Back to Journals » Cancer Management and Research » Volume 12
Evaluation of p16 in Epithelial Ovarian Cancer for a 10-Year Study in Northeast China: Significance of HPV in Correlation with PD-L1 Expression
Authors Yang X , You Q, Yao G, Geng J, Ma R, Meng H
Received 7 June 2020
Accepted for publication 16 July 2020
Published 3 August 2020 Volume 2020:12 Pages 6747—6753
DOI https://doi.org/10.2147/CMAR.S262678
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Eileen O'Reilly
Xinxin Yang1 ,* Qi You2,3 ,* Guodong Yao,1 Jingshu Geng,1 Rong Ma,4 Hongxue Meng1,2
1Department of Pathology, Harbin Medical University Cancer Hospital, Harbin, People’s Republic of China; 2Department of Pathology, Harbin Medical University, Harbin, People’s Republic of China; 3Department of Gastroenterology, Harbin Medical University Cancer Hospital, Harbin, People’s Republic of China; 4Department of Gynaecology, Harbin Medical University Cancer Hospital, Harbin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hongxue Meng Department of Pathology
Harbin Medical University, 150 Haping Road, Harbin, People’s Republic of China
Tel +86-451-85718261
Email [email protected]
Rong Ma Department of Gynaecology
Harbin Medical University Cancer Hospital, 150 Haping Road, Harbin, People’s Republic of China
Tel +86-451-85718263
Email [email protected]
Background: Human papillomavirus (HPV) is a high-risk etiological factor for cervical and ovarian carcinomas. p16 protein can be used as a surrogate biomarker for HPV infection in high-risk tumors. A strong correlation between HPV infection and programmed death ligand 1 (PD-L1) protein expression has consistently been reported.
Objective: Given this background, this study investigates the prevalence, prognostic and clinicopathologic features of HPV-related epithelial ovarian cancer (EOC) for the last 10 years in Northeast China to elucidate the involvement of p16 in the PD-L1 protein expression, tumorigenesis, and progression of EOC.
Methods: Specimens from 310 patients diagnosed with EOC collected from 2006 to 2016 were analyzed by polymerase chain reaction (PCR) for HPV DNA, and overexpression of p16 by immunohistochemistry was also evaluated. Statistical analysis was performed to estimate the significant difference between HPV positive and negative patients, the correlation among HPV state, p16 and PD-L1 expression, and clinical presentation.
Results: Overexpression of p16 protein and HPV DNA were present in 100 (32.3%) of the 310 cases, and correlated with high PD-L1 expression. There was a good concordance between HPV positivity, p16 protein overexpression and PD-L1 expression. The etiological fraction of HPV in EOC is substantially higher in Northeast China than other cohorts previously reported.
Conclusion: Our study demonstrates that HPV infection and p16 overexpression is significantly associated with PD-LI expression in EOC, through the cooperative roles of dendritic cells (DCs) and IFN-γ, which may represent a promising strategy for therapeutic intervention in EOC.
Keywords: HPV, p16, PD-L1, ovarian cancer, pathogenesis, prognosis
Introduction
Ovarian cancer is the most common tumor of the female genital system worldwide, resulting in many deaths every year.1 Epithelial ovarian cancer (EOC) is the most lethal type of ovarian cancer.2 The disease is characterized by high nodal metastases and recurrence rates, because most cases are diagnosed in the later stage, its prognosis is usually poor, with overall 5-year survival rates of less than 30%.3 There is a group of EOC patients with a high risk for cancer recurrence or metastasis, even when surgery, chemotherapy, and radiotherapy are undertaken. Therefore, it is essential for the prevention and cure of EOC to finding a target or biomarker that could be used to individualize both patient prognosis and therapy.
Over the past decade, female malignant tumors such as cervical and ovarian cancer associated with human papillomavirus (HPV) infection has been declining in incidence.4–7 The prevalence of HPV-associated female malignant tumors has been increasing in multiple populations including the Western Europe, Australia, and United States.8–10 Nonetheless, few attempts have been made to analyze a panel of prevalence, significance, prognostic, and correlations of HPV infection in ovarian cancer in a Chinese cohort, account for 1/4 of the global population. The clinic pathologic features and precise pathogenesis of HPV-related ovarian cancer in Northeast China are still unclear.
