Back to Journals » International Journal of General Medicine » Volume 15
Evaluation of Foldable Capsular Vitreous Body Implantation Surgery
Authors Luo L, Wei Q, Liu Q, Wang L, Jiang Y
Received 5 July 2022
Accepted for publication 10 August 2022
Published 6 September 2022 Volume 2022:15 Pages 7077—7087
DOI https://doi.org/10.2147/IJGM.S380609
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Liying Luo, Qingquan Wei, Qing Liu, Li Wang, Yanyun Jiang
Department of Ophthalmology, Tongren Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China
Correspondence: Yanyun Jiang, Department of Ophthalmology, Tongren Hospital, Shanghai Jiao Tong University School of Medicine, 1111 Xianxia Road, Shanghai, People’s Republic of China, Email [email protected]
Background: Foldable capsular vitreous body (FCVB), a novel artificial vitreous substitute product, has been used clinically in recent years. The aim of this study was to evaluate the outcomes and complications of FCVB implantation surgery during the postoperative period.
Methods: We performed a prospective, nonrandomized study from November 2021 to March 2022. Eight patients with severe retinal detachment that could not be easily reattached were included in this study. Before and after surgery, visual acuity (VA), intraocular pressure (IOP), slit-lamp microscopy, optical coherence tomography (OCT), B-scan and CT were performed.
Results: After the operation, the FCVB was well distributed in the vitreous cavity and supported the retina according to the B-scan and CT images. During the follow-up period, no vitreous hemorrhage or retinal detachment was found in any of the patients. On the first postoperative day, the average IOP increased from 9.6 ± 7.7 mmHg preoperatively to 13.8 ± 14.3 mmHg. Although the IOP of two patients fell outside the normal range, IOP was finally held steady after the fifth postoperative day in all cases. In addition, three patients (37.5%) experienced eye ache, and after taking a Saridon tablet, the pain was greatly alleviated. Moreover, no adverse events, such as silicone oil (SO) spillage and emulsification or serious complications, were observed.
Conclusion: The current vitreous substitute FCVB is effective and safe for treating complicated retinal detachments in ophthalmic applications. Further multiple-center clinical designs should focus on indications and complications of FCVB during long-term follow-up periods.
Keywords: foldable capsular vitreous body, visual acuity, intraocular pressure
Introduction
Currently, pars plana vitrectomy (PPV) is the main approaches for severe retinal diseases such as diabetic retinopathy, retinal detachment and traumatic retinopathy. Since the vitreous is not actively regenerated, a temporary vitreous substitute in the form of gas or SO is injected into the vitreous cavity to support the retina until the retina becomes fully attached and healed.1 In some complicated cases, following the removal of SO or the absorption of gas, the retina detaches again;2 therefore, more than one operation is needed, and SO needs to be placed in the vitreous cavity for the long term. However, the disadvantages of the emulsification and toxicity of SO limit its clinical application.3 For the patients who cannot tolerate SO complications, a permanent or long-term vitreous substitute is in great need. In order to use for long-term, the vitreous substitute has to be nondegradable, transparent, biocompatible, and in stable conditions, which is challenging to achieve and need further evaluation.
Foldable capsular vitreous body (FCVB), a novel artificial vitreous substitute product, has been used clinically in recent years.4 The application of FCVB provides effective strategies for novel artificial vitreous substitutes.5 In some clinical trials, FCVB was applied to maintain eyeball integrity and save partial sight, showing good stability and efficacy after implantation surgery. After foldable capsule put into the eyeball, SO is injected through the tubevalve system to inflate the capsule. According to the Chinese State Food and Drug Administration (No. G20080656) and additional tests conducted in accordance with International Standardization Organization guidelines, the FCVB has good mechanical, optical, and biocompatible properties.6 Optical properties showed that the material has high light transmission and laser irradiation stability.6
In this study, we reviewed previous studies on the clinical application of FCVB and evaluated the feasibility of application in eight eyes after FCVB implantation.
