Back to Journals » Journal of Experimental Pharmacology » Volume 12
Evaluation of in vitro and in vivo Anti-Diabetic, Anti-Hyperlipidemic and Anti-Oxidant Activity of Flower Crude Extract and Solvent Fractions of Hagenia Abyssinica (Rosaceae)
Authors Kifle ZD , Yesuf JS , Atnafie SA
Received 15 February 2020
Accepted for publication 27 May 2020
Published 9 June 2020 Volume 2020:12 Pages 151—167
DOI https://doi.org/10.2147/JEP.S249964
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bal Lokeshwar
Zemene Demelash Kifle, Jibril Seid Yesuf, Seyfe Asrade Atnafie
University of Gondar, College of Medicine and Health Sciences, School of Pharmacy, Department of Pharmacology, Gondar, Ethiopia
Correspondence: Zemene Demelash Kifle
University of Gondar, College of Medicine and Health Sciences, School of Pharmacy, Department of Pharmacology, Gondar, Ethiopia
Tel +251918026724
Email [email protected]
Background: The flower of Hagenia abyssinica (Rosaceae) has been used in traditional health systems to treat diabetes mellitus in Ethiopia and Tanzania. However, the antidiabetic activity of this medicinal plant is not scientifically validated and authenticated. The present study aimed to investigate the in vitro and in vivo anti-diabetic activity of flower crude extract and solvent fractions of Hagenia abyssinica.
Methods: The in vitro α–amylase inhibition and antioxidant activity of the crude extract and solvent fractions of Hagenia abyssinica were evaluated by using 3,5-dinitrosalicylic acid (DNSA) and diphenyl-2-picrylhydrazyl (DPPH) assay model, respectively. Blood glucose lowering activity of 80% methanolic flower crude extract and solvent fraction was studied in four animal models: normoglycemic mice model, oral glucose loaded mice model, single dose-treated streptozotocin-induced diabetic mice model, and repeated dose-treated streptozotocin-induced diabetic mice model. The effect of the crude extract and solvent fraction of Hagenia abyssinica on diabetic lipid profile and body weight was also studied.
Results: The acute toxicity study of Hagenia abyssinica flower extract did not show mortality in the animals at the limit dose of 2g/kg during the observation period. The result of α–amylase enzyme inhibition activity was found in a dose-dependent manner, the strongest activity was shown by ethyl acetate fraction (54.23% inhibition at 800 μg/mL) compared to the standard acarbose having 91.87% inhibition at 800 μg/mL. Among these extracts, the crude extract had the highest antioxidant activity (58.38% inhibition at 500 μg/mL). The crude extract of H. abyssinica showed significant blood glucose-lowering effect on normoglycemic mice and oral glucose loaded mice. In streptozotocin-induced diabetic mice model, the crude extract and ethyl acetate fraction significantly decreased the fasting blood glucose level after 14 days of treatment. There were significant reductions in serum total cholesterol, serum triglycerides, very low-density lipoprotein, and low-density lipoprotein. However, there were significant increments in body weight and high-density lipoprotein as compared to untreated diabetic mice.
Conclusion: The result demonstrated the beneficial biochemical effects of Hagenia abyssinica extract by inhibiting α–amylase, scavenging diphenyl-2-picrylhydrazyl (DPPH) and improving serum lipid profile levels. The flower crude extract and solvent fractions of Hagenia abyssinica are effective in lowering blood glucose levels in diabetic and normoglycemic mice. The claimed traditional use as antidiabetic has scientific ground.
Keywords: antidiabetic, anti-hyperlipidemic, antioxidant, α-amylase, Hagenia abyssinica, streptozotocin
Introduction
Diabetes mellitus is a serious, chronic and heterogeneous group of metabolic disorders characterized by persistent hyperglycemia due to alteration in carbohydrate, fat and protein metabolism caused by inherited and/or acquired deficiency in production of insulin and/or by the ineffectiveness of the insulin produced.1–3 Chronic hyperglycemia during diabetes is associated with dysfunction of eyes, kidneys, nerves, heart, and blood vessels. Its complication mostly associated with dyslipidemia increased oxidative stress and consequently an alteration in the body’s antioxidant defense system.4 Worldwide, the number of people with diabetes has considerably increased between 1980 and 2014, rising from 108 million to around four times higher. The global prevalence of diabetes has grown-up from 4.7% in 1980 to 8.5% in 2014, during which time prevalence has increased or at best remained unchanged in every country.5
Alternative systems of medicine based on plant extracts have thrived through the ages and are still practiced by a large population for the management of diabetes.6 Globally, medicinal plants have been used as a source of medicine and 80–85% of populations rely on these medicinal plants using the extracts or their active components as a traditional medicine to meet their primary health-care needs.7 The world health organization expert committee on diabetes also suggested that medicinal herbs be further investigated as they are repetitively considered to be less toxic and side effects.5 Recently, herbal medicines are ahead importance due to their high margin of safety. There are several medicinal plants well recognized for their medicinal usage for treating diabetes mellitus in the traditional system of medicine. However, some of them have been studied systematically and scientifically for their antidiabetic efficacy.8 Some of herbal drugs with proven antidiabetic and related beneficial effects used in treatment of diabetes are Allium sativum, Aloe vera, Coccinia indica, Eugenia jambolana, Ficus benga/ensis, Momordica charantia, Ocimum sanctum syn., Trigonella, foenum graecum, Vernonia amygdalina, Falcaria vulgaris, and Stevia rebaudiana.9–12
Ethnomedicinal uses among rural communities of Ethiopia report showed that the Bark part of H. abyssinica is used traditionally to treat fever/cough, stomachache, bronchitis, livestock disease (thin/skinny body), dermatology and malaria. The Flower part of H. abyssinica is used to treat tapeworm, healing wound, epilepsy, evil eye, hepatitis, sexually transmitted diseases and problems related to bile. The Root part is used to treat severe abdominal pain, stomachache, throat disease, cancer, and the leaf part is used to treat diarrhea, typhoid, cough, livestock disease (mixed with juniperus procera), cancer, hypertension, bone fracture, allergic dermolitia, and wound.13,14 Pharmacological study showed that plants belong to the Rosaceae family have significant insulinomimetic and antidiabetic activity.15,16 H. abyssinica is one of the monotypic genus in the family Rosaceae.17 So, the study plant may have antidiabetic properties like another plant from this family.
The antidiabetic activity of medicinal plants is due to the presence of phenolic compounds (Anthraquinones, C-glycosylated anthrones, 2-hydroxy-3-methyl-anthraquinone, Physcion, etc.), Flavonoids, Terpenoids, Alkaloids, Glycosides, Steroid, Peptides, Lipids and other constituents.18–20 The presence of other bioactive phytochemicals, Phloroglucinol,21 Amino acids,22 Minerals,23 and Weak Organic acids,22 may also be attributed to the antihyperglycemic effect of the plant. Plant extracts with cytotoxic actions are also therapeutically useful for diabetic treatment.24,25 In line with these, the essential oil and other constituent of H. abyssinica has confirmed the cytotoxic effect in vitro.21,26 As a result, these Phytoconstituents may contribute to antidiabetic activity.
The practice of using plants for management of diabetes is also documented in Ethiopia and Tanzania just like other ailments. The flower of H. abyssinica (family, Rosaceae) has been used in the treatment of Diabetes mellitus in Ethiopian folk-medicine without any scientific verification for safety and efficacy.27–30 Thus, the present study aimed to investigate the in vitro and in vivo anti-diabetic activity of flower crude extract and solvent fractions of H. abyssinica.
Methods
Plant Materials
The Fresh flower of H. abyssinica was collected from Kosoye area (located in the Amhara region, northwest Ethiopia), in February 2019. The collected plant material was wrapped with plastic sheets during transportation. The botanical identification and authentication of the plant materials were performed by Mr. Abiyu Enyew (botanist) and the voucher specimen (002ZDK/2019) was deposited in Herbarium of Biology Department, Faculty of Natural and Computational Science, University of Gondar.