Female malignant tumors have increased mainly due to high-risk HPV infection. HPV prevalence of EOC being zero in North America, 1.1% in Western Europe, 18.5% in Eastern Europe, and 45.6% in Asia.11–13 High risk HPV16 and HPV18 are the dominant subtypes that have been identified in HPV-related cancers. Viral oncoproteins E6 and E7 are always expressed in HPV-related cancers and inhibit the activity of tumor suppressor retinoblastoma, leading to p16 overexpression.14
It has been reported that p16 overexpression is closely correlated with HPV infection in HPV-related cancers. Because of this, p16 could be used as a diagnostic biomarker for HPV infection in these cancer patients.15 Moreover, p16 is a potential independent prognostic factor for progression-free survival and overall survival of cervical cancer.16 However, whether p16 IHC is a good discriminator of clinical outcome in patients with ovarian cancer has not been defined. Larger studies are required to determine whether p16 IHC could be used in addition to established prognostic variables such as T category, nodal status and depth of invasion for progression of ovarian cancer.
HPV infection may result in a large spectrum of epithelial lesions. Previous studies have indicated that p16 may enhance the immunogenicity of dendritic cells (DCs), through cyclin-dependent pathways and Th1 cytokine secretion in HPV-induced cancers.17 Little literature has been published correlating HPV positivity and immune-checkpoint inhibitors.18 Since active DCs may regulate IFN-γ expression, which is crucial for programmed death ligand 1 (PD-L1) expression.19 Recent studies reported that PD-L1 overexpression in many solid tumors, to safeguard cancer cells and also to simultaneously suppress the activity of PD-1 expressing adjoining tumor-infiltrating effector CD4/CD8 T cells.20,21 Manipulation of the PD-1 and PD-L1 interaction is one of the most promising concepts of cancer checkpoint inhibitor therapies.22 The immune-biomarkers are defined for providing suitable patients for immune therapy. Additionally, the impact of PD-L1 of deciphering among p16 positive cancer patients could be of interest for patients' tailored treatments. However, little is known about the complex interrelationship between HPV infection and tumor PD-L1 expression in ovarian cancer.
The incidence of HPV-related female malignant tumors has increased in recent decades, though the precise pathogenesis and clinicopathologic features of HPV-related ovarian cancer in Northeast China are still unclear. The objective of this study was to investigate the prevalence, prognostic and clinicopathologic features of HPV-related ovarian cancer in Northeast China and to elucidate the involvement of p16 in the PD-L1 expression, Clinicopathological Characteristics and progression of ovarian cancer.
Methods
Patients
This study enrolled 310 patients with pathology-proven ovarian cancer. Patients were recruited from Harbin Medical University Cancer Hospital from January 2006 to February 2016.
Clinical Parameters and Histopathological Diagnosis
The clinical parameter of patients was collected, including age, CA-125 level and clinical stage. All patients were diagnosed and assessed according to the WHO classification. No patients had history of cervical tumors. All the slides were reviewed to score the pathological variables by two pathologists. International Federation of Gynecology and Obstetrics (FIGO) criteria were used to assess the tumor stage.
Antibodies and Immunohistochemistry (IHC)
Formalin-fixed and paraffin-embedded sections (4 µm thick) were blocked with 1% H2O2. Then samples were treated by trypsin at 37°C for 30 min, followed by immersion using citrate buffer (pH 6.0) at 120°C for 20 min in an autoclave. A Protein Blocking Agent (Beckman Coulter, France) was used to block the sections, then the following primary antibodies were used to incubate sections overnight at 4°C: anti-human p16 (1:100, Zhongshan, China), anti-human DC-sign (1:100, abcam, USA), anti-human IFN-γ (1:100, abcam, USA), anti-human PD-L1 (1:200, abcam, USA). After that, sections were incubated with secondly antibody (Beckman Coulter, France) and visualized by DAB. Phosphate-buffered saline omitting primary antibodies was used as negative control.
