Back to Journals » Journal of Experimental Pharmacology » Volume 11
Evaluation of in vivo antidiabetic, antidyslipidemic and in vitro antioxidant activities of hydromethanolic root extract of Datura stramonium L. (Solanaceae)
Authors Belayneh YM , Birhanu Z , Birru EM , Getenet G
Received 26 October 2018
Accepted for publication 5 February 2019
Published 23 April 2019 Volume 2019:11 Pages 29—38
DOI https://doi.org/10.2147/JEP.S192264
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bal Lokeshwar
Yaschilal Muche Belayneh,1 Zewdu Birhanu,2 Eshetie Melese Birru,2 Gedefaw Getenet1
1Department of Pharmacy, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia; 2Department of Pharmacology, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Background: The global morbidity and mortality rates of diabetes mellitus are persistently increasing. There is a demand for new antidiabetic drugs because the safety and efficacy of currently available medications are limited. The present study was therefore conducted to study the antidiabetic activities of the hydromethanolic root extract of Datura stramonium L. (Solanaceae) in mice.
Methods: Blood glucose lowering activity of three doses (100, 200, and 400 mg/kg) of the hydromethanolic root extract of D. stramonium was tested on normoglycemic, oral glucose-loaded, and streptozotocin (STZ)-induced diabetic mice models. The effect of the extract on body weight and diabetic dyslipidemia was also studied on STZ-induced diabetic mice. Additionally, antioxidant activity of the plant extract was determined using 2,2-diphenyl-1-picrylhydrazine free radical scavenging assay. Data were analyzed using one way ANOVA followed by Tukey’s post hoc multiple comparison test.
Results: The hydromethanolic root extract did not show significant hypoglycemic activity in normoglycemic mice. The plant extract at doses of 100, 200, and 400 mg/kg significantly (P<0.05) reduced blood glucose levels of oral glucose-loaded and diabetic mice. All the three doses of the root extract significantly improved diabetic dyslipidemia and the body weight of diabetic mice. Free radical scavenging activity of the root extract was found to be comparable to ascorbic acid with an IC50 of 13.47 µg/mL.
Conclusion: This study demonstrated that the hydromethanolic root extract of D. stramonium possesses significant antidiabetic, antidyslipidemic, and antioxidant activities.
Keywords: diabetes mellitus, Datura stramonium, antidiabetic, antidyslipidemic, antioxidant
Introduction
Diabetes mellitus (DM) is a chronic disorder of carbohydrate, protein, and fat metabolism characterized by persistent hyperglycemia secondary to insulin secretion, insulin action, or both.1 Chronic hyperglycemia leads to microvascular complications affecting the eyes, kidneys, and nerves and increased risk for cardiovascular disease.1,2 In addition, hypercholesterolemia and hypertriglyceridemia are common complications of DM. Elevated serum triglyceride, total cholesterol, and low serum high-density lipoprotein cholesterol (HDL-C) levels occur in diabetic patients compared to nondiabetic individuals.3,4
The global prevalence of DM was 11.4% with a treatment rate of 48.2% and a mortality rate of 2.7% in 2017.5 According to a report of the International Diabetes Federation, there were 425 million people (aged 20–79 years) with diabetes globally in 2017 and this number will rise to 629 million in 2045.6
Plants have always been a source of drugs for the treatment of human ailments, and millions of people in the world rely on medicinal plants for primary health care, income generation, and livelihood improvement.7,8 There are about 800 plants which have been reported to show antidiabetic activity implying that there is a great demand for research on plant-derived compounds to develop new antidiabetic medications.8 Different clinical trials have shown that plants cause significant antidiabetic and antidyslipidemic activities in humans.9–14
The currently available antidiabetic drugs are expensive, limited in efficacy, and possess severe side effects.15 Moreover, commonly used oral antidiabetic agents (biguanides, sulfonylureas, and thiazolidinediones) are contraindicated in diabetic patients with renal, hepatic, and cardiac failure.16–18 Thus, the use of plant-derived bioactive compounds which are effective, accessible, safe, and less expensive is an important treatment option for DM.
Traditionally, Datura stramonium has been used in the treatment of DM in Ethiopia. An ethnobotanical survey which was carried out in Nekemte town (East Wollega, Ethiopia) reported that the root of the plant is taken orally to treat DM.19 However, the antidiabetic activity of this medicinal plant is not scientifically validated. More importantly, previous studies have shown that crude aqueous leaf extract and methanolic seed extract of D. stramonium possess in vitro α-amylase enzyme inhibitory activity suggesting the plant to be a potential candidate for DM.20,21 Datura metel, a similar plant species in the genus Datura, has been reported to have significant hypoglycemic activity.22 Consequently, D. stramonium may have a similar effect as both plants belong to the same genus.
Scientific evidence has suggested that induction of oxidative stress and inflammation is a major process in the pathogenesis of DM and its complications.2,23–26 The protective effects of antioxidants and anti-inflammatory agents have been explained in different studies.27–29 Interestingly, the leaf extract of D. stramonium showed in vitro antioxidant and in vivo anti-inflammatory activities30,31 suggesting that the plant may demonstrate antidiabetic activity. Thus, this study was conducted to evaluate the antidiabetic activities of the hydromethanolic root extract of D. stramonium L. (Solanaceae) in mice.
