Back to Journals » Clinical Ophthalmology » Volume 14
Evaluation of Nd:YAG Laser Capsulotomy Rates in a Real-Life Population
Authors Ling R, Borkenstein EM, Borkenstein AF
Received 13 August 2020
Accepted for publication 5 October 2020
Published 13 October 2020 Volume 2020:14 Pages 3249—3257
DOI https://doi.org/10.2147/OPTH.S276329
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Roland Ling,1 Eva-Maria Borkenstein,2 Andreas F Borkenstein2
1The Medical Eye Clinic, Royal Devon and Exeter Hospital, Exeter, UK; 2Privatklinik der Kreuzschwestern Graz, Private Practice Borkenstein & Borkenstein, Graz, Austria
Correspondence: Andreas F Borkenstein
Privatklinik der Kreuzschwestern Graz, Private Practice Borkenstein & Borkenstein, Graz 8010, Austria
Tel +43 316 331 3880
Email [email protected]
Objective: The objective of this study was to assess the rate of posterior capsule opacification (PCO), under “real-life” conditions, as measured by rates of Nd:YAG laser intervention, rather than from a controlled study from which patients with conditions predisposing to PCO have been excluded.
Methods and Analysis: This was a retrospective, multicenter study in an unselected consecutive cohort of patients undergoing surgery for senile cataract. Patients aged 18 years and older, previously implanted with the CT LUCIA 611P IOL, were contacted at 12, 18 and 24 months to ascertain if they had received Nd:YAG laser treatment. There was an additional assessment at 36 months at the Austrian centre.
Results: A total of 200 patients were recruited at two centers. Laser capsulotomy rates were 4.5% at 1 year and 10% by year 2 and 12% by year 3. Three Nd:YAG capsulotomies, carried out at other external centers, were performed for reasons other than PCO, including astigmatism, epiretinal membrane and ARMD. If these patients are excluded, the true rate of Nd:YAG carried out for PCO at 1 year was 3.5% and at 2 years was 8.5%.
Conclusion: It is critical to ensure that Nd:YAG capsulotomies are being performed only for the correct clinical reason. Carrying out unnecessary procedures places the patient at risk of adverse events, is a cost to the healthcare system, and is likely to have no direct visual benefit for the patient. In PCO studies, it should be a requirement to document the fibrosis grade to confirm that Nd:YAG capsulotomy was correctly indicated.
Keywords: Nd:YAG laser capsulotomy, PCO, cataract, CT LUCIA 611P
Plain Language Summary
Posterior capsular opacification (PCO) happens when cells in the eye grow across an implanted lens and adversely affect the ability of the patient to see through the lens. The only way to remove the cells is to use a technique called laser capsulotomy where a Nd:YAG laser is used to ablate the cells. Although PCO rates have been falling with developments to IOLs and surgical technique, Nd:YAG rates have not fallen at the same rate and we wanted to explore the reasons why this might be. In clinical trials Nd:YAG treatment is usually used only when the PCO has had a significant effect on a patient’s vision. However, we found that, outside of the controlled world of clinical trials, Nd:YAG laser capsulotomy is sometimes performed without confirmation that clinically significant PCO is the cause of impaired vision. As a result, the treatment does not bring about any improvement in visual acuity and is also a waste of resources. Our findings highlight the importance of reaching a proper diagnosis of PCO before undertaking Nd:YAG in order to conserve healthcare resources, and to avoid placing the patient at risk. Studies reporting Nd:YAG rates in clinical trials should be obliged to report the grade and extent of PCO so that other readers can see that the procedure was necessary. Unfortunately, there is currently no standardized method of evaluation for severity prior to a planned capsulotomy in standard clinical practice.
Introduction
Posterior capsule opacification (PCO) remains the most common long-term post-operative complication of cataract surgery.1,2 Posterior capsule opacification arises from the growth and abnormal proliferation of residual lens epithelial cells (LECs) which migrate from the equator of the capsule over the posterior capsule, where they eventually obscure the visual axis.2,3 Due to the detrimental effects on vision and the costs of treating PCO, a great deal of research has been carried out to identify the main causative factors for its development, and strategies to limit its growth.