For calculating tumor cells with p16 overexpression, we classified nuclear and cytoplasmic positivity as positive reactions and semiquantitatively scored p16 positive cells described by previous study.17 The principle of p16 positive was defined as strong and diffuse nuclear and cytoplasmic staining in at least 80% or more tumor cells. The number of positive cells for DC-sign, IFN-γ and PD-L1 were scored and defined as: 0 (absent), 1+ (< 25% of cells), 2+ (25–50% of cells), 3+ (50–75% of cells) or 4+ (> 75% of cells).
DNA Extraction and PCR Analysis
Formalin-fixed, paraffin-embedded tissues (10 FFPE 4 µm thick slices) were used to extract and purify total DNA using DNeasy Micro kit (Qiagen, Germany). The extracted DNAs were as the templates for polymerase chain reaction (PCR) analysis. The DNA was amplified after 35 cycles for PCR analysis. The forward and reverse primers were listed as follows: HPV GP5+/GP6+ 5′- CGT CCM ARR GGA WAC TGA TC 3′ (forward); 5′- GCM CAG GGW CAT AAY AAT GG −3′ (reverse); β-globin 5′- GAA GAG CCA AGG ACA GGT AC −3′ (forward); 5′- CAA CTT CAT CCA CGT TCA CC −3′ (reverse). The cycling conditions of HPV and β-globin are: the denaturation temperature is at 94°C for 1 minute, the annealing temperature is 56°C for 1 minute, the extension temperature is 72°C for 1 minute, and finally extended for 10 minutes at 72°C. The PCR products were analyzed by 4% agarose gel electrophoresis, and observed on the imager using ethidium bromide.
Statistical Analysis
The significant difference between HPV positive patients and HPV negative patients was estimated by a Mann–Whitney U-test. The correlation among HPV state, clinical presentation, and p16 positivity was analyzed by Spearman correlation analysis (SAS Institute Inc., Cary, NC, USA). Differences with p-values of less than 0.05 were considered significant.
Results
Clinical and Pathological Parameters
The clinicopathological findings of the patients are shown in Table 1. The patients were predominantly older patients with a high serum CA-125 level. Based on the histologic typing, 208 (67.1%) of the 310 cases were serious adenocarcinoma and 48 (15.5%) cases were mucinous adenocarcinoma. For the differentiation, 134 (43.2%) cases were well, 102 (32.9%) cases were moderate and 74 (23.9%) cases were poor. Patients with low clinical stage (I/II) were 76 (24.5%) cases and patients with high clinical stage (III/IV) were 234 (75.5%) cases.
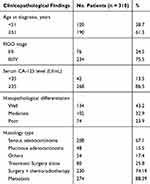 |
Table 1 Profiles and Clinicalpathological Parameters of Patients |
p16 Protein Overexpression and HPV DNA-PCR
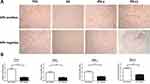
By IHC, we detected that p16 was overexpressed in 100 (32.3%) of the 310 cases. HPV was positive in 78 cases (25.16%) of the 310 cases by PCR (Figure 1). HPV was positive in 76 cases of the 100 p16 positive cases, and HPV was negative in 208 cases of the 210 p16 negative cases (Table 2). In these p16 positive tumor samples, more than 80% of carcinoma cells could be positively stained by p16 antibody (Figure 2A). Furthermore, we also found that p16 overexpression was highly correlated with HPV positivity (Figure 2B).
 |
Table 2 The Relationship Between p16 and Clinicalpathological Parameters of Patients |
 |
Figure 1 HPV DNA-PCR of Ovarian cancer. Using PCR, HPV statue was detected in ovarian cancer patients. |
Table 2 represented the relationship between clinicopathological variables and HPV infection. Obviously, HPV infection was more frequently observed among the older patients (P<0.05) and high FIGO stage (P<0.01), well differentiation (P<0.01) cases.
Increased Expression of p16, DC-Sign, IFN-γ and PD-L1 Positive Cells in HPV Positive Epithelial Ovarian Cancer
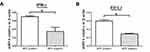
To understand the mechanisms of PD-L1 overexpression in HPV positive EOC, we assessed the expression of DC-sign and IFN-γ by IHC. Expression of p16, DC-sign, IFN-γ and PD-L1 were increased in HPV positive EOC than in those HPV negative (Figure 2B). More importantly, p16 levels correlated with DC-sign, IFN-γ and PD-L1 levels. Moreover, IFN-γ and PD-L1 mRNAs were increased in HPV positive EOC than in those HPV negative (Figure 3).