Materials and methods
Collection of plant material
Roots of D. stramonium L were collected from Gondar town (located in Amhara region, northwest Ethiopia) in December 2017. Taxonomic identification of the plant was done by a botanist Dr Getinet Masresha, and a specimen of the plant material was preserved with the voucher number GM004/2010 in the Herbarium of the Biology Department, University of Gondar for future reference.
Drugs, chemicals, and instruments
The following drugs, chemicals, and instruments were used during the study: streptozotocin (STZ; Sigma-Aldrich Co, St Louis, MO, USA), 2,2-diphenyl-1-picrylhydrazine (DPPH; 97%), glibenclamide (Julphar Pharmaceuticals, Ras Al Khaimah, UAE), l-ascorbic acid (99%), citric acid monohydrate (Lab Tech Chemicals, Mumbai, India), trisodium citrate dihydrate (Blulux Laboratories, Faridaban, India), methanol absolute (AppliChem GmbH, Darmstadt, Germany), ferric chloride (Thermo Fisher Scientific, Waltham, MA, USA), sodium hydroxide (Suppertek Chemical, Belapur, India), hydrochloric acid (Suppertek Chemical), 40% glucose solution (Reyoung Pharmaceuticals, Shandong, China), analytical balance, pH meter, i-QARE DS-W® blood glucose meter, and test strips (Alliance International, New Taipei City, Taiwan), lyophilizer, UV spectrophotometer, distilled water (DW), automated chemistry analyzer (Shenzhen Mindray Bio-medical Electronics Co, Ltd, Shenzhen, China), Whatman filter paper no 1, gauze, beakers, funnels, measuring cylinder, glass rod, gloves, spatula, pipettes, gavage (oral feeding syringe), scissors, mask, animal cages, insulin syringe with needle, oven, desiccators, and a refrigerator. Analytical grade chemicals were used in the experiment.
Preparation of the plant extract
The roots of the plant were thoroughly washed with DW to remove dirt and soil, and then dried under shade at room temperature with optimal ventilation. The dried roots were pulverized. Then, 700 gm coarse powder of the roots was macerated in 80% methanol for 72 hours at room temperature and then the solvent was decanted through gauze followed by filtration via Whatman filter paper no 1. The residue was re-macerated twice with fresh solvent (80% methanol) each for 72 hours and the filtrates obtained from the successive maceration were dried using an oven at 40°C to remove methanol and a lyophilizer to remove water. The dried root extract was then kept in a refrigerator at −4°C until further use.32
Experimental animals
Healthy male Swiss albino mice (body weight, 20–35 g; age 8–12 weeks) were used in the study except for the acute oral toxicity study wherein healthy female mice (body weight, 20–35 g; age, 8–12 weeks) were used. The mice were purchased from the Ethiopian Public Health Institute, kept at the animal house of the Pharmacy Department, Wollo University under standard conditions (at a temperature of 25±2°C, and with a 12-hour light–dark cycle), and provided with free access to standard pellet laboratory diet and water ad libitum. Animals were acclimatized to the laboratory conditions for 1 week before the initiation of the experiment. The experiment was conducted in accordance with the guide for the care and use of laboratory animals.33
Phytochemical analysis of the hydromethanolic root extract of D. stramonium
Qualitative preliminary phytochemical screening tests were carried out for 80% methanol root extract of D. stramonium using standard procedures34,35 to determine the presence or absence of alkaloids, phenols, flavonoids, tannins, saponins, anthraquinones, terpenoids, glycosides, and steroids.
Acute toxicity study
Acute oral toxicity study was carried out based on the protocols of the Organization for Economic Cooperation and Development guideline no 425.36 One female Swiss albino mouse was fasted for 4 hours and the fasting body weight of the animal was measured. Then, the hydromethanolic root extract was administered sequentially to the fasted mouse at a dose of 2,000 mg/kg. The mouse was then kept under strict observation for physical and behavioral changes for 24 hours with special attention during the first 4 hours. Following the results from the first mouse, four other mice were recruited and allowed to fast for 4 hours and their body weight was measured. The root extract was administered separately to each mouse at a dose of 2,000 mg/kg, and each mouse was observed in the same manner. The observation was continued for 14 days for any sign of overt toxicity.
Grouping and dosing of animals
Male Swiss albino mice were recruited and used in all animal models of this study since female mice are insensitive to STZ and insulin.37–39 In normoglycemic and oral glucose-loaded models, overnight fasted mice (for 16 hours)40 were randomly divided into five groups (each group containing six mice). Group I (negative control) was treated with 10 mL/kg DW; groups II, III, and IV were treated with 100, 200, and 400 mg/kg hydromethanolic root extract, respectively; and group V was treated with 5 mg/kg glibenclamide. In the STZ-induced diabetic animal model, overnight fasted (for 16 hours) mice were randomly divided into six groups (five groups of diabetic and one group of normal mice, each group containing six mice). Groups I and II (normal and diabetic control) were treated with 10 mL/kg DW; groups III, IV, and V (diabetic test groups) were treated with 100, 200, and 400 mg/kg root extract, respectively; and group VI was treated with 5 mg/kg glibenclamide. All the treatments (glibenclamide, plant extract, and DW) were administered orally to the mice since people traditionally use the root of D. stramonium orally for the treatment of DM.19
Measurement of blood glucose level
Blood samples were withdrawn from the tail vein of each fasted mouse by cutting the tip of the tail aseptically in all animal models. Blood glucose level was measured using a glucometer and test strip. Measurements were carried out in triplicates and the average value was taken.