Experimental Animals
Healthy male Swiss albino mice (weighing 20–28 g and age of 6–10 weeks) were purchased from Ethiopian Public Health Institute (EPHI). Female rodents were excluded for greater compatibility nature of males for the models,31 except for oral toxicity study. The animals were housed in cages under standard conditions and provided with pellet diet and water ad libitum. The animals were acclimatized to the laboratory conditions before one week of the beginning of the experiments. Animal handling and care was carried out throughout the experiment according to international laboratory animal use and care guidelines,32 and approved by the research and ethics committee, Department of pharmacology, University of Gondar with a Reference number of SOP 4/105/11. Anesthetic chambers were clean to minimize odors that might distress animals subsequently euthanized. The mice were placed in a closed container containing cotton soaked with 2% halothane (and the halothane was applied onto the cotton in the cassette use of 0.1 mL liquid halothane per 1000 mL chamber volume and was given about a 2% concentration of gas). Mice were uncovered only to vapors. Euthanasia procedure was conducted in a chemical fume hood to prevent inhalation of halothane by workers. Sufficient O2 was accessible due to large container during the induction phase. Mice were monitored for rising and falling of chest, palpable heartbeat, mucous membrane color, response to toe pinch, and color change in eyes for death confirmation. Finally, cervical dislocation was used on mice to ensure that they have been properly euthanized.33
Preparation of Plant Crude Extract
The collected plant flowers were cleaned with distilled water and air dried at room temperature under shade (25–27°C) and reduced to appropriate size. The powdered plant materials were packed in plastic bag and kept until extraction. The powdered plant materials were weighed by sensitive digital weighing balance and a total of 1.2 g of powdered flowers were macerated with 80% methanol (250 g in 1500 mL) in Erlenmeyer flask for 72 hrs at room temperature (25–27°C). The extraction process was facilitated by occasional shaking. After 72 hrs, the extract was separated from the marc using gauze and further filtered by Whatman filter paper No. 1. The residue was re-macerated for another 72 hrs three times using the same volume of 80% methanol to exhaustively extract the plant material. The filtrates obtained from the successive maceration were concentrated under reduced pressure using rotary evaporator (Hamato, Japan) followed by hot air oven (Medit-Medizin Technik, Germany) set at 40°C. The extract was further concentrated to dryness by freeze drying using lyophilizer (Labfreez, China). After drying, the amount of dry extract obtained was harvested and the dried extract was transferred into airtight bottles and stored in a refrigerator at −4°C until used. The weight of the dry extract was expressed as percentage of the total mass of dry plant matter to determine the percentage yield.34
Fractionation of Crude Extract
Solvent fractionation of flower crude extract was carried out using water, ethyl acetate, and chloroform. Briefly, the flower crude extract was dissolved in 400 mL of distilled water and this solution was transferred to a separating funnel. An equal volume of chloroform was added to it and was shaken vigorously. The mixture was separated into two layers, waited for a while and then chloroform fraction was removed. The partition with chloroform was repeated two times. The chloroform layer was combined and subjected to evaporation using hot air oven set at 40°C to get the chloroform fraction. To the separating funnel containing aqueous layer, 400 mL of ethyl acetate was added. The mixture was separated into two layers, and then the ethyl acetate was separated and the procedure was repeated two times. The ethyl acetate layer was pooled and concentrated using hot air oven set at 40°C to obtain the ethyl acetate fraction. The remaining aqueous layer was concentrated using hot air oven set at 40°C and frozen in the refrigerator overnight and then, concentrated in a lyophilizer to remove the water. The % yield of the dried fractions were calculated and the fractions obtained were put in airtight bottles and stored in a refrigerator at −4°C until used.35,36
Preliminary Phytochemical Screening of Flower Crude Extracts
Standard Preliminary phytochemical qualitative analysis of the extract was carried out for various plant constituents. The crude extract was screened for the presence or absence of secondary metabolites such as Reducing sugars, Alkaloids, Steroidal compounds, Phenolic compounds, Cardiac glycosides, Flavonoids, Saponins, Tannins and Anthraquinones using standard procedures. Detection of Alkaloids (Mayer’s Test): About 0.2g H. abyssinica crude extract was dissolved individually in dilute hydrochloric acid and filtered. Filtrate was treated with Mayer’s reagent (Potassium Mercuric Iodide). Formation of a yellow-colored precipitate indicates the presence of Alkaloids; Detection Saponins (Foam Test): 0.5 g of H. abyssinica crude extract was shaken with 2 mL of water. If foam was produced which persists for 10 mins it indicates the presence of Saponins; Detection of Tannins (Braymer’s Test: 1 mL of the H. abyssinica crude extract was diluted with distilled water and added with 2 drops of ferric chloride). A transient greenish to black color indicates the presence of Tannins; Detection of Terpenoids (Copper acetate Test): An amount of 0.8 g of H. abyssinica crude extract was taken in a test tube, then poured 10 mL of methanol in it, shaken well and filters to take 5 mL extract of plant sample. Then, 2 mL of chloroform was mixed in extract of selected plant sample and 3 mL of sulphuric acid was added in selected sample extract. Formation of reddish brown color indicates the presence of Terpenoids in the selected plants; Detection of Phenols (Ferric Chloride Test): H. abyssinica crude extract was treated with 3 to 4 drops of ferric chloride solution. Formation of bluish-black color indicates the presence of Phenols; Detection of Flavonoids (Alkaline Reagent Test): H. abyssinica crude extract was treated with few drops of sodium hydroxide solution. Formation of intense yellow color, which was become colorless on addition of dilute hydrochloric acid, indicates the presence of Flavonoids; Detection of Anthraquinones: H. abyssinica crude extract was (equivalent to 100 mg) shaken vigorously with 10 mL of benzene, filtered and 5 mL of 10% ammonia solution was added to the filtrate. The mixture was shaken and observed for the presence of a pink, red or violet color in the ammonia (lower) phase that indicates the presence of free Anthraquinones; Cardiac glycosides (Keller killianis test): About 100mg of H. abyssinica crude extract was dissolved in 1mL of glacial acetic acid containing one drop of ferric chloride solution. This was then under layer with 1mL of concentrated sulphuric acid. A brown ring obtained at the interface would indicate the presence of a de-oxy sugar characteristic of cardenolides; and Steroids (Salkowski’s test): About 100mg of H. abyssinica crude extract was dissolved in 2mL of chloroform. Sulphuric acid was carefully added to form a lower layer. A reddish-brown color at the interface was an indicative of the presence of steroidal ring.37–39
Grouping and Dosing of Animals
For all animal models (normoglycemic, oral glucose loaded and streptozotocin-induced diabetic model) male mice were used. In all cases, mice were assigned randomly into five groups of 6 mice each (n=6). In the normoglycemic and oral glucose loaded animal models, there was a negative control group (groups I) which received distilled water (DW); a positive control group (group II) which received glibenclamide (Sanofi-aventis, France) 5 mg/kg (GLC 5 mg/kg); test groups (group III–V) received three different doses of the H. abyssinica crude extract (HAC). In the single dose-treated diabetic animal model, there was a negative control group (group I) which received the vehicle (distilled water=DW); a positive control group (group II) which received the standard drug, Glibenclamide 5 mg/kg (GLC 5 mg/kg); twelve test groups (groups III–XIV) received different doses of the H. abyssinica crude extract (HAC); Chloroform fraction (CFF); Ethyl acetate fraction (EAF); and Aqueous fraction (AQF). In the repeated daily dose-treated a diabetic animal model of crude extract and solvent fractions, mice were randomly divided into nine groups (8 groups of diabetic mice and 1 additional group of normal mice, 6 mice per group). Group I (diabetic mice) was used as diabetic control (DC) that was received vehicle orally (distilled water=DW), Group II (positive control) received a standard drug, glibenclamide5 mg/kg (GLC 5 mg/kg) and Groups III–VIII (test groups) received different doses of the H. abyssinica crude extract (HAC) and Ethyl acetate fraction (EAF) with (100 mg/kg, 200 mg/kg and 400 mg/kg) doses; and Group IX (non-diabetic mice) were used as negative control (NC) that received vehicle orally (distilled water=DW).40,41 The extract doses to be administered are determined based on the acute toxicity study and the volume of administration was 1 mL/100 g of body weight of the mouse.42 The middle dose was one-tenth of the limit dose, the higher dose was twice the middle dose, and the lower dose was calculated as half of the middle dose. The study was conducted using the oral route of administration since the plant materials are traditionally used by people via the oral route.29
Determinations of α-Amylase Inhibition Activity
The α-amylase inhibition assay was performed using the 3.5-dinitrosalicylic acid (DNSA) method.43 The crude extract and solvent fractions of H. abyssinica were dissolved in buffer (Na2HPO4/NaH2PO4 (0.02 M), NaCl (0.006 M) at pH 6.9) to give concentrations ranging from 25 to 800 μg/mL. A volume of 200 μL of α-amylase solution (Molychem, India) (2 units/mL) was mixed with 200 μL of the extract and was incubated for 10 mins at 30°C. Thereafter, 200 μL of the starch solution (1% in water (w/v)) was added to each tube and incubated for 3 mins. The reaction was terminated by the addition of 200 μL DNSA reagent (12 g of sodium potassium tartrate tetrahydrate in 8.0 mL of 2 M NaOH and 20 mL of 96 mM of 3.5-dinitrosalicylic acid solution) and was boiled for 10 mins in a water bath at 85°C. The mixture was cooled to ambient temperature and was diluted with 5 mL of distilled water, and the absorbance was measured at 540 nm using a UV-Visible spectrophotometer (Agilent Technologies, Malaysia). The blank with 100% enzyme activity was prepared by replacing the plant extract with 200 μL of the buffer. A blank reaction was similarly prepared using the plant extract at each concentration in the absence of the enzyme solution. A positive control sample was prepared using acarbose (Bayer, Germany) and the reaction was performed similarly to the reaction with plant extract as mentioned above. The inhibition of α-amylase was expressed as percentage of inhibition and was calculated by the following equation: Inhibition (%) = [(Ac − Acb) − (As − Asb)/(Ac − Acb)] × 100, where Ac- absorbance of control; Acb-absorbance of control blank; As-absorbance of sample; and Asb-absorbance of sample blank. The % α-amylase inhibition was plotted against the extract concentration and the IC50 values were obtained from the graph.