In the early 1990s, IOL material and strength of adhesion of the IOL to the capsule were thought to be the most significant factors in prevention of PCO.4 Studies comparing material types reported rates of PCO for hydrophobic IOLs which were generally much lower than for hydrophilic IOLs.5–9 Since then it has been acknowledged that IOL design is a much more important factor in the development of PCO. Recent reviews have not found any significant differences in PCO scores for different IOL materials, but have found significantly lower PCO rates in IOLs of any material, that are constructed with sharp posterior edges.1
A 360 degree sharp posterior optic edge which presses against the capsule can induce a bend which provides a barrier to the migration of epithelial cells across the lens,10–14 many studies have now confirmed this benefit.4,14–23 The geometry of the optic-haptic junction is important in ensuring that the sharp edge extends around the entire posterior edge of the IOL: some one-piece IOLs with thick optic-haptic junctions have no sharp edge at the haptic junction (interrupted square edge) resulting in incomplete adhesion of the posterior capsule at the junction and allowing LECs to migrate behind the IOL, beginning at the optic-haptic junction.24–26
The definitive treatment for PCO is Nd:YAG laser capsulotomy.1 However, the requirement to perform Nd:YAG is a considerable financial burden,27–29 is not without risks and is not always available in the developing world. Increased awareness of the role played by the geometry of the IOL, and the importance of surgical techniques has contributed to a general reduction in Nd:YAG rates since the 1990s. Nd:YAG rates commonly reported before 1992 were between 20.3% and 33.4%; while Nd:YAG rates for IOLs implanted ten years later were below 17.1%.30,31 Even lower rates, often in single figures, were reported for hydrophobic IOLs by 2017.32
However, most published Nd:YAG rates are derived from controlled studies from which patients with conditions predisposing to PCO have been excluded. The objective of this study was to assess the rate of PCO, under “real-life” conditions, as measured by rates of Nd:YAG laser intervention, following implantation of a one-piece, square-edge hydrophobic IOL, the CT Lucia 611P, in an unselected population of patients undergoing routine surgery for senile cataract.
Methods
Study Design
This was a retrospective, multicenter study investigating the rate of PCO based on Nd:YAG laser intervention in a consecutive cohort of patients who had undergone surgery for senile cataract. Study physicians with extensive experience of implanting the CT LUCIA 611P IOL at two facilities in the UK and Austria were involved in the study.
Suitable study subjects who had previously been implanted with the IOL were identified from patient registers at the participating institutions and were contacted for recruitment into the study. Patients aged 18 years and older, implanted with the IOL between October 2016 and June 2017, were contacted at post-operative intervals to ascertain if they had received Nd:YAG laser treatment as a result of PCO.
The study was carried out in compliance with the Helsinki Declaration and the subsequent modifications regarding Good Clinical Practice. Approval for the study center in Austria was obtained from the Ethics Committee of the Medical University of Graz. In the UK, patients gave informed consent for their information to be used anonymously. All products (implants) were CE-marked and standard state-of-the-art procedures were performed.
Patient and Public Involvement
The concept of patient involvement was applied to the study design and execution phases of the research. We provided basic educational materials and a research tutorial to help to encourage familiarity with research concepts and terminology and discussed the planned study with the patients.
Patients
The subject population comprised individuals aged 18 years and older who met the criteria for cataract surgery and had undergone implantation of the LUCIA 611P in the previous 18 months.
Inclusion criteria included cataract outcomes without pseudoexfoliation (PEX) or other post-operative complications, the ability and willingness to make the required study visits, give verbal informed consent and follow study instructions.
As the intention of the study was to reflect the “real-life” clinical situation, patients were not excluded if they had pre-operative conditions such as diabetes, uveitis or ARMD which would pre-dispose them to the development of PCO, but they were excluded if ocular complications such as uveitis or PEX developed during or after surgery.
This includes complications such as active infection of the anterior or posterior segments, any ocular pathology affecting the anterior segment, any ocular surgery other than laser refractive surgery, and use of post-operative drops. Patients with a reduced VA (<6/9 at 6 weeks) were also excluded in order to exclude early PCO that may have been due to surgical factors or co-existing conditions, rather than to the design of the IOL.
Both eyes were selected for inclusion in the study if both qualified for the study.