Induction of experimental diabetes
Diabetes was induced using STZ which was dissolved in 0.1 M fresh cold citrate buffer (pH 4.5). Freshly prepared STZ solution was injected intraperitoneally (ip) in overnight (16 hours) fasted mice at a dose of 150 mg/kg.41–43 Food consumption was allowed after 30 minutes of STZ injection. After 6 hours, the animals were given free access to 5% glucose solution for the next 24 hours to prevent death secondary to hypoglycemic shock. They were screened for diabetes after 72 hours of STZ injection and mice with fasting blood glucose levels >200 mg/dL were considered diabetic and used in the study.44–46 Bedding of the cages was changed every day after STZ injection to maintain dry bedding for polyuric diabetic animals.47
Evaluation of hypoglycemic effect of the extract in normoglycemic mice
Overnight fasted normal mice were randomly divided into five different groups. Then, mice were treated with DW, plant extract, and glibenclamide according to their respective grouping as mentioned earlier. The blood glucose level of each mouse was measured before treatment as baseline and then at 1, 2, 4, and 6 hours posttreatment.
Evaluation of the antihyperglycemic effect of the extract in oral glucose-loaded mice
Overnight fasted mice were used for the oral glucose tolerance test.40,48–52 Fasted mice were randomly divided into five groups and then DW, plant extract, and glibenclamide were administered according to their respective grouping. After 30 minutes of treatment administration, 40% glucose solution was loaded into each mouse orally at a dose of 2 g/kg.43,51 Blood glucose level of each mouse was measured just before oral glucose loading and then at 30, 60, and 120 minutes following glucose loading.46,53,54
Evaluation of the effects of repeated daily doses of the plant extract on blood glucose, serum lipid, and body weight of diabetic mice
Overnight fasted diabetic and normal mice were randomly grouped into six groups (five groups of diabetic and one group of normal mice, each group containing six mice). Then, mice were treated with DW, plant extract, and glibenclamide according to their respective grouping once daily for 14 days. Fasting blood glucose level and body weight of the mice were measured on day 1 (3 days after STZ injection immediately before the first day of treatment) as baseline, day 7, and day 14. Measurements of blood glucose level and body weight of overnight fasted mice were carried out 2 hours after treatment.51,55 On day 15, overnight fasted mice were sacrificed with an overdose of pentobarbitone (150 mg/kg, ip),56 and then blood samples were collected in a sterile tube from each mouse through cardiac puncture. The blood samples were kept at room temperature for 2 hours to allow coagulation, and then centrifuged at 2,000 rpm for 10 minutes. The supernatant was decanted into test tubes for serum sample preparation to determine the level of total cholesterol, triglyceride, and high HDL-C by using an automated chemistry analyzer.
Evaluation of in vitro antioxidant activity of the root extract using DPPH assay
Free radical scavenging activity of the root extract of D. stramonium was evaluated by using DPPH free radical scavenging assay.57 Four milligrams of DPPH were dissolved in 100 mL methanol in the dark and 3.9 mL of a 0.1 mM methanolic solution of DPPH was mixed with a 100 µL methanolic solution of different concentrations (12.5, 25, 50, 100, 200, and 400 µg/mL) of the root extract. It was allowed to mix well and incubated in the dark for 30 minutes at room temperature. Ascorbic acid at the same concentration was used as positive control (standard antioxidant). DPPH solutions without the tested samples were used as negative control. After 30 minutes, the absorbance of the mixture and the control at 517 nm were recorded using a UV spectrophotometer. The test was repeated with the same concentration of each sample in triplicate and the average value was taken. The percentage scavenging of DPPH free radical was calculated by the formula: [(A0−A1)/A0] ×100, where A0 is the absorbance of the control and A1 is the absorbance of the plant extract or standard drug containing sample.
Ethical considerations
Ethical approval for this study was obtained from the ethical review committee of the College of Medicine and Health Sciences, Wollo University before the commencement of the study.
Statistical analysis
Data are presented as mean ± standard error of mean. All parameters between groups and within a group were compared using one way ANOVA followed by Tukey’s post hoc multiple comparison test. P-values <0.05 were considered statistically significant. SPSS version 23 software (IBM Corporation, Armonk, NY, USA) was used for the statistical analysis.
Results
Percentage yield of the plant extract
The percentage yield of the hydromethanolic root extract of D. stramonium was found to be 7.14% (w/w). The extract was a reddish semisolid at room temperature, and it solidified when stored in a refrigerator.
Acute oral toxicity test
The root extract was found to be safe at the dose of 2,000 mg/kg. There was no sign of acute toxicity and mortality in extract-fed female mice in the 2-week observation period. This shows that LD50 of hydromethanolic root extract of D. stramonium is >2,000 mg/kg. Based on the acute toxicity test result, 100, 200, and 400 mg/kg doses of hydromethanolic root extract were selected for hypoglycemic and antihyperglycemic studies.