In-vitro Antioxidant Activity in DPPH Assay Model
The free radical scavenging activity of the plant crude, solvent fractions, and ascorbic acid were determined in vitro by diphenyl-2-picrylhydrazyl (DPPH) (Sigma Aldrich, Germany) assays according to the method described earlier.44 The potential antioxidant activity of plant crude extract and solvent fractions were determined based on the scavenging activity of the stable DPPH free radical. Aliquots of 100 µL of a methanolic solution containing different concentrations ranging from 15.625 to 500 μg/mL were added to 3.9 mL of a 0.004% methanolic solution of DPPH. Absorbance at 517 nm was determined after 30 mins, and the percent inhibition activity was calculated. IC50 values denote the concentration of the sample required to scavenge 50% DPPH-free radicals. The percentage (%) of the scavenging of the DPPH-free radical was calculated by the formula: (A0-A1)/A0 X 100. A0 is absorbance of the control and A1 is absorbance of the extract/standard.
Acute Toxicity Study
Acute oral toxicity of the crude extract was performed on healthy female Swiss albino mice as per OECD-425 guideline limit test procedure. Accordingly, five female albino mice of 6–8 weeks were used. All mice were fasted (food but not water) for 3 hrs before and 1 hr after administration of the extract. A limit dose of 2g/kg was given for the first animal. Based on the result, four additional animals were dosed sequentially. The animals were housed separately and observed for manifestation of gross behavioral and physical toxicities like changes in skin, urination, lacrimation, reduction in feeding activity, excitation, paw licking, increased respiratory rate, decreased motor activity, diarrhea, weight loss and paralysis continuously for the first 30 mins and intermittently for 4 hrs, over a period of 24 hrs and later followed for 14 days for an interval of 24 hrs for any lethality.45
Effect of the Extract in Blood Glucose Level of Normoglycemic Mice
Healthy normal mice were fasted overnight for 14 hrs, but the water was permitted ad libitum and then randomly divided into five different groups (6 mice in each). Mice were grouped and treated as described above. Using aseptic conditions, blood samples were collected from tips of the tail of each mouse to determine blood glucose level (BGL). Baseline blood glucose level of each mouse was measured just before treatment (at 0 hr). Then, blood glucose level of each mouse was measured at 1, 2, 4 and 6 hrs after-treatment.46
Effect of the Extract on Blood Glucose After Oral Glucose Tolerance Test (OGTT)
Mice were used for the OGTT after fasting overnight for 14 hrs, as there is increased insulin sensitivity (insulin-stimulated glucose utilization) specifically in mice;47,48 mice were grouped and treated as described above and the baseline blood glucose level was determined. Then, 2 g/kg of glucose solution was administered orally to each mouse after 30 mins of extract administration. As usual blood glucose level was measured for each animal just before treatment (at 0 min) as baseline, and then at 30 min, 1 hr and 2 hrs following glucose administration.40
Induction of Experimental Diabetes
In this experimental study, diabetes was induced using streptozotocin (STZ). The drug was dissolved in 0.1 M citrate buffer (pH=4.5). The prepared streptozotocin solution at 150 mg/kg dose was given through intraperitoneal rout,31 which were fasted overnight for 14 hrs before administration. After 6 hrs of STZ injection, the mice were allowed to receive glucose solution for about 24 hrs to avoid death secondary to hypoglycemic shock. After 72 hrs of STZ injection, the mice were screened for DM. Mice that showed FBG level >200 mg/dl were a candidate for the study.49
Anti-Hyperglycemic Activity of a Single Dose and Repeated Dose of the Extracts in STZ-Induced Diabetic Mice
For single-dose streptozotocin-induced diabetic model, mice were assigned randomly into five groups (n=6) after fasting for 14 hrs. Then, mice were treated with distilled water, glibenclamide, and 80% methanolic crude extract and solvent fractions according to their respective grouping as described above. Blood glucose level was measured just before treatment (at 0 hr) as a baseline, and then at 2, 4, 6 and 8 hrs post-treatment.46,50 For repeated-dose streptozotocin-induced diabetic model, mice were divided in six groups (n=6). The vehicle, standard drug, crude extract, and solvent fraction were administered once daily for 14 days. Fasting blood glucose level and the bodyweight of mice were measured just before starting treatment (day 0), 7th day and 14th day of treatment following overnight fasting for 14 hrs.50,51
Effect on Serum Lipid Level of Diabetic Mice
On the 15th day, blood samples were collected in a sterile tube by cardiac puncture beneath halothane anesthesia from the overnight fasted (14 hrs) diabetic mice. The blood samples were left at room temperature for 2 hrs and then centrifuged at 2500 rpm at 30°C for 15 mins. The supernatant was instantly separated from the precipitate to get ready serum samples to find out the level of triglyceride, total cholesterol, high-density lipoprotein and low-density lipoprotein.47,48
Statistical Analysis
The IC50 values were calculated from plots of log concentration of inhibitor concentration versus percentage inhibition curves. The data were expressed as mean ± standard error of mean (SEM). All the results were articulated as the mean ± standard error of means (SEM) for six mice in each group. Statistical analysis was performed by using statistical package for social sciences (SPSS) version 24 software. The group analysis was conceded out using one-way ANOVA, followed by Tukey’s multiple comparison tests. The result was considered significant when p < 0.05.
Results
Yield of Crude Extract and Fractions
From 211.2 grams (17.6% yield) of 80% flower crude extract, and a yield of chloroform, ethyl acetate and aqueous fractions of flower H. abyssinica were 24.62%, 30.54%, and 42.43%, respectively.
Preliminary Phytochemical Screening
Preliminary phytochemical screening was done for the flower crude extract of H. abyssinica resulted in the presence of Saponins, Tannins, Terpenoids, Phenols, Flavonoids, Glycosides, Steroids, and Anthraquinones. Alkaloids were not present in the phytochemical screening (Table 1).
 |
Table 1 Phytochemical Screening of Flower Crude Extract |
In vitro α-Amylase Inhibition Activity of Crude Extract and Solvent Fractions
In this study, one crude extract and the three solvent fractions were evaluated for their possible α-amylase inhibitory activities alongside acarbose as a positive control. The α-amylase inhibitory activities and IC50 values of the acarbose, crude extract and fractions are summarized in Table 2. As the results showed, the inhibitory activities of all the extracts were varied IC50 values from 20.78 to 52.11μg/mL. Concentration-dependent inhibition was observed for various concentrations of the tested extracts and the standard. Among the extracts, the ethyl acetate flower fraction of H. abyssinica showed the highest alpha–amylase enzyme inhibition activity with an IC50 value of 20.78μg/mL (54.23% inhibition at 800μg/mL). Similarly, the IC50 values of water fraction, chloroform fraction, and Crude flower extract were 52.11±0.63, 49.08±0.97, and 28.09±0.75 μg/mL, respectively. The standard positive control Acarbose showed an IC50 value of 1.24±0.67 μg/mL (91.87±0.54% inhibition at 800µg/mL)
 |
Table 2 α-Amylase Inhibitory Activities of the Crude Extract and Solvent Fractions |
Antioxidant Activity of Crude Extract and Solvent Fractions
Antioxidant activity of the crude extract and solvent fractions of H. abyssinica were tested for antioxidant activity using DPPH free radical. The extracts showed antioxidant activity as shown in Table 3. There was a dose-dependent increase in the percentage of antioxidant activity for all concentrations tested. Ascorbic acid was used as the standard drug for the determination of the antioxidant activity by DPPH method. The concentration of ascorbic acid and the extracts varied from 15.625 to 500 µg/mL. Among extracts, the highest inhibitory activities were shown by the crude extract with an IC50 value of 9.41±0.14 µg/mL (58.38% inhibition at 500µg/mL). Likewise, the IC50 values of water fraction, chloroform fraction, and ethyl acetate fraction were 16.96±0.13, 12.14±0.64, and 10.78±0.54 μg/mL, respectively. The standard positive control Ascorbic acid showed an IC50 of 3.59±0.94 µg/mL (89.72±0.86% inhibition at 500µg/mL).
 |
Table 3 Antioxidant Activities of the Crude Extract and Solvent Fractions |
Acute Toxicity Test
The acute toxicity study indicated that the crude extract caused no mortality in limit dose of 2 g/kg within the first 24 hrs as well as for the following 14 follow-up days. Physical and behavioral observations of the experimental mice also revealed no visible signs of overt toxicity. This indicates that Median Lethal Dose (LD50) of the extract is greater than 2 g/kg.
