with sensitivity 86.57%, specificity 97.43%, PPV 95%, and NPV 92.6%.27 Wicheta et al showed the sensitivity of LSGB was 82.3%, specificity 90%, PPV 66.6%, and NPV 95.4%.28 Another study of Wicheta et al found that a positive LSGB result allowed for a definitive diagnosis of SS in 80% of biopsied patients.29
There are some limitations of our study. The diagnostic values of objective tests for diagnosis of SS are not accurate, due to small number of SS patients detected in this study, LSGBs being performed for only seven patients. Assessment of keratoconjunctivitis sicca was done by vBS (maximum score 9) instead of SICCA OSS (maximum score 12). Accordingly, some patients who had negative vBS might have had positive SICCA OSS, so they might have been missed for SS diagnosis. There are no studies validating the conversion of SICCA score with the traditional vBS technique.30 Another problem is lack of use of ultrasound of salivary glands, which has proved to be a simple noninvasive method for early diagnosis of SS with high diagnostic value.31,32 Also, the inability to use tests for detection of novel antibodies, including anti-SP1, anti-CA6, and anti-PSP was a drawback. These autoantibodies were recently involved in SS diagnosis, particularly in the early stage of disease.33–35
Conclusion
SS was detected with reasonable frequency among dry-eye patients, particularly pSS. Screening of DES for SS can pick patients of SS early in the disease course. We recommend that clinical evaluation, ocular tests, and serological analysis be performed with any dry-eye patient for early diagnosis of SS.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Nelson JD, Craig JP, Akpek EK, et al. TFOS DEWS II Introduction. Ocul Surf. 2017;15(3):269–275. doi:10.1016/j.jtos.2017.05.005
2. Rouen PA, White L. Dry eye disease: prevalence, assessment, and management. Home Healthc Now. 2018;36(2):74–83. doi:10.1097/NHH.0000000000000652
3. Lemp MA, Baudouin C, Baum J, et al. The definition and classification of dry eye disease: report of the definition and classification subcommittee of the international dry eye workshop. Ocul Surf. 2007;5(2):75–92.
4. Tincani A, Andreoli L, Cavazzana I, et al. Novel aspects of Sjögren’s syndrome in 2012. BMC Med. 2013;11(1):93. doi:10.1186/1741-7015-11-93
5. Beckman KA, Luchs J, Milner MS. Making the diagnosis of Sjögren’s syndrome in patients with dry eye. Clin Ophthalmol. 2016;10:43–53. doi:10.2147/OPTH.S80043
6. Akpek EK, Klimava A, Thorne JE, Martin D, Lekhanont K, Ostrovsky A. Evaluation of patients with dry eye for presence of underlying Sjögren’s syndrome. Cornea. 2009;28(5):493–497. doi:10.1097/ICO.0b013e31818d3846
7. Romão VC, Talarico R, Scirè CA, et al. Sjögren’s syndrome: state of the art on clinical practice guidelines. RMD Open. 2018;4(Suppl 1):e000789. doi:10.1136/rmdopen-2018-000789
8. Baer AN, Walitt B. Sjögren syndrome and other causes of sicca in older adults. Clin Geriatr Med. 2017;33(1):87–103. doi:10.1016/j.cger.2016.08.007
9. Akpek EK, Mathews P, Hahn S, et al. Ocular and systemic morbidity in a longitudinal cohort of Sjögren’s syndrome. Ophthalmology. 2015;122(1):56–61. doi:10.1016/j.ophtha.2014.07.026
10. Shiboski S, Shiboski C, Criswell L, et al. American College of Rheumatology classification criteria for Sjögren’s syndrome: a data‐driven, expert consensus approach in the Sjögren’s International Collaborative Clinical Alliance Cohort. Arthritis Care Res. 2012;64(4):475–487. doi:10.1002/acr.21591
11. Funovits J, Aletaha D, Bykerk V, et al. The 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for rheumatoid arthritis: methodological report phase I. Ann Rheum Dis. 2010;69(9):1589–1595. doi:10.1136/ard.2010.130310
12. Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–2686. doi:10.1002/art.34473
13. van den Hoogen F, Khanna D, Fransen J, et al. Classification criteria for systemic sclerosis: an ACR-EULAR collaborative initiative. Arthritis Rheum. 2013;65(11):2737. doi:10.1002/art.38098
14. Daniels TE, Cox D, Shiboski CH, et al. Associations between salivary gland histopathologic diagnoses and phenotypic features of Sjögren’s syndrome among 1,726 registry participants. Arthritis Rheum. 2011;63(7):2021–2030. doi:10.1002/art.30381
15. Zhang M, Kim E, Akpek EK. Prevalence and predictors of Sjögren’s syndrome in a prospective cohort of patients with aqueous-deficient dry eye. Br J Ophthalmol. 2012;96(12):1498–1503. doi:10.1136/bjophthalmol-2012-301767