Preliminary qualitative phytochemical screening
Qualitative preliminary phytochemical analysis indicated the presence of flavonoids, phenols, tannins, alkaloids, steroids, glycosides, and anthraquinones in the hydromethanolic root extract of D. stramonium (Table 1).
  | Table 1 Preliminary qualitative phytochemical screening of the hydromethanolic root extract of Datura stramonium Abbreviations: +, present; –, absent. |
Hypoglycemic effect of hydromethanolic root extract of D. stramonium in normoglycemic mice
In normoglycemic mice, all the three doses of the D. stramonium root extract did not show significant (P>0.05) hypoglycemic effect at all time points compared to the negative control (Table 2). Glibenclamide significantly reduced the blood glucose at all time points (P<0.01 at 1 hour, and P<0.001 at 2, 4, and 6 hours).
Effect of hydromethanolic root extract of D. stramonium on blood glucose level of oral glucose-loaded mice
It has been found that before oral glucose loading, there were no significant differences in the fasting blood glucose level among all groups (P>0.05). The peak blood glucose level was measured 0.5 hours after glucose loading in all groups confirming induction of hyperglycemia (Figure 1). The extract and standard drug did not significantly reduce the increased blood glucose level after 30 minutes of oral glucose loading as compared to the negative control. It was revealed that the root extract at doses of 100 mg/kg (P<0.05), 200 mg/kg (P<0.05), and 400 mg/kg (P<0.01) significantly reduced the blood glucose level at 1 and 2 hours post oral glucose loading compared to the negative control. In addition, glibenclamide (5 mg/kg) significantly (P<0.001) reduced the blood glucose level at 1 and 2 hours post oral glucose administration.
Effect of hydromethanolic root extract of D. stramonium on blood glucose level of diabetic mice
There was significant (P<0.001) elevation in the baseline blood glucose level of diabetic mice compared to normal controls on day 0 (Table 3). Groups of diabetic mice which were treated with the plant extract and glibenclamide showed significant reduction of blood glucose level on day 7 and day 14 of treatment compared to the diabetic control (Table 3). The study showed that the root extract at doses of 100 mg/kg (P<0.05 on day 7 and 14), 200 mg/kg (P<0.05 on day 7 and 14), and 400 mg/kg (P<0.05 on day 7, P<0.01 on day 14) significantly reduced blood glucose levels compared to the diabetic control. Glibenclamide significantly (P<0.001 on day 7 and 14) reduced the blood glucose level compared to the diabetic control. Intergroup analysis showed that there was no significant difference in the blood glucose level among the extract and standard drug-treated groups.
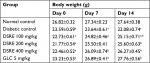
Effect of hydromethanolic root extract of D. stramonium on body weight of diabetic mice
The study showed that there was significant (P<0.01) reduction in the body weight of the diabetic control compared to normal control on days 0, 7, and 14 of treatment. Administration of the root extract significantly (P<0.05) improved body weight of diabetic mice as compared to the diabetic control (Table 4). The root extract at doses of 100 and 200 mg/kg showed significant improvement in body weight of the diabetic mice on day 14, whereas 400 mg/kg showed significant improvement in body weight on day 7 and 14 compared to the diabetic control. Additionally, glibenclamide significantly (P<0.05) improved the body weight of diabetic mice on day 7 and 14 of treatment compared to the diabetic control.
Effect of hydromethanolic root extract of D. stramonium on serum lipid level of diabetic mice
There was a significant (P<0.01) elevation of serum total cholesterol and triglyceride level with significant reduction in HDL cholesterol in diabetic controls compared to the normal control, confirming induction of diabetic dyslipidemia (Table 5). On administration of the root extract, the serum lipid level improved. The result showed that the root extract at doses of 100, 200, and 400 mg/kg significantly (P<0.05) decreased the level of total cholesterol and triglycerides compared to the diabetic control. Similarly, the level of HDL cholesterol significantly (P<0.05) increased after 14 days of treatment by all the doses of the root extract compared to the negative control. In addition, glibenclamide also improved (P<0.01) the lipid profile in diabetic mice compared to the diabetic control.
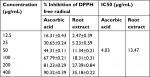
Antioxidant activity of hydromethanolic root extract of D. stramonium
Antioxidant activity of the hydromethanolic root extract was studied using DPPH free radical scavenging assay. This study showed that free radical scavenging activity of the root extract is concentration dependent and comparable to ascorbic acid. IC50 of the root extract and ascorbic acid in the assay was found to be 13.47 and 4.03 µg/mL, respectively (Table 6).
Discussion
DM is a chronic disorder of carbohydrate, protein, and fat metabolism characterized by persistent hyperglycemia resulting from abnormalities in insulin secretion, insulin action, or both.1,58,59 Potential plant-derived antidiabetic compounds that can act on multiple disease-related drug targets with proven long-term safety are needed for the treatment of DM.60 Bioactive compounds that are obtained from various medicinal plant sources, including flavonoids, phenolic compounds, alkaloids, terpenoids, saponins, tannins, glycosides, glycolipids, dietary fibers, carotenoids, anthocyanins have been reported to have potent antidiabetic activity.43,61,62 In the present study, preliminary qualitative phytochemical screening of the hydromethanolic root extract of D. stramonium revealed the presence of phenols, flavonoids, tannins, alkaloids, glycosides, and steroids implying that blood glucose lowering activity of the plant extract is possibly due to the presence of these phytochemicals.