Hypoglycemic Activity on Normoglycemic Mice
The effect of 80% methanolic flower crude extract on normoglycemic mice is shown in Table 4. Ahead of initiating the treatment, there were no statistically significant differences in blood glucose level among the whole groups (P>0.05). In normoglycemic mice, 80% methanolic flower crude extract at HAC100 did not significantly decrease the plasma glucose in normal mice at all time points compared to the negative control group. However, blood glucose level was significantly reduced by GLC5 at the 1st (p <0.05), 2nd (p<0.001), 4th (p < 0.001), and 6th (p<0.001) hours compared to the negative control. Likewise, the extract HAC200 at 6th hours (p<0.05) and HAC400 at 4th and 6th hours (p< 0.05 and p < 0.001, respectively) significantly decreased BGL compared to the negative control. Similarly, comparing GLC5 treated group with extract-treated groups, it was revealed that GLC5 significantly reduced the BGL at the 4th (p<0.01) and 6th hours (p < 0.001) compared to HAC100 treated group; at the 4th (p<0.05) and 6th hours (p<0.01) compared to HAC200 treated group. Within-group analysis showed that treatment with the standard drug reduced the BGL significantly at the 1st (p < 0.05), 2nd (p < 0.001), 4th (p < 0.001), and 6th (p<0.001) hours compared to the baseline blood glucose level with percentage reduction of 16.03%, 25.85%, 33.47%, and 41.48%, respectively. While extract HAC100 at the 6th (p<0.05) hours with percentage reduction of 14.22%; HAC200 at the 4th (p < 0.05) and 6th (p<0.01) hours with percentage reduction of (15.85%, 22.76%, respectively); and HAC400 at the 4th (p < 0.001), and 6th (p<0.001) hours with percentage reduction of (27.73%, 31.68%, respectively), reduced the BGL compared to the baseline BGL. By contrast, DW10 did not produce any significant reduction in BGL across all time points compared to the baseline (t=0).
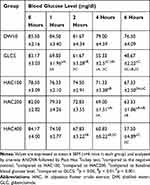 |
Table 4 Hypoglycemic Activity of the Flower Crude Extract in Normoglycemic Mice |
Anti-Hyperglycemic Activity of the Plant Extract on Oral Glucose Loaded Mice
The result of H. abyssinica crud extract on oral glucose tolerance test of normoglycemic mice is summarized in Table 5. Oral Administration of 2g/kg of glucose to normoglycemic mice 30 mins after treatment with distilled water, test extracts, and glibenclamide showed peak blood glucose level in 30 mins (Figure 1). Initially, there was no significant difference in blood glucose level of mice (p>0.05). The hyperglycemia was not significantly reduced with HAC100 at 60 and 120 mins compared to the negative control. Similarly, HAC200 and HAC400 failed to produce detectable changes at 60 mins compared to the negative control group. However, HAC200 and HAC400 significantly (p < 0.05 and p < 0.01, respectively) improved oral glucose loading at 120 mins compared to the negative control group. Likewise, GLC5 produced significant improvement of hyperglycemia at 60 mins (p < 0.001) and 120 mins (p < 0.001) compared to the negative control group. Within-group analysis showed that oral glucose loading results a statistically significant reduction in BGL at 60 and 120 mins in all groups including the negative control compared to the respective BGL at 30 mins after glucose administration.
 |
Table 5 Effect of the Flower Crude Extract in Oral Glucose Loaded Mice |
Anti-Hyperglycemic Activity of Single Doses in STZ-Induced Diabetic Mice
The result of H. abyssinica crude extract and solvent fractions on STZ-induced diabetes is summarized in Table 6. There was no significant disparity between baseline blood glucose levels crosswise all groups. Likewise, there was no significant disparity in blood glucose level at all time points when groups received the plant extract were compared to each other.
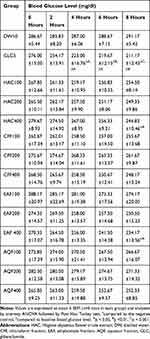 |
Table 6 Antihyperglycemic Activity of a Single Dose of Flower Crude Extract and Solvent Fractions in Diabetic Mice |
Within a group, the analysis showed that there was no significant blood glucose level reduction showed in all doses of crude extract and solvent fractions treated groups at all time points compared to the baseline fasting blood glucose level. However, the greatest percent reduction in BGL was recorded as 5.08% in AQF400, 7.57% in CHF400, 12.46% in HAC400, 13.38% in EAF400 and 23.49% in GLC5 treated group at the 8th hour compared to the respective baseline fasting level. The standard drug glibenclamide produced a significant blood glucose level reduction at the 4th (p<0.05), 6th (p < 0.01), and 8th (p<0.001) hours compared to the negative control. In the same way, HAC400 and EAF400 showed a significant (p < 0.05) blood glucose level reduction at 8th hours compared to the negative control. The standard drug, glibenclamide also produced a significant blood glucose level reduction at the 4th (p<0.05), 6th (p < 0.05) and 8th (p<0.01) hour compared to baseline blood glucose level.
Anti-Hyperglycemic Activity of the Repeated Doses in STZ-Induced Diabetic Mice
Blood glucose levels were measured once weekly in normal and diabetic mice given HAC, EAF, DW10, and GLC5 after the induction of diabetes. The results are summarized in Table 7. After induction, diabetic mice showed significant differences in blood glucose levels compared to normal mice (p<0.001), but no significant difference in baseline fasting BGL across all groups of diabetic mice. Treatment with HAC200 and EAF200 (p < 0.05, on the 7th and 14th days); HAC400 (p<0.05 and p < 0.01, on the 7th and 14th days, respectively); and EAF400 (p < 0.01 and p<0.001, on the 7th and 14th days, respectively) resulted in a significant reduction in BGL compared to diabetic control. Similarly, GLC5 treated group revealed significant (p < 0.001) reduction in BGL on the 7th and 14th days. In contrast, HAC100 and EAF100 failed to show a reduction of BGL on the 7th and 14th days at all time points compared to diabetic control. Within a group analysis revealed that both HAC400 and EAF400 significantly (P<0.05 and P<0.001) reduced the BGL on the 7th and 14th days, respectively, compared to the baseline level, but the remaining extract-treated, diabetic control and the normal control did not show a significant alteration in BGL on 7th and 14th days compared to the respective baseline level. Similarly, the standard drug reduced the BGL significantly (P<0.01 and P<0.001) on the 7th and 14th days, respectively, compared to the baseline BGL. The greatest reduction in fasting BGL was attained at the 14th days 20.68%, 23.12%, and 35.93%, respectively, for HAC400, EAF400, and GLC5 (Table 7).
 |
Table 7 Antihyperglycemic Activity of Repeated Daily Doses of Flower Crude Extract and Ethylacetate Fraction in Diabetic Mice |
Effect of the Repeated Daily Doses on Body Weight of Diabetic Mice
The bodyweight of diabetic and normal mice prior to and gone the induction of diabetes are specified in Table 8. As baseline, the weight of mice was measured after 3 days of streptozotocin injection and was no significant difference between diabetic groups but had significant weight loss compared to normal control. Streptozotocin fashioned a significant loss of body weight in the diabetic control on the 7th and 14th days of treatment compared to the normal control. Significant body weight increment was recorded for HAC200 (p < 0.01), HAC400 (p < 0.001), EAF400 (p < 0.01) and GL5 (p < 0.001) at the 7th day of treatment compared to diabetic control group. All doses of the crude extract, ethyl acetate fraction and GL5 showed a significant (p < 0.001) increment in body weight at the 14th days when compared to diabetic control. By contrast, the bodyweight of the diabetic control group was significantly decreased (p<0.05 and p<0.001) at the 7th and 14th days, respectively, compared to normal control group. Within a group analysis revealed that the HAC400 and GL5 showed a significant increment in body weight on the 7th and 14th days of treatment compared to baseline body weight. In the same way, HAC200 and EAF400 showed a significant increment in body weight on the 14th day compared to baseline body weight.