Discussion
High risk HPV infection is usually an important factor of epithelial malignant tumors, which is infected in squamous epithelium by a non-enveloped DNA virus.8–10 Previous studies have indicated numerous pathogenesis of EOC, but HPV infection has been pointed out by many scholars as an necessary pathogenic factor, and PCR is usually used as its detection method.10 Women of gender are more likely to contract HPV and the prevalence rate exceeds 80%. Some researchers suggest that from a pathophysiological point of view, HPV rises from the cervix to infect the fallopian tubes and ovaries in ovarian cancer.11–13
Here we studied HPV prevalence in ovarian cancers for more than 10 years and found some inherent deviations representative of a retrospective cohort. Interestingly, we found that overexpression of p16 were frequently present in HPV positive tumor. It suggested that overexpression of p16 could be used to predict HPV infection. As far as we know, this is the first research to build a link between HPV infection and p16 expression in ovarian cancer in North China. And p16 may be a potential biomarker or therapeutic target in cancer therapy.
It is important to note that compared with the recent meta-analysis results of HPV in EOCS, the HPV positive EOCs in our study was significantly higher.18 Different geographic sources of samples, different health conditions of cancer patients, and differences in laboratory procedures used in research may all be the reasons for the discrepancy in results. The results also indicated that the estimated HPV-AFs of the elderly are much higher. HPV infection was significantly correlated with the older, high FIGO stage and well differentiation cases.
The association between HPV and PD-L1 has recently attracted the attention of scholars.14,15 In our study, expression of p16, DC-sign, IFN-γ and PD-L1 were increased in HPV positive EOC than in those HPV negative, and p16 levels correlated with DC-sign, IFN-γ and PD-L1 levels. To our knowledge, infection with HPV may enhance the immunogenicity of DCs, which is the main source of IFN-γ secretion. While IFN-γ is crucial for PD-L1 expression.17,18 This is the first report to demonstrate that HPV infection and p16 overexpression is significantly associated with PD-LI expression in EOC, through the cooperative roles of DC and IFN-γ, which may be a significant treatment strategy for EOC in the future.
Conclusion
The significant correlation between p16 and PD-L1 expression in ovarian cancer was confirmed in our study. Furthermore, the etiological fraction of HPV is higher in Northeast China than both Western Europe and the United States had previously reported in ovarian cancer. Predicting the contribution of HPV to the development of ovarian cancer is very important for predicting the future burden of these high-risk cancers and informing the global potential preventive effects of HPV vaccines. Further study is required to determine the detailed mechanism of HPV involvement in ovarian cancer.
Abbreviations
HPV, human papillomavirus; PD-L1, programmed death ligand 1; EOC, epithelial ovarian cancer; PCR, polymerase chain reaction; IHC, immunohistochemistry; DC, dendritic cell.
Data Sharing Statement
The clinical and experimental data used to support the findings of this study are included within the article.
Ethics Statement
All procedures performed in this study were were performed according to institutional protocols and approved by the Medical Ethics Committee of the Harbin Medical University Cancer Hospital.