Intraocular Lens
The CT LUCIA 611P IOL (Carl Zeiss Meditec AG, Jena, Germany) is a heparin-coated, monofocal, aspheric, one-piece hydrophobic acrylic lens with C-loop haptics for implantation in the capsular bag (Figures 1 and 2). The optic diameter is 6mm and the total diameter is 13mm. The IOL is available in a range of diopters from +4.0 to 34.0D in 0.5D increments. The lens has step-vaulted haptics to translate the optic in a posterior direction for better contact with the posterior capsule, and has a 360° square edge design, with a radius of <3µm, including at the optic-haptic junction.
 |
Figure 1 The CT LUCIA 611P IOL. |
 |
Figure 2 Scanning Electron Microscopy of the Special Design of the Haptics and the Optic-haptic junction of the CT Lucia 611P (AFB, Graz). |
Nd:YAG Capsulotomy
Where patients had undergone Nd:YAG capsulotomy at one of the participating centers, they received bromfenac eye drops twice daily for 14 days.
Assessments
All patients who had received Nd:YAG surgery, either at a participating center or elsewhere, were invited to the clinic for assessment at 12, 18 and 24 months following surgery, with an additional assessment at 36 months at the Austrian center.
During the visit, intraocular pressure and visual acuity (ETDRS charts) were measured and the eyes underwent slit lamp examination. If the patient had received Nd:YAG surgery at another non-participating center, the notes were examined to identify and record the reason for carrying out surgery.
Subjects were informed of any abnormalities found during the testing and advised to consult their ophthalmologist or optometrist. Test results were made available to the patient’s GP upon written request.
Endpoints
Data was analysed on the basis of whether patients implanted with the CT Lucia IOL had Nd:YAG treatment at any interval visit either at the participating centers or at others. Where patients had undergone Nd:YAG at another center, the indication for treatment was sought through examination of the patient file.
Statistical Methods
The study aimed to include 200 eyes. There was no data available from previous studies.
A non-parametric pairwise comparison (Wilcoxon signed rank test) of 200 eyes per group to allow for a 17% drop-out (power 80% and alpha <0.05) was performed.
Missing, unused, or spurious data which did not meet the pre-determined quality criteria was noted in the study records and removed.
Patients were not involved in the research process, the development, design of, or recruitment for the study. We have no plans at present to involve patients in the dissemination of the study results.
Results
A total of 200 patients were recruited at the two centers, see Table 1. Four patients had bilateral implantation of the IOL and 204 eyes were therefore included in the study. The two centers are experienced with the device and have implanted this IOL in approximately 2000 patients. All of the patients who were contacted agreed to participate.
 |
Table 1 Patient Demographics at Baseline and Nd:YAG Rates at Scheduled Follow-Up Visits |
The mean age of the patients in the two centres was 74.3 years, with ages ranging from 58 to 87 years.
At one of the centers, there were 18 patients with diabetes mellitus at baseline, and four cases of uveitis. Following surgery, there were no serious complications.
Laser capsulotomy rates were 4.5% at 1 year and 10% by year 2, 12% by year 3.
Three Nd:YAG capsulotomies were performed for reasons other than PCO (Table 2) so the true rate of Nd:YAG carried out for PCO at 1 year is lower at 7/200 (3.5%) and at 2 years it is 17/199, or 8.5%.
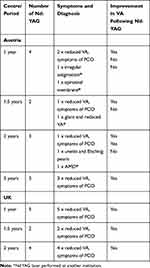 |
Table 2 Detail of Nd:YAG Procedures |
Anonymised patient-level data is available upon request from the corresponding author.
Discussion
In this study, we aimed to establish a typical “real world” rate for Nd:YAG capsulotomies in patients implanted with the CT LUCIA 611P IOL. In most prospective studies, patients are excluded if they have pre-existing conditions such as ARMD, diabetes, uveitis and glaucoma which are known to increase the risk of developing PCO33,34 therefore rates from trials may not be representative of those encountered in clinical practice. We did not collect data on rates of PCO as this measure can be largely meaningless if it is not accompanied by an analysis of the severity of the PCO, and the effect that the PCO has on visual acuity. As Nd:YAG capsulotomy is the ultimate measure of PCO that has become visually significant, we confined our study to an assessment of Nd:YAG rates in an unselected patient population.