16. Lee JS, Choi W, Lee SS, Yoon KC. Prevalence and clinical aspects of Sjögren syndrome in dry eye patients. J Korean Ophthalmol Soc. 2012;53(4):499–504. doi:10.3341/jkos.2012.53.4.499
17. Kan E, Demirag MD, Beyazyildiz E. Presence of Sjogrenʼs syndrome in dry eye patients. Rheumatology (Sunnyvale). 2014;4:137. doi:10.4172/2161-1149.1000137
18. Henrich CF, Ramulu PY, Akpek EK. Association of dry eye and inflammatory systemic diseases in a tertiary care–based sample. Cornea. 2014;33(8):819–825. doi:10.1097/ICO.0000000000000173
19. Yen JC, Hsu CA, Li YC, Hsu M-H. The prevalence of dry eye syndrome’s and the likelihood to develop Sjögren’s syndrome in Taiwan: A population-based study. Int J Environ Res Public Health. 2015;12(7):7647–7655. doi:10.3390/ijerph120707647
20. Versura P, Frigato M, Cellini M, Mulè R, Malavolta N, Campos EC. Diagnostic performance of tear function tests in Sjogren’s syndrome patients. Eye. 2007;21(2):229. doi:10.1038/sj.eye.6702204
21. Jonsson R, Theander E, Sjöström B, Brokstad K, Henriksson G. Autoantibodies present before symptom onset in primary Sjögren syndrome. JAMA. 2013;310(17):1854–1855. doi:10.1001/jama.2013.278448
22. Theander E, Jonsson R, Sjöström B, Brokstad K, Olsson P, Henriksson G. Prediction of Sjögren’s syndrome years before diagnosis and identification of patients with early onset and severe disease course by autoantibody profiling. Arthritis Rheumatol. 2015;67(9):2427–2436. doi:10.1002/art.39214
23. Hernández-Molina G, Leal-Alegre G, Michel-Peregrina M. The meaning of anti-Ro and anti-La antibodies in primary Sjögren’s syndrome. Autoimmun Rev. 2011;10(3):123–125. doi:10.1016/j.autrev.2010.09.001
24. Davidson B, Kelly C, Griffiths I. Primary Sjögren’s syndrome in the North East of England: a long-term follow-up study. Rheumatology (Oxford). 1999;38(3):245–253. doi:10.1093/rheumatology/38.3.245
25. Solomon DH, Kavanaugh AJ, Schur PH, Guidelines A. Evidence‐based guidelines for the use of immunologic tests: antinuclear antibody testing. Arthritis Rheum. 2002;47(4):434–444. doi:10.1002/art.10561
26. Guellec D, Cornec D, Jousse-Joulin S, et al. Diagnostic value of labial minor salivary gland biopsy for Sjögren’s syndrome: a systematic review. Autoimmun Rev. 2013;12(3):416–420. doi:10.1016/j.autrev.2012.08.001
27. Giovelli RA, Santos MC, Serrano ÉV, et al. Clinical characteristics and biopsy accuracy in suspected cases of Sjögren’s syndrome referred to labial salivary gland biopsy. BMC Musculoskelet Disord. 2015;15:16–30.
28. Wicheta S, Faquin W, August M. Evaluation of minor salivary gland biopsy in the diagnosis of Sjögren’s syndrome. J Oral Maxillofac Surg. 2017;46:151. doi:10.1016/j.ijom.2017.02.521
29. Wicheta S, Van der Groen T, Faquin WC, August M. Minor salivary gland biopsy an important contributor to the diagnosis of Sjögren syndrome. J Oral Maxillofac Surg. 2017;75(12):2573–2578. doi:10.1016/j.joms.2017.05.021
30. Rasmussen A, JA I, Li H, et al. Comparison of the American-European Consensus Group Sjögren’s syndrome classification criteria to newly proposed American College of Rheumatology criteria in a large carefully characterised sicca cohort. Ann Rheum Dis. 2014;73(1):31–38. doi:10.1136/annrheumdis-2013-203845
31. Cornec D, Jousse‐Joulin S, Pers JO, et al. Contribution of salivary gland ultrasonography to the diagnosis of Sjögren’s syndrome: toward new diagnostic criteria? Arthritis Rheum. 2013;65(1):216–225. doi:10.1002/art.37698
32. Abd-Allah NM, Omar G, Elameen N, Mousa RA. Diagnostic value of salivary gland ultrasonography for Sjӧgren’s syndrome in patients with sicca symptoms. Egyp Rheumatologist. 2018;40(3):191–195. doi:10.1016/j.ejr.2017.09.003
33. Shen L, Suresh L, Lindemann M, et al. Novel autoantibodies in Sjogren’s syndrome. Clin Immunol. 2012;145(3):251–255. doi:10.1016/j.clim.2012.09.013
34. Suresh L, Malyavantham K, Shen L, Ambrus JL. Investigation of novel autoantibodies in Sjogren’s syndrome utilizing Sera from the Sjogren’s international collaborative clinical alliance cohort. BMC Ophthalmol. 2015;15(1):38. doi:10.1186/s12886-015-0023-1
35. Martín-Naresa E, Hernández-Molinaa G. Novel autoantibodies in Sjögren Syndrome: A comprehensive review. Autoimmun Rev. 2019;18(2):192–198.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.