Materials and Methods
This study was reviewed and approved by the ethics committee of Shanghai Tongren Hospital (NO.2022–013). The clinical trials complied with the principles of the World Medical Association Declaration of Helsinki, according to CONSORT guidelines. All patients were informed and signed an informed consent form. The FCVB implantation surgery was conducted according to the standard manufacturer’s procedure. In addition, this surgery is conducted regularly for patients meet the clinical indications at our hospital.
Patients
We performed a prospective nonrandomized study at the Department of Ophthalmology, Tongren Hospital, Shanghai Jiao Tong University School of Medicine from November 2021 to March 2022. Eight patients with severe retinal detachment that could not be easily reattached were included in this study. Among the eight patients, three had ocular rupture, two had ocular atrophy caused by endophthalmitis, and three had recurrent retinal detachment. The demographic and clinical characteristics of the various patients are shown in Table 1.
 |
Table 1 Clinical Characteristics of Patients |
At our clinic, clinically assessed and eligible patients were asked if they were interested in participating in the study. Before applying, they decided whether they would like to participate, and if so, they provided their written informed consent.
The inclusion criteria were as follows: (1) a minimum age of 18; (2) treatment for eye vision, including hand movement (HM), light perception (LP), and no light perception (NLP); (3) axial length between 16 and 28 mm; (3) severe retinal detachment caused by ocular rupture or endophthalmitis; and (4) recurrent retinal detachment with SO tamponade.
The exclusion criteria were as follows: (1) suitable silicone oil-filled eyes; (2) serious heart, lung, liver, or kidney diseases; (3) pregnancy or brest-feeding female; (4) allergic to rubber.
FCVB Implantation Protocol
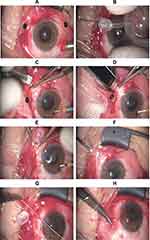
We performed all the FCVB implantation surgery by the same experienced surgeon. The surgical procedures are presented in previous studies.7 (Figure 1A) A standard three-channel 23G PPV was performed to remove the original or residual vitreous, SO, or vitreous hemorrhage and layers of proliferation membranes. Then, we attempted to reattach the retina as well as possible. (Figure 1B) A suitable type of FCVB capsule was prepared preoperatively according to the ocular axial length. A gas-tightness test was performed, and the structural integrity of FCVB was checked. (Figure 1C) An approximately 4 mm vertical incision on the sclera was made at the superior temporal position 4 mm from the limbus. (Figure 1D) An FCVB was folded and implanted into the ocular through the incision with a specific injector. (Figure 1E) The lens surface side faced the lens, and the drainage valve was fixed with 8–0 prolene suture. (Figure 1F) The FCVB capsule was filled with SO using a syringe while monitoring intraocular pressure during the operation. The injection volumes of SO were set appropriately according to capsule type and ocular length before surgery. (Figure 1G) The drainage tube was ligated and fixed to the sclera. (Figure 1H) Conjunctival suture was performed using absorbable 8–0 prolene.
Data Collection
Basic data included preoperative and postoperative IOP and VA. The IOP was tested by the same technician using the Goldmann applanation measurement technique, and the average of the three results was the final value. The anterior and posterior segments were evaluated using slit-lamp microscopy. Retinal reattachments were analyzed by optical coherence tomography (OCT) (Visante, Carl Zeiss Meditec, Dublin, CA) and B-scan (Aviso, Quantel Medical, Cinescan, France). Lastly, CT was used to evaluate the morphology of the ocular and the position of the FCVB.
Statistical Analyses
All results are expressed as mean ± standard deviation (SD). The t-test was used for comparisons of normally distributed binocular parameters before and after surgery; the Wilcoxon matched-pair signed-rank test was used for the analysis of nonnormally distributed data. The quantitative parameters were calculated using SPSS 15.0 (SPSS Inc., Chicago, IL, USA). All results were repeated at least three times, and p < 0.05 was considered significant.