With this aim in mind, we also investigated the reasons for treating patients with Nd:YAG capsulotomy if that patient was treated at another center. In our study, three patients who received treatment in external centers due to a diagnosis of incipient PCO actually had secondary diagnoses of astigmatism, epiretinal membrane and ARMD. A fourth patient complaining of glare and reduced visual acuity was diagnosed with PCO at an external center and was subsequently treated with Nd:YAG capsulotomy. Following treatment, VA did not improve and the patient was found to have epiretinal membrane, which limited the ability to achieve a functional VA, and which was not amenable to treatment with Nd:YAG. All four patients who were given Nd:YAG treatment for reasons other than PCO failed to realise any improvement in VA.
If these four patients are excluded from our results, the actual combined (adjusted) rates of Nd:YAG intended for the treatment of PCO are 3.5% after 1 year and 8.5% at 2 years. These figures compare well with rates published in other retrospective and real-world studies.35–37
In studies reporting rates of PCO and Nd:YAG, particularly where these may take place at external clinics, it is important to ascertain the referral for capsulotomy in order to gain a true picture of the performance of the IOL. It is possible that Nd:YAG capsulotomy may be performed for secondary diagnoses such as macular changes. Ideally, PCO studies would document the grade of fibrosis with a visual record to confirm that Nd:YAG capsulotomy was an appropriate intervention in each case.
Techniques for assessing PCO should measure the area, the severity, and the relationship of the opacity to the visual axis, in order to distinguish between a thin monolayer of cells that do not disturb vision and a smaller area of dense opacification on or near to the visual axis that may cause significant vision loss. It is also desirable that an objective and standardised method be used in which the examiner would not be able to detect the identity of the IOL. This has proved challenging in practice and a number of systems are available, all of which have advantages and disadvantages.
The three main pattern analysis systems in use are EPCO, POCO and AQUA but many other systems have been proposed, which may or may not gain wider circulation over time (OSCA [Open-access Systematic Capsule Assessment],38 OQAS [Optical Quality Analysis System],39 ADOS [Automated Detector Opacification Software],40 and CPCO [Contourlet-based PCO quantification system]).41
EPCO (Evaluation of Posterior Capsule Opacification) is a qualitative system in which the user manually defines the area of the PCO and subjectively grades each segment from 1 (minimal) to 4 (severe). The EPCO score for each segment is calculated by multiplying the opacification grade by the fraction of capsule area affected, and the total score is obtained by summing the individual scores.42
The POCO (Posterior Capsule Opacity) software uses pixel analysis of the image to identify texture differences, resulting in an estimate of the percentage area affected by PCO. Different densities of opacification were initially not accounted for, but recent upgrades have been able to provide a measure of the severity of the opacification through texture analysis.43 POCOman (manual POCO) is a more subjective and simpler system.44
AQUA I (Automated Quantification of After-Cataract) also uses texture analysis based on the inhomogeneity of each pixel relative to its neighbours but gives no information on the localisation of the PCO.45 AQUA II is a fully automated qualitative and quantitative development of the measurement system.46
Laser treatment with Nd:YAG is an effective, quick and relatively easy outpatient procedure, but it can produce complications such as a transient rise in IOP (5%), drop in VA (4%), cystoid macular oedema (<1.5%) and retinal detachment (<1.5%).47 It has been calculated that a second procedure may be required in around 25% of patients.48 Although the rate of adverse events associated with Nd:YAG may be relatively low, due to the large number of patients undergoing the procedure, the absolute number of patients experiencing a post-procedure adverse event is high. One group has estimated that, over a period of 9 years, up to 11,500 adverse events for every 400,000 cataract extractions could be avoided if capsulotomy rates were reduced.49
The procedure is also a major financial burden: in the US health system, Nd:YAG accounts for $500 million expenditure per year, second only to the costs of cataract surgery itself.29
It is therefore critical to ensure that Nd:YAG capsulotomies are being performed only for the correct clinical reason. A thorough diagnostic procedure should be carried out beforehand to confirm that a drop in VA is due to the presence of PCO and not to another pathology. Carrying out unnecessary procedures places the patient at risk of adverse events, is a cost to the healthcare system, and is likely to have no direct visual benefit for the patient.27,50 Therefore, measures are usually taken during surgery to prevent or delay the onset of PCO.