Plant-derived phytochemicals affect glucose metabolism by increasing insulin secretion and action, inhibiting α-glucosidase and α-amylase, decreasing gluconeogenesis, protecting pancreatic β-cells against oxidative stress and inflammation, enhancing expression and translocation of glucose transporters, promoting pancreatic β-cell proliferation, and inhibiting apoptosis.61 These mechanisms of action of phytochemicals provide an insight to new antidiabetic drug designs. Flavonoids and other polyphenols show blood glucose lowering activities mainly by enhancing GLUT-2 expression in pancreatic β-cells and increasing expression and promoting translocation of GLUT-4.63–65
In the oral glucose-loaded mice model, all three doses (100, 200, and 400 mg/kg) of 80% methanolic root extract of D. stramonium significantly reduced raised blood glucose level. In this model, the blood glucose lowering activity of the leaf extract of D. stramonium could be associated with the presence of different phytomolecules like flavonoids, phenols, and others that act individually or synergistically in glucose homeostasis by different mechanisms of action. Previous studies reported that various plant extracts show significant blood glucose lowering activities on oral glucose-loaded animal models.45,51,55
STZ is a widely used diabetogenic agent for the induction of experimental diabetes in animals due to its toxic effect on pancreatic β-cells, resulting in loss of insulin secretion. It is a structural analog of glucose; as a result, it is taken up by pancreatic β-cells through GLUT 2 and destroys beta cells via DNA methylation and free radical generation.66,67 Pancreas contains stable (quiescent) β-cells which have regenerative capacity after being damaged by chemicals (STZ, alloxan) through neogenesis or replication of the preexisting differentiated cells.47,68 The leaf extract of Moringa stenopetala, as reported by Toma et al, and other plant extracts enhance proliferation/regeneration of β-cells of the pancreas resulting in increased insulin secretion and action in STZ-induced diabetic animals.55 In the present study, all the three doses of the hydromethanolic root extract of D. stramonium showed significant antihyperglycemic activities in STZ-induced diabetic mice suggesting that pancreatic β-cell regeneration might be one possible mechanism for the antidiabetic activity of the root extract.
Oxidative stress and inflammation involving the pancreas are the key factors in the pathogenesis and progression of diabetes. Excessive free radicals generated from hyperglycemia-induced glucose autoxidation and protein glycosylation play an important role in DM pathogenesis.26,70,71 Pancreas β-cells are sensitive to damage by nitric oxide and other free radicals which can be generated by STZ.66,72,73 Previous studies revealed that various plant extracts have shown pancreas β-cell protective effects due to their antioxidant activities.27–29 Similarly, the root extract of D. stramonium showed antioxidant activity in the current study. This suggests that the β-cell protective effect secondary to the antioxidant activity of the root extract may have contributed to its antihyperglycemic activity in STZ-induced diabetic mice.
The root extract did not show hypoglycemic effect in normal mice even though it demonstrated significant antihyperglycemic activity in oral glucose-loaded and STZ-induced diabetic mice. Since normoglycemic mice demonstrate normal homeostasis, the extract could cause less suppression of normal regulatory mechanisms involved in carbohydrate metabolism.74,75
In the present study, STZ-induced diabetic mice developed significant hyperglycemia, body weight loss, and diabetic dyslipidemia. Body weight loss in STZ-induced diabetic mice is due to increased protein catabolism secondary to insulin deficiency.70,76 Previous studies demonstrated that various plant extracts improved body weight loss in STZ-induced diabetic animals.40,43,51 In this study, the hydromethanolic root extract-treated diabetic mice showed significant (P<0.05) improvement in body weight suggesting that the plant extract might have protective effects against protein catabolism and muscle wasting possibly due to the enhancement of insulin secretion and/or action.
Insulin activates lipoprotein lipase which can degrade triglycerides and very low-density lipoprotein cholesterol. In diabetic patients, lipoprotein lipase is less active due to insulin deficiency, and this can lead to the development of diabetic dyslipidemia. Additionally, persistent hyperglycemia activates transcription factors for fatty acid synthase which causes increased lipid biosynthesis.69,77 Thus, augmenting insulin secretion and/or action is a potential approach to treat diabetic dyslipidemia. In this study, repeated daily dose administration of D. stramonium root extract for 14 days significantly (P<0.05) reduced serum triglyceride and total cholesterol levels while it significantly (P<0.05) increased HDL-cholesterol at least due to improved glucose utilization in the diabetic mice. Additionally, the plant extract may have a direct effect on lipid absorption and metabolism that can lead to the improvement of diabetic dyslipidemia.78
Conclusion
This study demonstrated that the hydromethanolic root extract of D. stramonium has antihyperglycemic and antioxidant activities. Additionally, the extract significantly improved diabetic dyslipidemia.
Acknowledgment
We are grateful to Wollo University for funding this study.
Disclosure
The authors report no conflicts of interest in this work.