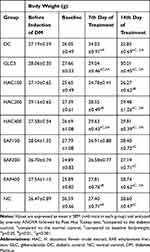 |
Table 8 Effect of Repeated Daily Doses of the Flower Crude Extract and Ethylacetate Fraction on Bodyweight of Diabetic Mice |
Effect of Repeated Daily Doses on Serum Lipid Level of Diabetic Mice
After the induction of diabetes and succeeding treatment with all the three doses of the crude extract and the ethyl acetate fraction, there was a significant (p<0.001) increase in serum level of TC, TG, VLDL-c and LDL, and a significant (p<0.001) decrease in HDL cholesterol when compared to normal mice. EAF100 treated group did not show a significant reduction in the serum level of TG and VLDL-c; and did not show a significant increment in the serum level of HDL-c compared to diabetic control. Correspondingly, EAF200 treated group did not show a significant decrease in serum level of VLDL-c, but showed a significant (p<0.05) reduction in serum level of TG and a significant increment in serum level of HDL-c compared to diabetic control. However, administration of all the three doses of flower crude extract (HAC100, HAC200, and HAC400), ethyl acetate fraction (EAF400), and GLC5 to diabetic mice for 14 days showed a significant (p<0.001) dose-dependent reduction in the serum level of TC, TG, LDL, and VLDL whilst increasing the levels of HDL-c compared to the diabetic control (Table 9).
 |
Table 9 Effect of Repeated Daily Doses of Flower Crude Extract and Ethyl Acetate Fraction on Serum Lipid Level of Diabetic Mice |
Discussion
Numerous new bioactive phytochemicals isolated from the plants having hypoglycemic and anti-hyperglycemic effects prove the same anti-diabetic activity and sometimes even more potent than already recognized oral hypoglycemic agents.52,53 The present study investigated the in vitro and in vivo antidiabetic, antihyperlipidemic, and antioxidant activity of the 80% methanolic crude extract and solvent fractions of H. abyssinica on normal and diabetic mice.
In the acute oral toxicity study, administering hydromethanolic crude extract at single dose of 2 g/kg body weight orally did not cause any major toxicity and death of mice during observation. This finding revealed that the LD50 of the plant extract is greater than 2 g/kg. This result supports the study which presents the tough evidence of the non-toxic outcome of the plant.26,54
Various secondary metabolites isolated from diverse plant species have been anticipated to have potent hypoglycemic, anti-hyperglycemic and glucose suppressive effects. These secondary metabolites include Flavonoids,55,56 Sterols/Triterpenoids,57 Alkaloids and Phenolics.58 Possessions might be achieved by stimulating insulin release from pancreatic ß-cells, inhibiting glucose absorption in the gut, stimulating glycogenesis in the liver and/or increasing glucose utilization by the body.59 Apart from lowering blood glucose effect, these phytochemicals are recognized to regenerate the damaged beta cells and stopping oxidative stress on beta cells in experimental diabetic rats.60 A study with Artemisia afra has been reported to possess hypoglycemic activity in diabetic rabbits due to the presence of Saponins which might be acting as a stimulant for the release of insulin following the repair of pancreatic beta cells by the extract.61 The preliminary phytochemical screening of the 80% methanolic crude extract of H. abyssinica, showed the likely existence of Saponins, Tannins, Terpenoids, Phenols, Flavonoids, Glycosides, Steroids, and Anthraquinones. This finding was similar to the reports by Wolde T et al, 2016.62 As a result, these secondary metabolites which were found in H. abyssinica crude extracts and solvent fractions may, therefore, be accountable for the observed glucose suppressive and anti-hyperglycemic activity of the extract and some of the bioactive constituents in this study could act synergistically or independently enhancing the action of glycolytic and glyconeogenic enzymes.
Previous chemical composition studies of flower extract of H. abyssinica have shown the existence of phloroglucinols, specific amino acids, minerals, and weak organic acids.21–23,63 These findings advocate that the component of H. abyssinica may have a protective or therapeutic potential for the treatment of diabetes via numerous likely mechanisms.63–68
It is well known that reduction of postprandial hyperglycemia can be achieved by inhibiting intestinal α-glucosidase and pancreatic α-amylase activity via delayed carbohydrate digestion.69 The plant crude extract and solvent fractions showed pancreatic α-amylase inhibitory activity. The search for new group of agents from natural resources, especially from medicinal plants, becomes an attractive approach for the treatment of postprandial hyperglycemia. As shown in Table 2, all doses of the crude extract and solvent fractions of H. abyssinica demonstrated a dose-dependent reduction in α-amylase activity. The most important inhibition appeared in the ethyl acetate solvent fraction (54.23%) while the aqueous fraction (26.18%) showed the weakest effect. The α amylase inhibitory activity in ethyl acetate extract is most likely to be due to semi-polar compounds and is worth investigating further and isolating pure active compounds. Flavonoids, Tannins and Phenolic acids are a major group of polyphenolic compounds that have been reported to possess inhibitory activity against α-amylase.70 In this study, the phytochemical analysis revealed that the extracts are rich in polyphenolic components, this suggests that the bioactive exerting the inhibitory effect against α-amylase may be present in all plant extracts at different concentrations. The greatest anti-oxidant activity (58.38%) was observed in the crude extract while the lowest antioxidant activity was observed in aqueous fraction (36.52%). The meticulous results of both plants are shown in Table 3. The attractive fact of our study was that the crude extract showed outstanding antioxidant potential than solvent fractions. The result of the anti-oxidant activity of H. abyssinica extract showed a dose-dependent antioxidant activity.
In the hypoglycemic activity of the flower crude extract, DW10 did not significantly change the blood glucose concentrations of fasted normoglycemic mice. However, crude extract of HAC200 (p < 0.05) at 4th hours and HAC400 (p<0.05 and p<0.001, respectively) at 4th and 6th hours significantly reduced the blood glucose level of non-diabetic mice and it was observed that the extract exerted its action in a dose-dependent manner. The crude extract produced a delayed but significant hypoglycemia; this showed that the anti-diabetic activity of the extract amplified with time, as the maximal effect was achieved at the 6th hours (Table 4). This could mean that the active constituents in the extract need time to attain adequate concentration at the target site, as a similar pattern was observed with other plants displaying anti-diabetic activity.71 The plant extract showed comparatively slower hypoglycemic onset of action than the standard drug. The hypoglycemic effect of glibenclamide was apparent due to the stimulation of insulin release from pancreatic β-cells and inhibition of glucagon secretion.72 The crude extract might have an insulin-like effect or stimulate insulin secretion from β-cells. Compounds of a natural product such as Flavonoids and Tannins isolated from medicinal plants are reported to stimulate insulin secretion from pancreatic β–cells.73 Since these active constituents exist in H. abyssinica, the probable mechanism of action of plant extract is similar to GLC5. So the HAC200 and HAC400 crude extract were shown a significant BGL reduction as compared to the negative control.
OGTT is a measure of the body’s ability to utilize glucose and it is seen as the “gold standard” in diagnosing diabetes mellitus.74 The effect of extracts on glucose tolerance test in normal mice is shown in Table 5. At 30 mins of glucose administration, the peak of blood glucose level became higher and then consequently lowered. This confirms the physiologic induction of hyperglycemia due to oral glucose loading. Statistical analysis showed that there was no significant difference among the groups at 0 min. Similarly, statistical analysis at 30 mins showed that there was a significant difference among the groups. Additional, statistical analysis at 60 mins showed that there was a significant difference among the groups at 60 mins. Post hoc test revealed that GLC5 showed significant (P<0.001) reduction in the plasma sugar level compared to negative control at 60 mins. Moreover, Post hoc test revealed that GLC5, HAC200, and HAC400 showed significant (P<0.05 and P<0.01) decrease in the plasma sugar level compared to glucose loaded control group at 120 mins. In this test, glucose tolerance was enhanced significantly (p < 0.001) in GLC5 and all doses of the crude extract-treated groups from 60 mins onwards compared to the blood glucose level at 30 mins. In general, in OGTT the crude extract showed a significant reduction in blood glucose level from 120 mins compared to negative control. Mice treated with crude extract have better glucose utilization capacity. The postprandial glucose-lowering ability of the extract may be accredited to embarrassment of glucose absorption, stimulation of peripheral glucose utilization, reduction in glycogenolysis, and gluconeogenesis.75 This suggests that the extract is capable with the ability to get better regulatory mechanisms, indicative of a potential advantage of the extract in minimizing hyperglycemia-related complications of diabetes. This pattern of blood glucose modulation was similar to the finding by Yaschilal et al, 2018,76 and M. Anitha et al, 2012.77 This finding provides confident evidence that the claimed medicinal plant has an antihyperglycemic activity.