Factors that can reduce the rates of PCO and therefore the need for Nd:YAG is important for patient outcomes but also to reduce the costs involved in management of cataracts. Surgical techniques to reduce the development of PCO are now well known and include cortical clean up, limiting the size of the continuous curvilinear capsulorhexis (CCC) and 360 degree overlap of the CCC edge on the IOL optic.28 Equally, there is no doubt that a sharp posterior edge is critical in limiting the development of PCO and reducing the risk of having to undergo Nd:YAG treatment.51 Studies have consistently demonstrated that IOLs with a square edge optic profile are associated with lower rates of PCO than those with round edges.10,12,22,52–54
However, while most IOLs are now designed with a square-edge optic profile, it is possible that there are differences between IOLs regarding the degree of sharpness,33 which in turn, has some impact on the development of PCO.36 Using scanning electron microscopy (SEM) and computer-aided imaging, the extent to which the area of the edge deviates from a perfect square can be calculated.36 Area-measurement values for hydrophilic IOLs are higher than those for hydrophobic IOLs, indicating a more rounded edge. This is reflected in the radius of curvature: one study reported that IOLs with a radius of curvature <10.0mm appear to have a good PCO performance.55
The encouragingly low rates of Nd:YAG capsulotomy found in this real-world study may be partly due to the nature of the IOL. The CT LUCIA 611P has an uninterrupted square edge profile around the posterior face of the optic, extending over the haptics. The haptics themselves are thick and rigid with a solid optic-haptic junction, providing good stability.
This study was carried out on a real-life cohort, and is therefore representative of rates encountered in a typical clinic, rather than in the environment of a trial with controlled inclusion criteria. It also has the advantage of being able to follow patients for an extended length of time to obtain robust longitudinal data, a factor which is often limited by costs in a prospective controlled trial.
One limitation is that some of the data is self-reported and may not be reliable. However, before cataract surgery took place, the patient was fully briefed on the possible adverse consequences, including the formation of PCO and the possibility that Nd:YAG treatment may be required. In this conversation, the phenomenon of PCO and the nature of Nd:YAG treatment is described to them in detail. During the follow-up process for this study, the person contacting the patient goes through the same process, so we can be very confident that the patient is reporting a Nd:YAG capsulotomy rather than another procedure on the eye.
Conclusion
Posterior capsule opacification is the most common complication of cataract surgery. Nd:YAG is a reliable and speedy treatment to improve visual acuity following the development of PCO but a confirmed diagnosis of PCO is not always made before Nd:YAG is carried out. The diagnosis should always be made first and the influence on visual acuity discussed with the patient. In future PCO studies, it should be a requirement to document the fibrosis grade to confirm that Nd:YAG capsulotomy was correctly indicated. This will help to ensure that reported PCO and Nd:YAG rates more accurately reflect the nature of the IOL under assessment. In our study an IOL with a sharp posterior edge, a rigid optic-haptic junction and good stability in the eye was associated with low rates for Nd:YAG.
Declaration of Helsinki
This retrospective study was performed in accordance with the ethical standards of the institutional research committee (approved by the ethic committee of Medical University Exeter and Graz) and of the Declaration of Helsinki and its later amendments or comparable ethical standards and used only de-identified patient data. All patients signed an informed consent document. Standard procedures and CE-marked products were used.
Acknowledgments
The authors would like to thank Jude Douglass of Healthcom Partners Ltd, Oxfordshire, UK for her assistance in preparing this manuscript, Christina Krasser, Lead Assistant to Drs Borkenstein at the Privatklinik der Kreuzschwestern Graz, Austria and Lisa Marie-Melville, Lead Nurse, Medical Eye Clinic, Royal Devon and Exeter Hospital, Exeter, UK for their tremendous assistance with the study.
Author Contributions
All authors have met all five conditions of the IMCJE Authorship Guidelines. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Dr. Roland Ling is the director of the Medical Eye Clinic, Royal Devon and Exeter Hospital, Exeter UK. Dr. Eva-Maria Borkenstein and Dr. Andreas Borkenstein are the co-directors of Borkenstein & Borkenstein, private practice at the Privatklinik der Kreuzschwestern, Graz, Austria. The authors report no other conflicts of interest in this work.