References
Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. | ||
Skyler JS, Bakris GL, Bonifacio E, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. 2017;66(2):241–255. | ||
Schofield JD, Liu Y, Rao-Balakrishna P, Malik RA, Soran H. Diabetes dyslipidemia. Diabetes Ther. 2016;7(2):203–219. | ||
Vijayaraghavan K. Treatment of dyslipidemia in patients with type 2 diabetes. Lipids Health Dis. 2010;9(1):144. | ||
Ge Q, Chen L, Chen K. Treatment of diabetes mellitus using iPS cells and spice polyphenols. J Diabetes Res. 2017;2017(1):1–11. | ||
International Diabetes Federation. IDF Diabetes Atlas. 8th ed. Brussels, Belgium: IDF; 2017. | ||
Uprety Y, Asselin H, Dhakal A, Julien N. Traditional use of medicinal plants in the boreal forest of Canada: review and perspectives. J Ethnobiol Ethnomed. 2012;8(1):7. | ||
Khera N, Bhatia A. Medicinal plants as natural anti-diabetic agents. Int J Pharm Sci Res. 2014;5(3):713. | ||
Eldin IMT, Ahmed EM, Abd EH. Preliminary study of the clinical hypoglycemic effects of Allium cepa (red onion) in type 1 and type 2 diabetic patients. Environ Health Insights. 2010;4:EHI.S5540EHI. | ||
Hosseini S, Jamshidi L, Mehrzadi S, et al. Effects of Juglans regia L. leaf extract on hyperglycemia and lipid profiles in type two diabetic patients: a randomized double-blind, placebo-controlled clinical trial. J Ethnopharmacol. 2014;152(3):451–456. | ||
Jandaghi P, Noroozi M, Ardalani H, Alipour M. Lemon balm: a promising herbal therapy for patients with borderline hyperlipidemia—A randomized double-blind placebo-controlled clinical trial. Complement Ther Med. 2016;26:136–140. | ||
Okolie UV, Okeke CE, Oli JM, Ehiemere IO. Hypoglycemic indices of Vernonia amygdalina on postprandial blood glucose concentration of healthy humans. African J Biotech. 2008;7(24):4581–4585. | ||
Serraclara A, Hawkins F, Pérez C, Domínguez E, Campillo JE, Torres MD. Hypoglycemic action of an oral fig-leaf decoction in type-I diabetic patients. Diabetes Res Clin Pract. 1998;39(1):19–22. | ||
Yongchaiyudha S, Rungpitarangsi V, Bunyapraphatsara N, Chokechaijaroenporn O. Antidiabetic activity of Aloe vera L. juice. I. clinical trial in new cases of diabetes mellitus. Phytomedicine. 1996;3(3):241–243. | ||
Rao MU, Rao MU, Sreenivasulu M, et al. Herbal medicines for diabetes mellitus: a review. Int J PharmTech Res. 2010;2(3):1883–1892. | ||
Tschöpe D, Hanefeld M, Meier JJ, et al. The role of co-morbidity in the selection of antidiabetic pharmacotherapy in type-2 diabetes. Cardiovasc Diabetol. 2013;12(1):62. | ||
Varas-Lorenzo C, Margulis AV, Pladevall M, et al. The risk of heart failure associated with the use of noninsulin blood glucose-lowering drugs: systematic review and meta-analysis of published observational studies. BMC Cardiovasc Disord. 2014;14(1):129. | ||
Yousef F, Yousef N, Mansour O, Herbali J. Metformin: a unique herbal origin medication. Global J Med Res. 2017;17. | ||
Suleman S, Alemu T. A survey on utilization of ethnomedicinal plants in Nekemte Town, East Wellega (Oromia), Ethiopia. J Herbs Spices Med Plants. 2012;18(1):34–57. | ||
Shobha GSC, Shashidhara KS. Phytochemical profile, antibacterial and antidiabetic effects of crude aqueous leaf extract of Datura stramonium. Pharmacophore. 2014;5(2):273–278. | ||
Mehrabadi M, Bandani AR, Saadati F, Mahmudvand M. α-Amylase activity of stored products insects and its inhibition by medicinal plant extracts. J Agric Sci Technol. 2011;13:1173–1182. | ||
Krishna Murthy B, Nammi S, Kota MK, Krishna Rao RV, Koteswara Rao N, Annapurna A. Evaluation of hypoglycemic and antihyperglycemic effects of Datura metel (Linn.) seeds in normal and alloxan-induced diabetic rats. J Ethnopharmacol. 2004;91(1):95–98. | ||
Yan L-J. Pathogenesis of chronic hyperglycemia: from reductive stress to oxidative stress. J Diab Res. 2014;2014(4):1–11. | ||
Kaneto H, Katakami N, Matsuhisa M, Matsuoka TA. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators Inflam. 2010;2010(6865):1–11. | ||
Hameed I, Masoodi SR, Mir SA, Nabi M, Ghazanfar K, Ganai BA. Type 2 diabetes mellitus: from a metabolic disorder to an inflammatory condition. World J Diabetes. 2015;6(4):598. | ||
Chen L, Chen R, Wang H, Liang F. Mechanisms linking inflammation to insulin resistance. Int J Endocrinol. 2015;2015(1):1–9. | ||
Gothai S, Ganesan P, Park S-Y, Fakurazi S, Choi D-K, Arulselvan P. Natural phyto-bioactive compounds for the treatment of type 2 diabetes: inflammation as a target. Nutrients. 2016;8(8):461. | ||