Ethics and Consent
The study has obtained the written informed consent from the study participants. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mirza MR, Matulonis UA. Niraparib in recurrent ovarian cancer. N Engl J Med. 2017;376(8):801–802. doi:10.1056/NEJMc1616633
2. Holmes D. Ovarian cancer: beyond resistance. Nature. 2015;527(7579):S217. doi:10.1038/527S217a
3. Ferrarelli LK. Locking TNFR2 to kill ovarian cancer. Science. 2017;355(6322):257–258. doi:10.1126/science.355.6322.257-h
4. Scudellari M. HPV: sex, cancer and a virus. Nature. 2013;503(7476):330–332. doi:10.1038/503330a
5. Lew JB, Simms KT, Smith MA, et al. Primary HPV testing versus cytology-based cervical screening in women in Australia vaccinated for HPV and unvaccinated: effectiveness and economic assessment for the National Cervical Screening Program. Lancet Public Health. 2017;2(2):e96–e107. doi:10.1016/S2468-2667(17)30007-5
6. Campos NG, Tsu V, Jeronimo J, Mvundura M, Kim JJ. Estimating the value of point-of-care HPV testing in three low- and middle-income countries: a modeling study. BMC Cancer. 2017;17(1):791. doi:10.1186/s12885-017-3786-3
7. Chetty R. 70 years of the JCP-highly cited papers: the causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2017;70(12):997. doi:10.1136/jclinpath-2017-204867
8. Printz C. Women who undergo HPV testing receive earlier detection and treatment of cervical precancers. Cancer. 2017;123(24):4751. doi:10.1002/cncr.31149
9. Galati L, Peronace C, Fiorillo MT, et al. Six years genotype distribution of human papillomavirus in Calabria Region, Southern Italy: a retrospective study. Infect Agent Cancer. 2017;12:43. doi:10.1186/s13027-017-0154-5
10. de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–670. doi:10.1002/ijc.30716
11. Rosa MI, Silva GD, de Azedo Simões PW, et al. The prevalence of human papillomavirus in ovarian cancer: a systematic review. Int J Gynecol Cancer. 2013;23(3):437–441. doi:10.1097/IGC.0b013e318280f3e0
12. Svahn MF, Faber MT, Christensen J, Norrild B, Kjaer SK. Prevalence of human papillomavirus in epithelial ovarian cancer tissue. A meta-analysis of observational studies. Acta Obstet Gynecol Scand. 2014;93(1):6–19. doi:10.1111/aogs.12254
13. Arfi A, Hequet D, Bataillon G, et al. HPV DNA integration site as proof of the origin of ovarian metastasis from endocervical adenocarcinoma: three case reports. BMC Cancer. 2019;19:375. doi:10.1186/s12885-019-5582-8
14. Zhang PP, Zhou L, Cao JS, et al. Possible epithelial ovarian cancer association with HPV18 or HPV33 infection. Asian Pac J Cancer Prev. 2016;17(6):2959–2964.
15. Mills AM, Dirks DC, Poulter MD, Mills SE, Stoler MH. HR-HPV E6/E7 mRNA in situ hybridization: validation against PCR, DNA in situ hybridization, and p16 immunohistochemistry in 102 samples of cervical, vulvar, anal, and head and neck neoplasia. Am J Surg Pathol. 2017;41(5):607–615. doi:10.1097/PAS.0000000000000800
16. Stiasny A, Freier CP, Kuhn C, et al. The involvement of E6, p53, p16, MDM2 and Gal-3 in the clinical outcome of patients with cervical cancer. Oncol Lett. 2017;14(4):4467–4476. doi:10.3892/ol.2017.6752
17. Garcia-Bates TM, Kim E, Concha-Benavente F, et al. Enhanced Cytotoxic CD8 T cell priming using dendritic cell-expressing human papillomavirus-16 E6/E7-p16INK4 fusion protein with sequenced anti-programmed death-1. J Immunol. 2016;196(6):2870–2878. doi:10.4049/jimmunol.1502027
18. Lyford-Pike S, Peng S, Young GD, et al. Evidence for a role of the PD-1: PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73(6):1733–1741. doi:10.1158/0008-5472.CAN-12-2384
19. Ukpo OC, Thorstad WL, Lewis JS
20. Kim HS, Lee JY, Lim SH, et al. Association between PD-L1 and HPV status and the prognostic value of PD-L1 in oropharyngeal squamous cell carcinoma. Cancer Res Treat. 2016;48(2):527–536. doi:10.4143/crt.2015.249
21. Tan S, Zhang CW, Gao GF. Seeing is believing: anti-PD-1/PD-L1 monoclonal antibodies in action for checkpoint blockade tumor immunotherapy. Signal Transduct Target Ther. 2016;1:16029. doi:10.1038/sigtrans.2016.29
22. Kuol N, Stojanovska L, Nurgali K. Apostolopoulos V. PD-1/PD-L1 in disease. Immunotherapy. 2018;10(2):149–160. doi:10.2217/imt-2017-0120
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.