References
1. Findl O, Buehl W, Bauer P, Sycha T. Interventions for preventing posterior capsule opacification. Cochrane Database System Rev. 2010. doi:10.1002/14651858.CD14003738.pub14651853
2. Raj S, Vasavada A, Kaid Johar S, Vasavada V, Vasavada V. Post-operative capsular opacification: a review. Int J Biomed Sci. 2007;3(4):237–250.
3. Trivedi RH, Werner L, Apple DJ, Pandey SK, Izak AM. Post cataract-intraocular lens (IOL) surgery opacification. Eye. 2002;16:217–241. doi:10.1038/sj.eye.6700066
4. Nishi Y, Rabsilber TM, Limberger IJ, Reuland AJ, Auffarth GU. Influence of 360-degree enhanced optic edge design of a hydrophilic acrylic intraocular lens on posterior capsule opacification. JCRS. 2007;33(2):227–231.
5. Heatley CJ, Spalton DJ, Kumar A, Jose R, Boyce J, Bender LE. Comparison of posterior capsule opacification rates between hydrophilic and hydrophobic single-piece acrylic intraocular lenses. JCRS. 2005;31(4):718–724.
6. Vasavada AR, Raj SM, Shah A, Shah G, Vasavada V, Vasavada V. Comparison of posterior capsule opacification with hydrophobic acrylic and hydrophilic acrylic intraocular lenses. JCRS. 2011;37(6):1050–1059.
7. Iwase T, Nishi Y, Oveson BC, Jo YJ. Hydrophobic versus double-square-edged hydrophilic foldable acrylic intraocular lens: effect on posterior capsule opacification. JCRS. 2011;37(6):1060–1068.
8. Ursell PG, Spalton DJ, Pande MV, et al. Relationship between intraocular lens biomaterials and posterior capsule opacification. JCRS. 1998;24:352–360.
9. Hollick EJ, Spalton DJ, Ursell PG, et al. The effect of polymethylmethacrylate, silicone, and polyacrylic intraocular lenses on posterior capsular opacification 3 years after cataract surgery. Ophthalmology. 1999;106:49–54. doi:10.1016/S0161-6420(99)90047-7
10. Nishi O, Nishi K, Wickström K. Preventing lens epithelial cell migration using intraocular lenses with sharp rectangular edges. JCRS. 2000;26(10):1543–1549.
11. Nishi O, Nishi K. Effect of round-edge acrylic intraocular lenses on preventing posterior capsule opacification. JCRS. 2001;27:608–613.
12. Peng Q, Visessook N, Apple DJ, et al. Surgical prevention of posterior capsule opacification. Part 3: intraocular lens optic barrier effect as a second line of defense. JCRS. 2000;26:198–213.
13. Kohnen T, Fabian E, Gerl R, et al. Optic edge design as long-term factor for posterior capsular opacification rates. Ophthalmology. 2008;115(8):
14. Buehl W, Findl O. Effect of intraocular lens design on posterior capsule opacification. JCRS. 2008;34(11):1976–1985.
15. Buehl W, Findl O, Menapace R, et al. Effect of an acrylic intraocular lens with a sharp posterior optic edge on posterior capsule opacification. JCRS. 2002;28(7):1105–1111.
16. Buehl W, Findl O, Menapace R, et al. Long-term effect of optic edge design in an acrylic intraocular lens on posterior capsule opacification. JCRS. 2005;31(5):954–961.
17. Buehl W, Menapace R, Findl O, Neumayer T, Bolz M, Prinz A. Long-term effect of optic edge design in a silicone intraocular lens on posterior capsule opacification. Am J Ophthalmol. 2007;143(6):913–919. doi:10.1016/j.ajo.2007.02.017
18. Nishi O, Yamamoto N, Nishi K, Nishi Y. Contact inhibition of migrating lens epithelial cells at the capsular bend created by a sharp-edged intraocular lens after cataract surgery. JCRS. 2007;33(6):1065–1070.