Dal S, Sigrist S. The protective effect of antioxidants consumption on diabetes and vascular complications. Diseases. 2016;4(4):24. | ||
Masjedi F, Gol A, Dabiri S. Preventive effect of garlic (Allium sativum L.) on serum biochemical factors and histopathology of pancreas and liver in Streptozotocin-induced diabetic rats. Iran J Pharm Res. 2013;12(3):325. | ||
Rashid S, Ahmad M, Zafar M, et al. Ethnopharmacological evaluation and antioxidant activity of some important herbs used in traditional medicines. J Tradit Chin Med. 2016;36(5):689–694. | ||
Abbas DA. Analgesic, anti-inflammatory and antidiarrhoeal effects of Datura stramonium hydroalcoholic leaves extract in mice. IJRRAS. 2013;14(1):193–199. | ||
Satyajit Sarker DD, Gray AI. Natural Products Isolation. 2nd ed. UK: Humana Press Inc; 2006. | ||
Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, DC: Institute of Laboratory Animal Resources, National Research Council; 2010:248. Available from: https://grants.nih.gov/grants/olaw/Guide-for-the-Care-and-use-of-laboratory-animals.pdf. Accessed October 30, 2018. | ||
Pandey A, Tripathi S. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J Pharmacog Phytochem. 2014;2(5):115–119. | ||
Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: a review. Internationale Pharmaceutica Sciencia. 2011;1(1):98–106. | ||
OECD/OCDE. OECD guideline for the testing of chemicals. Acute oral toxicity; up-and-down procedure (UDP). Paris, France: OECD; 2008. No 4252008. | ||
Deeds MC, Anderson JM, Armstrong AS, et al. Single dose streptozotocin-induced diabetes: considerations for study design in islet transplantation models. Lab Anim. 2011;45(3):131–140. | ||
Furman BL. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol. 2015;5(47):1–20. | ||
Vital P, Hiriart M. Sexual dimorphism in insulin sensitivity and susceptibility to develop diabetes in rats. J Endocrinol. 2006;190(2):425–432. | ||
Zhang Y, Feng F, Chen T, Li Z, Shen QW. Antidiabetic and antihyperlipidemic activities of Forsythia suspensa (Thunb.) Vahl (fruit) in streptozotocin-induced diabetes mice. J Ethnopharmacol. 2016;192:256–263. | ||
Etuk E. Animal models for studying diabetes mellitus. Agric Biol JN Am. 2010;1(2):130–134. | ||
MMPC. Streptozotocin-induced type 1 diabetes model. Mouse Metabolic Phenotyping Centers Protocols. 2013. Available from: https://www.mmpc.org/shared/document.aspx?id=152&docType=Protocol. Accessed October 30, 2018. | ||
Sulyman AO, Akolade JO, Sabiu SA, Aladodo RA, Muritala HF. Antidiabetic potentials of ethanolic extract of Aristolochia ringens (Vahl.) roots. J Ethnopharmacol. 2016;182:122–128. | ||
Baquer NZ, Kumar P, Taha A, Kale RK, Cowsik SM, Mclean P. Metabolic and molecular action of Trigonella foenum-graecum (fenugreek) and trace metals in experimental diabetic tissues. J Biosci. 2011;36(2):383–396. | ||
Tamiru W, Engidawork E, Asres K. Evaluation of the effects of 80% methanolic leaf extract of Caylusea abyssinica (fresen.) fisch. & Mey. on glucose handling in normal, glucose loaded and diabetic rodents. BMC Complement Altern Med. 2012;12(1):151. | ||
Demoz MS, Gachoki KP, Mungai KJ, Negusse BG. Evaluation of the anti-diabetic potential of the methanol extracts of Aloe camperi, meriandra dianthera and a polyherb. J Diabetes Mellitus. 2015;5(04):267. | ||
King AJF. The use of animal models in diabetes research. Br J Pharmacol. 2012;166(3):877–894. | ||
Bowe JE, Franklin ZJ, Hauge-Evans AC, King AJ, Persaud SJ, Jones PM. Metabolic phenotyping guidelines: assessing glucose homeostasis in rodent models. J Endocrinol. 2014;222(3):G13–G25. | ||
Ayala JE, Samuel VT, Morton GJ, et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech. 2010;3(9–10):525–534. | ||
Zhang P. Glucose tolerance test in mice. Bio-Protocol. 2011;1(19):e159. | ||
Sharma S, Choudhary M, Bhardwaj S, Choudhary N, Rana AC. Hypoglycemic potential of alcoholic root extract of Cassia occidentalis Linn in streptozotocin induced diabetes in albino mice. Bull Fac Pharm Cairo Univ. 2014;52(2):211–217. | ||
Sharma B, Salunke R, Balomajumder C, Daniel S, Roy P. Anti-diabetic potential of alkaloid rich fraction from Capparis decidua on diabetic mice. J Ethnopharmacol. 2010;127(2):457–462. | ||
Rajurkar B. Phyto-pharmacological investigations of Clerodendrum infortunatum Gartn. Int J Pharm. 2011;2:130–132. | ||