Among the variety of single doses of the crude extract and solvent fractions, greatest activity was observed with ethyl acetate fraction. It is appealing to note that EAF400 was proficient of bringing down STZ-induced hyperglycemia by 13.38% as compared with the crude extract (12.46%) at a dose of 400 mg/kg of body weight 8 h after oral administration, while the AQF and chloroform fractions showed weak activity (5.08% and 7.57%, respectively), representing that the active constituent(s) of the plants are semi-polar. GLC5 showed a greatest reduction of 23.49% in blood glucose level 8 h after oral administration. As shown in Table 6, this anti-diabetic activity corresponds to GLC5 and all doses of the crude and solvent fractions showed a reduction in blood glucose levels after oral administration, indicating the improvement in blood glucose homeostasis was in a dose-dependent manner, this can be explained by the possible existence of a sufficient concentration of the active metabolite (s) in the higher dose 400 mg/kg in contrast to the lower 100mg/kg and middle 200mg/kg dose levels of plant extracts. The in vitro α amylase and single-dose anti-hyperglycemic activity (STZ-induced mice) in the ethyl acetate showed a superior anti-diabetic activity compared to other solvent fractions. This may indicate that the ethyl acetate fractions could contain compounds better than the HAC, CHF, and AQF. Due to its superior activity in their in vitro anti-diabetic and better bringing down of STZ-induced hyperglycemia, the ethyl acetate fraction was further investigated for their in vivo anti-hyperglycemic activity of repeated daily doses on STZ-induced diabetic mice. This finding was not in line with other study which reported that the aqueous fraction had significant glucose-lowering activity.78
In the anti-hyperglycemic activity of repeated daily doses of the HAC and EAF in STZ-induced diabetic mice, the maximum percent fall of fasting blood glucose was found at EAF400 (23.12%) as compared to HAC400 (20.68%) on day 14th. The result was comparable with the standard drug GLC5 which reduced fasting blood glucose level by 35.93% on the same day. Furthermore, significant fasting blood glucose fall in diabetic mice treated with HAC200 (p<0.05 in both cases); HAC400 (p<0.05 and p < 0.01, respectively in both cases); and EAF400 (p < 0.01 and p<0.001, respectively in both cases) resulted in a significant reduction in BGL at the 7th and 14th days, respectively, compared to diabetic control. Similarly, GLC5 treated group revealed significant (p<0.01 and p<0.001) reduction in BGL at the 7th and 14th days, respectively. It was observed that the extract exerted its action in a dose-dependent manner, this might be due to increasing dose contains a higher concentration of the active component(s) responsible for more fall of fasting blood glucose than the lower dose. Likewise, GLC5 lowers fasting blood glucose level via selective blockage of adenosine triphosphate (ATP) sensitive K+ (KATP) channels in the plasma membrane. This leads to membrane depolarization, activate voltage-gated Ca2+ channels, a rise in cytosolic (Ca2+) and release of endogenous insulin in β-cells of the pancreas,79 this suggests that Streptozotocin at 150 mg/kg i.p. might not adequate for the destruction of β-cells and/or few cells remained to have the capability to regenerate and secrete insulin. Oxidative stress and inflammation involving the pancreas are the key factors in the pathogenesis and progression of diabetes. Excessive free radicals generated from hyperglycemia-induced glucose autoxidation and protein glycosylation play an imperative role in DM pathogenesis.80,81 Pancreas β-cells are sensitive to damage by nitric oxide and other free radicals which can be generated by STZ.82 Preceding studies revealed that different plant extracts have shown pancreas β cell-protective activity due to their antioxidant activities.83,84 In the same way, the extract of H. abyssinica showed anti-oxidant activity in the present study. This suggests that the β-cell protective effect secondary to the antioxidant activity of the plant extract might contribute to its anti-hyperglycemic activity in STZ-induced diabetic mice.
Significant (p < 0.001) body weight loss was observed in STZ-induced diabetic control mice and more or less normalized by treatment with HAC and EAF. In diabetic mice, this loss of weight may be due to tissue protein break down and muscle wasting via unavailability of carbohydrates as an energy source and catabolism of fats.85 However, this finding revealed that GLC5 and all doses of the extract-treated mice showed significant weight gain (p < 0.001, p < 0.05) in contrast with the diabetic group and baseline body weight, respectively, after 14 days treatment. The protective effect of the extract on body weight loss may be due to its capability to decrease hyperglycemia. Here, the bioactive compounds of H. abyssinica may help in suppressing the free radicals generated due to hyperglycemia, and control over muscle wasting resulted from glycemic control in treated diabetic mice, and ultimately lead to body weight gain.
Activation of hormone-sensitive lipase during insulin insufficiency causes raise in free fatty acid mobilization from adipose tissue. Besides, hyperglycemia is accompanied by a rise in TC, TG, LDL-C and a fall in HDL-C.86 In the current study, STZ-induced diabetic control mice showed significantly increased serum STC, STG, VLDL-C, and LDL-C and decreased HDL-C as expected. Repeated administration of HAC and EAF for 14 days significantly decreased STC, STG, VLDL-C, and LDL-C levels and at the same time, HDL-C level was increased in a dose-dependent manner, and all the three doses of the HAC did show better improvement in diabetic dyslipidemia compared to EAF treated group. It is not notorious whether plant extract had a straight effect on lipid metabolism or the anti-dyslipidemic activity is achieved only due to the controlled hyperglycemia. But, it can be concluded that both the crude extract and the fraction improve diabetic dyslipidemia. This finding was also consistent with other findings that showed the ethanolic and aqueous extracts of Costus igneus showed significant reduction in blood glucose level, Cholesterol, Triglycerides, LDL and elevated the HDL level in streptozotocin-induced diabetes.61
Conclusions
This study revealed that the crude extract and solvent fractions of H. abyssinica have showed significant lowering of blood glucose level on diabetic, normoglycemic and oral glucose loaded mice and not permitted bodyweight loss of diabetic. The results also verified that inhibition of intestinal α-amylase and free radical scavenging activity by the extracts may contribute to the antihyperglycemic and anti-hyperlipidemic activity. The results give scientific support for the use of the plant in folk medicine for the management of diabetes and its associated complications. H. abyssinica would be promising for further clinical studies in the management of DM. Further studies to find out the mechanism of this plant for its antidiabetogenic effect and there is a need for bioactivity guided investigation to isolate the lead compound responsible for the antidiabetic activity.
Abbreviations
BGL, blood glucose level; DNSA, 3,5-dinitrosalicylic acid; DM, diabetes mellitus; DPPH, 2,2-diphenyl-1-picrylhydrazyl; HDL, high-density lipoprotein; IC50, half-maximal inhibitory concentration; LDL, low-density lipoprotein; LD50, median lethal dose; OECD, Organization for Economic Cooperation and Development; OGTT, oral glucose tolerance test; STZ, streptozotocin; TC, total cholesterol; TG, triglycerides; VLDL, very-low-density lipoprotein.
Data Sharing Statement
Most of the data is included in the manuscript. Additional can be found from the corresponding author based on reasonable request.
Ethics Approval and Consent to Participate
Ethical clearance was obtained from the research and ethics committee, department of pharmacology, University of Gondar with a Reference number of SOP 4/105/11 to conduct the study in animal model. Apart from that, all possible steps were taken to avoid animal suffering at each stage of the experiment. However, no consent was needed for this study.
Acknowledgment
Authors would like to acknowledge University of Gondar for material support and for allowing to use the laboratory facility.
Disclosure
The authors declare that they have no competing interests.
References
1. Okur ME, Karantas ID, Siafaka PI. Diabetes mellitus: a review on pathophysiology, current status of oral pathophysiology, current status of oral medications and future perspectives. Acta Pharm Sci. 2017;55(1):61. doi:10.23893/1307-2080.APS.0555
2. Shewasinad A, Bhoumik D, ZERO HISHE H, Masresha B. Antidiabetic activity of methanol extract and fractions of thymus schimperi ronniger leaves in normal and streptozotocin induce diabetic mice. Iran J Pharmacol Ther. 2018;16(1):1–8.
3. Wadkar K, Magdum C, Patil S, Naikwade N. Anti-diabetic potential and Indian medicinal plants. J Herb Med Toxicol. 2008;2(1):45–50.
4. Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Supplement 1):S81–S90. doi:10.2337/dc14-S081
5. Organization WH. Global Report on Diabetes. World Health Organization; 2016.
6. Rizvi SI, Matteucci E, Atukeren P. Traditional medicine in management of type 2 diabetes mellitus. J Diabetes Res. 2013;2013.
7. Elujoba AA, Odeleye O, Ogunyemi C. Traditional medicine development for medical and dental primary health care delivery system in Africa. Afr J Tradit Complement Altern Med. 2005;2(1):46–61.
8. DeyL AA, Yuan CS. Alternative therapies for type 2 diabetes. Altern Med Rev. 2002;7(1):45–58.
9. Mamun-or-Rashid A, Hossain MS, Hassan N, Dash BK, Sapon MA, Sen MK. A review on medicinal plants with antidiabetic activity. Int J Pharmacogn Phytochem. 2014;3(4):149–159.