19. Auffarth G, Golescu A, Becker K, Völcker H. Quantification of posterior capsule opacification with round and sharp edge intraocular lenses. Ophthalmology. 2003;110(4):772–780. doi:10.1016/S0161-6420(02)01980-2
20. Findl O, Buehl W, Menapace R, et al. Long-term effect of sharp optic edges of a polymethyl methacrylate intraocular lens on posterior capsule opacification: a randomized trial. Ophthalmology. 2005;112:2004–2008. doi:10.1016/j.ophtha.2005.06.021
21. Mester U, Fabian E, Gerl R, et al. Posterior capsule opacification after implantation of CeeOn Edge 911A, PhacoFlex SI-40NB, and AcrySof MA60BM lenses: one-year results of an intraindividual comparison multicenter study. JCRS. 2004;30:978–985.
22. Tetz M, Wildeck A. Evaluating and defining the sharpness of intraocular lenses Part 1: influence of optic design on the growth of the lens epithelial cells in vitro. JCRS. 2005;31(11):2172–2179.
23. Buehl W, Menapace R, Sacu S, et al. Effect of a silicone intraocular lens with a sharp posterior optic edge on posterior capsule opacification. JCRS. 2004;30(8):1661–1667.
24. Ness PJ, Werner L, Maddula S, et al. Capsular bag opacification and sites of square-edged barrier breach. JCRS. 2011;37:923–930.
25. Apple D, Peng Q, Visessook N, et al. Surgical prevention of posterior capsule opacification. Part 1: progress in eliminating this complication of cataract surgery. JCRS. 2000;26:180–187.
26. Nishi O, Nishi K, Osakabe Y. Evaluation of posterior capsule opacification using a new posterior view method in rabbits: single-piece acrylic versus 3-piece acrylic intraocular lens. JCRS. 2005;31:2369–2374.
27. Boureau C, Lafuma A, Jeanbat V, Smith AF, Berdeaux G. Cost of cataract surgery after implantation of three intraocular lenses. Clin Ophthalmol. 2009;3:277–285.
28. Cullin F, Busch T, Lundstrom M. Economic considerations related to choice of intraocular lens (IOL) and posterior capsule opacification frequency – a comparison of three different IOLs. Acta Ophthalmol. 2014;92:179–183. doi:10.1111/aos.12026
29. Kossack N, Schindler C, Weinhold I, et al. German claims data analysis to assess impact of different intraocular lenses on posterior capsule opacification and related healthcare costs. J Public Health. 2018;26(1):81–90. doi:10.1007/s10389-017-0851-y
30. Apple DJ, Peng Q, Visessook N, et al. Eradication of posterior capsule opacification: documentation of a marked decrease in Nd: YAG laser posterior capsulotomy rates noted in an analysis of 5416 pseudophakic human eyes obtained postmortem. Ophthalmology. 2001;108:505–518. doi:10.1016/S0161-6420(00)00589-3
31. Schaumberg D, Dana M, Christen W, Glynn R. A systematic overview of the incidence of posterior capsule opacification. Ophthalmology. 1998;105(7):1213–1221. doi:10.1016/S0161-6420(98)97023-3
32. Zhao Y, Yang K, Li J, Huang Y, Zhu S. Comparison of hydrophobic and hydrophilic intraocular lens in preventing posterior capsule opacification after cataract surgery: an updated meta-analysis. Medicine. 2017;96(44):e8301. doi:10.1097/MD.0000000000008301
33. Vasavada A, Shetal MR, Shah GD, Nanavaty MA. Posterior capsule opacification after lens implantation. Expert Rev Ophthalmol. 2013;8:141–149. doi:10.1586/eop.12.80
34. Chan E, Mahroo OA, Spalton DJ. Complications of cataract surgery. Clin Exp Optom. 2010;93(6):379–389. doi:10.1111/j.1444-0938.2010.00516.x
35. Boureau C, Lafuma A, Jeanbat V, Berdeaux G, Smith AF. Incidence of Nd: YAG laser capsulotomies after cataract surgery: comparison of 3 square-edged lenses of different composition. Can J Ophthalmol. 2009;44(2):165–170. doi:10.3129/i09-007