Tesfaye A, Makonnen E, Gedamu S. Hypoglycemic and antihyperglycemic activity of aqueous extract of Justicia Schimperiana leaves in normal and streptozotocin-induced diabetic mice. Int J Pharm Sci Res. 2016;7(02):110–113. | ||
Toma A, Makonnen E, Mekonnen Y, Debella A, Adisakwattana S. Antidiabetic activities of aqueous ethanol and n-butanol fraction of Moringa stenopetala leaves in streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2015;15(1):242. | ||
Vogel HG. Drug Discovery and Evaluation: Pharmacological Assays. Berlin, Heidelberg: Springer-Verlag; 2002. | ||
Macdonald-Wicks LK, Wood LG, Garg ML. Methodology for the determination of biological antioxidant capacityin vitro: a review. J Sci Food Agri. 2006;86(13):2046–2056. | ||
Cernea S, Dobreanu M. Diabetes and beta cell function: from mechanisms to evaluation and clinical implications. Biochem Med. 2013;23(3):266–280. | ||
Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414(6865):799–806. | ||
Nicolle E, Souard F, Faure P, Boumendjel A. Flavonoids as promising lead compounds in type 2 diabetes mellitus: molecules of interest and structure-activity relationship. Curr Med Chem. 2011;18(17):2661–2672. | ||
Sayem A, Arya A, Karimian H, Krishnasamy N, Ashok Hasamnis A, Hossain C. Action of phytochemicals on insulin signaling pathways accelerating glucose transporter (GLUT4) protein translocation. Molecules. 2018;23(2):258. | ||
Upadhyay S, Dixit M. Role of polyphenols and other phytochemicals on molecular signaling. Oxid Med Cell Longev. 2015;2015(3, supplement):1–15. | ||
Romano B, Pagano E, Montanaro V, Fortunato AL, Milic N, Borrelli F. Novel insights into the pharmacology of flavonoids. Phytother Res. 2013;27(11):1588–1596. | ||
Kennedy DO. Polyphenols and the human brain: plant “secondary metabolite” ecologic roles and endogenous signaling functions drive benefits. Adv Nutr. 2014;5(5):515–533. | ||
Jiang C-S, Liang L-F, Guo Y-W. Natural products possessing protein tyrosine phosphatase 1B (PTP1B) inhibitory activity found in the last decades. Acta Pharmacol Sin. 2012;33(10):1217–1245. | ||
Maritim AC, Sanders RA, Watkins JB, Watkins J 3rd. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17(1):24–38. | ||
Oltésová D, Herichova I. On the mechanisms of diabetogenic effects of alloxan and streptozotocin. Diabetologie, Metabolismus, Endokrinologie, Výživa. 2011;14:130–138. | ||
Weir GC, Bonner-Weir S. GABA signaling stimulates β-cell regeneration in diabetic mice. Cell. 2017;168. | ||
Moodley K, Joseph K, Naidoo Y, Islam S, Mackraj I. Antioxidant, antidiabetic and hypolipidemic effects of Tulbaghia violacea Harv. (wild garlic) rhizome methanolic extract in a diabetic rat model. BMC Complement Altern Med. 2015;15(1):408. | ||
Stirban A, Gawlowski T, Roden M. Vascular effects of advanced glycation endproducts: clinical effects and molecular mechanisms. Mol Metab. 2014;3(2):94–108. | ||
Hameed I, Masoodi SR, Mir SA, Nabi M, Ghazanfar K, Ganai BA. Type 2 diabetes mellitus: from a metabolic disorder to an inflammatory condition. World J Diabetes. 2015;6(4):598. | ||
Spinas GA. The dual role of nitric oxide in islet β-cells. Physiology. 1999;14(2):49–54. | ||
Oltésová D, Herichová I. On the mechanisms of diabetogenic effects of alloxan and streptozotocin. Diabetologie, Metabolismus, Endokrinologie, Výživa. 2011;14:130–138. | ||
Vats V, Grover JK, Rathi SS. Evaluation of anti-hyperglycemic and hypoglycemic effect of Trigonella foenum-graecum Linn, Ocimum sanctum Linn and Pterocarpus marsupium Linn in normal and alloxanized diabetic rats. J Ethnopharmacol. 2002;79(1):95–100. | ||
Kameswara Rao B, Renuka Sudarshan P, Rajasekhar MD, Nagaraju N, Appa Rao C. Antidiabetic activity of Terminalia pallida fruit in alloxan induced diabetic rats. J Ethnopharmacol. 2003;85(1):169–172. | ||
Eleazu CO, Iroaganachi M, Okafor PN, Ijeh II, Eleazu KC. Ameliorative potentials of ginger (Z. officinale Roscoe) on relative organ weights in streptozotocin induced diabetic rats. Int J Biomed Sci. 2013;9(2):82. | ||
Poungvarin N, Lee JK, Yechoor VK, et al. Carbohydrate response element-binding protein (ChREBP) plays a pivotal role in beta cell glucotoxicity. Diabetologia. 2012;55(6):1783–1796. | ||
Rouhi-Boroujeni H, Rouhi-Boroujeni H, Heidarian E, Mohammadizadeh F, Rafieian-Kopaei M. Herbs with anti-lipid effects and their interactions with statins as a chemical anti- hyperlipidemia group drugs: a systematic review. ARYA Atheroscler. 2015;11(4):244–251. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.