10. Shukia R, Sharma S, Puri D, Prabhu K, Murthy P. Medicinal plants for treatment of diabetes mellitus. Indian J Clin Biochem. 2000;15(1):169–177. doi:10.1007/BF02867556
11. Zangeneh MM, Zangeneh A, Tahvilian R, Moradi R. Antidiabetic, hepatoprotective and nephroprotective effects of the aqueous extract of Falcaria vulgaris in diabetic male mice. Arch Biol Sci. 2018;70(4):655–664. doi:10.2298/ABS180222027Z
12. Hagh-Nazari L, Goodarzi N, Zangeneh MM, Zangeneh A, Tahvilian R, Moradi R. Stereological study of kidney in streptozotocin-induced diabetic mice treated with ethanolic extract of Stevia rebaudiana (bitter fraction). Comp Clin Path. 2017;26(2):455–463. doi:10.1007/s00580-016-2398-7
13. Assefa B, Glatzel G, Buchmann C. Ethnomedicinal uses of Hagenia abyssinica (Bruce) JF Gmel. among rural communities of Ethiopia. J Ethnobiol Ethnomed. 2010;6(1):20. doi:10.1186/1746-4269-6-20
14. Enyew A, Asfaw Z, Kelbessa E, Nagappan R. Ethnobotanical study of traditional medicinal plants in and around Fiche District, Central Ethiopia. Curr Res J Biol Sci. 2014;6(4):154–167.
15. Bnouham M, Ziyyat A, Mekhfi H, Tahri A, Legssyer A. Medicinal plants with potential antidiabetic activity-a review of ten years of herbal medicine research (1990–2000). Int J Diabetes Metab. 2006;14(1):1.
16. Patel D, Prasad S, Kumar R, Hemalatha S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac J Trop Biomed. 2012;2(4):320.
17. Feyissa T. Micropropagation, Transformation and Genetic Diversity of Hagenia Abyssinica (Bruce) Jf Gmel. Vol. 2006. 2006.
18. Demissew S, Friis I, Awas T, et al. Four new species of Aloe (Aloaceae) from Ethiopia, with notes on the ethics of describing new taxa from foreign countries. Kew Bull. 2011;66(1):111–121. doi:10.1007/s12225-011-9263-2
19. Grover J, Yadav S, Vats V. Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 2002;81(1):81–100. doi:10.1016/S0378-8741(02)00059-4
20. Vuksan V, Sievenpiper JL. Herbal remedies in the management of diabetes: lessons learned from the study of ginseng. Nutr Metab Cardiovasc Dis. 2005;15(3):149–160. doi:10.1016/j.numecd.2005.05.001
21. Nibret E, Wink M. Trypanocidal and antileukaemic effects of the essential oils of Hagenia abyssinica, Leonotis ocymifolia, Moringa stenopetala, and their main individual constituents. Phytomedicine. 2010;17(12):911–920. doi:10.1016/j.phymed.2010.02.009
22. Jansen PCM. Spices, Condiments and Medicinal Plants in Ethiopia, Their Taxonomy and Agricultural Significance. Pudoc; 1981.
23. Amde M, Megersa N, Taddesse AM, Bedassa T. Determination of the levels of selected metals in seeds, flowers and fruits of medicinal plants used for tapeworm treatment in Ethiopia. Toxicol Environ Chem. 2013;95(1):82–100. doi:10.1080/02772248.2012.744022
24. Chai -T-T, Yeoh L-Y, Ismail NIM, Ong H-C, Manan FA, Wong F-C. Evaluation of glucosidase inhibitory and cytotoxic potential of five selected edible and medicinal ferns. Trop J Pharm Res. 2015;14(3):449–454. doi:10.4314/tjpr.v14i3.13
25. Surh Y-J. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3(10):768. doi:10.1038/nrc1189
26. Woldemariam TZ, Fell AF, Linley PA, Bibby MC, Phillips RM. Evaluation of the anti-tumour action and acute toxicity of cousins from Hagenia abyssinica. J Pharm Biomed Anal. 1992;10(8):555–560. doi:10.1016/0731-7085(92)80080-7
27. Lunyera J, Wang D, Maro V, et al. Traditional medicine practices among community members with diabetes mellitus in Northern Tanzania: an ethnomedical survey. BMC Complement Altern Med. 2016;16(1):282.
28. Mekuria AB, Belachew SA, Tegegn HG, et al. Prevalence and correlates of herbal medicine use among type 2 diabetic patients in teaching hospital in Ethiopia: a cross-sectional study. BMC Complement Altern Med. 2018;18(1):85. doi:10.1186/s12906-018-2147-3
29. Habte BM, Kebede T, Fenta TG, Boon H. Explanatory models of adult patients with type 2 diabetes mellitus from urban centers of central Ethiopia. BMC Res Notes. 2016;9(1):441. doi:10.1186/s13104-016-1938-1
30. Habte BM, Kebede T, Fenta TG, Boon H. Use of medicinal plants among Ethiopian patients with diabetes: a qualitative exploration. Ethiop J Health Dev. 2017;31(1):18–26.
31. Etuk E. Animals models for studying diabetes mellitus. Agr Biol J N Am. 2010;1(2):130–134.
32. Care IoLARCo, Animals UoL, Resources NIoHDoR. Guide for the Care and Use of Laboratory Animals. National Academies; 1985.
33. Leary SL, Underwood W, Anthony R, et al. AVMA Guidelines for the Euthanasia of Animals: 2013 Edition. 2013.
34. Mathewos Anza FW, Libsu S, Mamo F, Endale M. Phytochemical screening and antibacterial activity of leaves extract of Bersama abyssinica. J Adv Botany Zool. 2015;3(2):1–5.
35. Molla M, Gemeda N, Abay SM. Investigating potential modes of actions of mimusops kummel fruit extract and solvent fractions for their antidiarrheal activities in mice. Evid Based Complement Alternat Med. 2017;2017:1–11. doi:10.1155/2017/4103410
36. Kashimawo A, Kolawole J, Ahmadu A. Bioassay Guided Fractionation and α-Amylase Inhibitory Activity of Lupeol from the Stem Bark of Faidherbia Albida Del. Mimosaceae.
37. Trease G, Evans M. Text Book of Pharmacognosy 13th Edition Bailiere Tindall. London, Toronto: Tokyo. Pgs; 1989:200–201.
38. Pandey A, Tripathi S. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. Int J Pharmacogn Phytochem. 2014;2(5).
39. Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: a review. IPS. 2011;1(1):98–106.
40. Tesfaye A, Makonnen E, Gedamu S. Hypoglycemic and anti-hyperglycemic activity of aqueous extract of Justicia Schimperiana leaves in normal and streptozotocin-induced diabetic mice. Int J Pharm Sci Res. 2016;7(2):107–113.
41. Tamiru W, Engidawork E, Asres K. Evaluation of the effects of 80% methanolic leaf extract of Caylusea abyssinica (fresen.) fisch. & Mey. on glucose handling in normal, glucose loaded and diabetic rodents. BMC Complement Altern Med. 2012;12(1):151. doi:10.1186/1472-6882-12-151
42. OCDE O. Acute oral toxicity: up and down procedure. OECD Guideline Test Chem. 2008;425:1–2.
43. Wickramaratne MN, Punchihewa J, Wickramaratne D. In-vitro alpha amylase inhibitory activity of the leaf extracts of Adenanthera pavonina. BMC Complement Altern Med. 2016;16(1):466. doi:10.1186/s12906-016-1452-y
44. Brand-Williams W, Cuvelier M-E, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28(1):25–30. doi:10.1016/S0023-6438(95)80008-5
45. The OECD guidelines for testing of chemicals. Acute Oral Toxicity: Up- and- Down procedure. 2008. 1–27
46. Birru EM, Abdelwuhab M, Shewamene Z. Effect of hydroalcoholic leaves extract of Indigofera spicata Forssk. on blood glucose level of normal, glucose loaded and diabetic rodents. BMC Complement Altern Med. 2015;15(1):321. doi:10.1186/s12906-015-0852-8
47. Bowe JE, Franklin ZJ, Hauge-Evans AC, King AJ, Persaud SJ, Jones PM. Metabolic phenotyping guidelines: assessing glucose homeostasis in rodent models. J Endocrinol. 2014;222(3):G13–G25. doi:10.1530/JOE-14-0182
48. Ayala JE, Samuel VT, Morton GJ, et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech. 2010;
49. Baquer NZ, Kumar P, Taha A, Kale R, Cowsik S, McLean P. Metabolic and molecular action of Trigonella foenum-graecum (fenugreek) and trace metals in experimental diabetic tissues. J Biosci. 2011;36(2):383–396. doi:10.1007/s12038-011-9042-0
50. Toma A, Makonnen E, Mekonnen Y, Debella A, Adisakwattana S. Antidiabetic activities of aqueous ethanol and n-butanol fraction of Moringa stenopetala leaves in streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2015;15(1):242. doi:10.1186/s12906-015-0779-0
51. Sharma S, Choudhary M, Bhardwaj S, Choudhary N, Rana AC. Hypoglycemic potential of alcoholic root extract of Cassia occidentalis Linn. in streptozotocin induced diabetes in albino mice. Bull Fac Pharm Cairo Univ. 2014;52(2):211–217. doi:10.1016/j.bfopcu.2014.09.003
52. Rai PK, Srivastava AK, Sharma B, Dhar P, Mishra AK, Watal G. Use of laser-induced breakdown spectroscopy for the detection of glycemic elements in Indian medicinal plants. Evid Based Complement Alternat Med. 2013;2013:1–9. doi:10.1155/2013/406365
53. Watal G, Dhar P, Srivastava SK, Sharma B. Herbal medicine as an alternative medicine for treating diabetes: the global burden. Evid Based Complement Alternat Med. 2014;2014:1–2. doi:10.1155/2014/596071
54. Jemal D Toxicological Study of Glinus Lotoides and Hagenia abyssinica: Traditionally Used Taenicidal Herbs in Ethiopia. A thesis, School of Graduate Studies, AAU; 2005. 43–74.