36. Morgan-Warren PJ, Smith JA. Intraocular lens-edge design and material factors contributing to posterior-capsulotomy rates: comparing Hoya FY60aD, PY60aD, and AcrySof SN60WF. Clin Ophthalmol. 2013;7:1661. doi:10.2147/OPTH.S48824
37. Ursell PG, Dhariwal M, Majirska K, et al. Three-year incidence of Nd: YAG capsulotomy and posterior capsule opacification and its relationship to monofocal acrylic IOL biomaterial: a UK real world evidence study. Eye. 2018;32:1579–1589. doi:10.1038/s41433-018-0131-2
38. Aslam TM, Patton N, Rose CJ. OSCA: a comprehensive open-access system of analysis of posterior capsular opacification. BMC Ophthalmol. 2006;6(1):30. doi:10.1186/1471-2415-6-30
39. Zhang H, Wang J. Visual quality assessment of posterior capsule opacification using Optical Quality Analysis System (OQAS). J Ophthalmol. 2017;2017:9852195. doi:10.1155/2017/9852195
40. Mastromonaco C, Balazsi M, Zoroquiain P, et al. Removing subjective post-mortem grading from posterior capsular opacification: a new automated detector opacification software, ADOS. Curr Eye Res. 2018;43(11):1362–1368. doi:10.1080/02713683.2018.1501071
41. Pourshahabi MR, Pourreza HR, Findl O, Daneshvar R, Buehl W. CPCO: countourlet based PCO quantification system. IEEE. 2009;409–413.
42. Tetz M, Auffarth G, Sperker M, Blum M, Völcker H. Photographic image analysis system of posterior capsule opacification. JCRS. 1997;23(10):1515–1520.
43. Barman SA, Hollick EJ, Boyce JF, et al. Quantification of posterior capsular opacification in digital images after cataract surgery. Invest Ophthalmol Vis Sci. 2000;41(12):3882–3892.
44. Bender L, Spalton DJ, Uyanonvara B, et al. POCOman. New system for quantifying posterior capsule opacification. JCRS. 2004;30(10):2058–2063.
45. Buehl W, Findl O, Menapace R, et al. Reproducibility of standardized retroillumination photography for quantification of posterior capsule opacification. JCRS. 2002;28(2):265–270.
46. Kronschläger M, Siegl H, Pinz A, et al. Automated qualitative and quantitative assessment of posterior capsule opacification by automated quantification of after-cataract II (AQUA II) system. BMC Ophthalmol. 2019;19(1):114. doi:10.1186/s12886-019-1116-z
47. Steinert RF, Puliafito CA, Kumar SR, Dudak SD, Patel S. Cystoid macular oedema, retinal detachment and glaucoma after Nd: YAG laser posterior capsulotomy. Am J Ophthalmol. 1991;112:373–378. doi:10.1016/S0002-9394(14)76242-7
48. Kato K, Kurosaka D, Bissen-Miyajima H, Negishi K, Hara E, Nagamoto T. Elschnig pearl formation along the posterior capsulotomy margin after neodymium: YAG capsulotomy. JCRS. 1997;23(10):1556–1560.
49. Billotte C, Berdeaux G. Adverse clinical consequences of neodymium: YAG laser treatment of posterior capsule opacification. JCRS. 2004;30(10):2064–2071.
50. Karahan E, Er D, Kaynak S. An overview of Nd: YAG laser capsulotomy. Med Hypothesis Discov Innov Ophthalmol. 2014;3:45–50.
51. Perez-Vives C. Biomaterial influence on intraocular lens performance: an overview. J Ophthalmol. 2018;2018:
52. Nishi O, Nishi K. Preventing posterior capsule opacification by creating a discontinuous sharp bend in the capsule. JCRS. 1999;25(4):521–526.
53. Hayashi K, Hayashi H. Posterior capsule opacification in the presence of an intraocular lens with a sharp versus rounded optic edge. Ophthalmology. 2005;112(9):1550–1556. doi:10.1016/j.ophtha.2005.03.024
54. Nagamoto T, Eguchi G. Effect of intraocular lens design on migration of lens epithelial cells onto the posterior capsule. JCRS. 1997;23:866–872.
55. Nanavaty MA, Spalton DJ, Boyce J, Brain A, Marshall J. Edge profile of commercially available square-edged intraocular lenses. JCRS. 2008;34:677–686.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