55. Sharma B, Salunke R, Balomajumder C, Daniel S, Roy P. Anti-diabetic potential of alkaloid rich fraction from Capparis decidua on diabetic mice. J Ethnopharmacol. 2010;127(2):457–462. doi:10.1016/j.jep.2009.10.013
56. Oliver-Bever B. Medicinal Plants in Tropical West Africa. Cambridge university press; 1986.
57. Ivorra M, Paya M, Villar A. A review of natural products and plants as potential antidiabetic drugs. J Ethnopharmacol. 1989;27(3):243–275. doi:10.1016/0378-8741(89)90001-9
58. Kameswara Rao BGR, Kesavulu MM, Appa Rao C. Herbal medicine: in the management of diabetes mellitus. Manphar Vaidhya Patrika. 1997;1(4):33–35.
59. Sezik E, Aslan M, Yesilada E, Ito S. Hypoglycaemic activity of Gentiana olivieri and isolation of the active constituent through bioassay-directed fractionation techniques. Life Sci. 2005;76(11):1223–1238. doi:10.1016/j.lfs.2004.07.024
60. Alexandru V, Balan M, Gaspar A, Coroiu V. Antioxidant activity, phenolics and flavonoid content of some selected Romanian medicinal plants. Planta Med. 2007;73(09):
61. Sunmonu TO, Afolayan AJ. Evaluation of antidiabetic activity and associated toxicity of artemisia afra aqueous extract in wistar rats. Evid Based Complement Alternat Med. 2013;2013:1–8. doi:10.1155/2013/929074
62. Wolde T, Behailu Bizuayehu TH, Tiruha K, Tiruha K. Phytochemical analysis and antimicrobial activity of Hagenia abyssinica. Indian J Pharm Pharmacol. 2016;3(3):127–134. doi:10.5958/2393-9087.2016.00028.5
63. Yoon J-Y, Choi H, Jun H-S. The effect of phloroglucinol, a component of Ecklonia cava extract, on hepatic glucose production. Mar Drugs. 2017;15(4):106.
64. van Loon LJ, Kruijshoop M, Menheere PP, Wagenmakers AJ, Saris WH, Keizer HA. Amino acid ingestion strongly enhances insulin secretion in patients with long-term type 2 diabetes. Diabetes Care. 2003;26(3):625–630. doi:10.2337/diacare.26.3.625
65. Newsholme P, Brennan L, Bender K. Amino acid metabolism, β-cell function, and diabetes. Diabetes. 2006;55(Supplement 2):S39–S47. doi:10.2337/db06-S006
66. Marunaka Y. The proposal of molecular mechanisms of weak organic acids intake-induced improvement of insulin resistance in diabetes mellitus via elevation of interstitial fluid pH. Int J Mol Sci. 2018;19(10):3244. doi:10.3390/ijms19103244
67. Ohly P, Dohle C, Abel J, Seissler J, Gleichmann H. Zinc sulphate induces metallothionein in pancreatic islets of mice and protects against diabetes induced by multiple low doses of streptozotocin. Diabetologia. 2000;43(8):1020–1030. doi:10.1007/s001250050009
68. Cefalu WT, Hu FB. Role of chromium in human health and in diabetes. Diabetes Care. 2004;27(11):2741–2751. doi:10.2337/diacare.27.11.2741
69. Aloulou A, Hamden K, Elloumi D, et al. Hypoglycemic and antilipidemic properties of kombucha tea in alloxan-induced diabetic rats. BMC Complement Altern Med. 2012;12(1):63.
70. Kim J-S, Kwon C-S, SoN KH. Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Biosci Biotechnol Biochem. 2000;64(11):2458–2461. doi:10.1271/bbb.64.2458
71. Sharma SB, Nasir A, Prabhu KM, Murthy PS. Antihyperglycemic effect of the fruit-pulp of Eugenia jambolana in experimental diabetes mellitus. J Ethnopharmacol. 2006;104(3):367–373. doi:10.1016/j.jep.2005.10.033
72. Ramkumar KM, Vanitha P, Uma C, Suganya N, Bhakkiyalakshmi E, Sujatha J. Antidiabetic activity of alcoholic stem extract of Gymnema montanum in streptozotocin-induced diabetic rats. Food Chem Toxicol. 2011;49(12):3390–3394. doi:10.1016/j.fct.2011.09.027
73. Tanko Y, Jimoh AG, Mohammed ADTA, Musa KY. Hypoglycaemic effects of the methanolic extract of aerial part of Chrysanthellum indicum in rats. J Nat Prod Plant Resour. 2011;1:1–7.
74. Sucharitha E, Estari M. Evaluation of antidiabetic activity of medicinal plant extracts used by tribal communities in rural areas of Warangal district, Andhra Pradesh, India. Biol Med. 2013;5:20.
75. Porchezhian E, Ansari S, Shreedharan N. Antihyperglycemic activity of Euphrasia officinale leaves. Fitoterapia. 2000;71(5):522–526. doi:10.1016/S0367-326X(00)00204-5
76. Yaschilal Muche EM. Antidiabetic activities of hydromethanolic leaf extract of Calpurnia aurea (Ait.) Benth. Subspecies aurea (Fabaceae) in mice. Evid Based Complement Alternat Med. 1918;2018:9.
77. Anitha M, Mohan VR. Effect of Cynoglossum zeylanicum (Vehl ex Hornem) Thunb. Ex Lehm on Oral glucose tolerance in rats. J Appl Pharm Sci. 2012;2(11):4.
78. Nardos A, Makonnen E, Debella A. Effects of crude extracts and fractions of Moringa stenopetala (Baker f) Cufodontis leaves in normoglycemic and alloxan-induced diabetic mice. Afr J Pharm Pharmacol. 2011;5(20):2220–2225.
79. TOMAI F, CREA F, GASPARDONE A, et al. Ischemic preconditioning during coronary angioplasty is prevented by glibenclamide, a selective ATP-sensitive K+ channel blocker. Am Heart Assoc. 1994;700–705.
80. Chen L, Chen R, Wang H, Liang F. Mechanisms linking inflammation to insulin resistance. Int J Endocrinol. 2015;2015.
81. Hameed I, Masoodi SR, Mir SA, Nabi M, Ghazanfar K, Ganai BA. Type 2 diabetes mellitus: from a metabolic disorder to an inflammatory condition. World J Diabetes. 2015;6(4):598. doi:10.4239/wjd.v6.i4.598
82. Šoltésová D, Herichová I. On the mechanisms of diabetogenic effects of alloxan and streptozotocin. Diabetol Metab Endokrinol výživa. 2011;14:130–138.
83. Gothai S, Ganesan P, Park S-Y, Fakurazi S, Choi D-K, Arulselvan P. Natural phyto-bioactive compounds for the treatment of type 2 diabetes: inflammation as a target. Nutrients. 2016;8(8):461.
84. Masjedi F, Gol A, Dabiri S. Preventive effect of garlic (Allium sativum L.) on serum biochemical factors and histopathology of pancreas and liver in streptozotocin-induced diabetic rats. Iran J Pharm Res. 2013;12(3):325.
85. Gougeon R, Morais JA, Chevalier S, Pereira S, Lamarche M, Marliss EB. Determinants of whole-body protein metabolism in subjects with and without type 2 diabetes. Diabetes Care. 2008;31(1):128–133. doi:10.2337/dc07-1268
86. Gao D, Li Q, Li Y, et al. Antidiabetic and antioxidant effects of oleanolic acid from Ligustrum lucidum Ait in alloxan‐induced diabetic rats. Phytother Res. 2009;23(9):1257–1262. doi:10.1002/ptr.2603
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