Results
Baseline Information
Overall, 23G PPV and FCVB implantation were performed on eight patients in our department between November 2021 and March 2022. Table 1 summarizes the demographic information and ocular condition of the patients. The patients included four males and four females with an average age of 52.1 ± 17.5 years (range, 28–71). Three patients (37.5%) presented with eyeball rupture, and they experienced scleral and corneal wound suturing shortly after the accident. Due to the terrible condition of both the anterior and posterior segments, 2 weeks later, a second procedure was performed to remove vitreous hemorrhage and support the retina with SO. Two patients (25.0%) presented with endophthalmitis with progressive ocular atrophy. One (12.5%) was diagnosed with SO-dependent eyes with recurrent retinal detachment. This patient had experienced PPV and SO injection twice before. Two (25.0%) presented with recurrent retinal detachment, high myopia, and SO tamponade, and also experienced PPV and encircling scleral buckling twice. We evaluated efficiency, reaction, and safety during the early postoperative period during 1 month.
Analysis of Postoperative Reactions to FCVB Implantation
No leakage of SO from the FCVB occurred. Inflammatory responses, such as inflammatory cells and keratic precipitates, were observed in the anterior chamber. The anterior chamber reaction was apparent on the first day after surgery and gradually declined and eventually disappeared completely in one week.
Among eight cases, two operations were induced with general anesthesia, while six were under retrobulbar nerve block anesthesia. The anesthetic mode was determined before the surgery, depending mainly on the patient’s preference and physical condition. Three patients (37.5%) experienced eye ache, and after taking a Saridon tablet, the pain was greatly alleviated.
Analysis of the Efficacy of FCVB Implantation
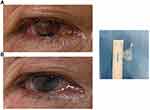
Corneal blood staining in the anterior chamber was found in three ocular rupture cases, and after surgery, hemorrhage in the anterior segment was obviously reduced, as shown in Case 1 (Figure 2). However, corneal degeneration remained in three patients.
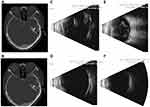
FCVB implantation surgery was performed successfully in all eight patients. The FCVB was well distributed in the vitreous cavity and evenly supported the retina according to the CT images after the operation (Figure 3A and B). Good profile and structure of the eyeballs were visible after FCVB implantation according to the B-scan, and no vitreous hemorrhage or retinal detachment was found in any of the patients (Figure 3C–F). Three patients were in the early stage of ocular atrophy before the operation. During the follow-up period, normal external appearances remained, and ocular atrophy made no progress in any of the cases.
As shown in Table 1, VA in Case 1 and Case 2 improved from NLP to LP after surgery. Besides, Case 8 improved VA significantly from LP to HM/45cm post-operation. The others showed no change in VA after surgery.
Analyzing the Safety of FCVB Implantation
No adverse events, such as SO spillage and emulsification or serious complications, occurred. Moreover, no rejection reaction was caused by the FCVB rubber. No drainage tube of FCVB was exposed outside the conjunctiva in any of the cases.
Figure 4 shows the IOP trends during the follow-up period. Before surgery, three patients had normal IOP, while two patients had ocular hypotony. On the first postoperative day, the average IOP increased from 9.6 ± 7.7 mmHg preoperatively to 13.8 ± 14.3 mmHg, and the IOP of two patients was outside the normal range. With the effects of ocular hypotensive agents, the IOP held steady since the 180 days postoperative day in all cases.
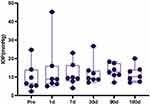 |
Figure 4 Changing trend of IOP before and after surgery. |
One eye showed postoperative hypotony (Case 1, Table 1). Before the FCVB implantation, his IOP was 5.3 mmHg. After implanting an AV-12P FCVB capsule, his IOP was 5 mmHg and 4 mmHg 1 and 3 days after surgery, respectively. After 1 month, his IOP was 9.2 mmHg and remained steady then.
One patient underwent acute high intraocular pressure with nausea and vomiting on the day of surgery. Her IOP was T + 3 six hours after the surgery and was 45.2 mmHg on the first postoperative day. After intravenous mannitol and medications of decreasing IOP, all symptoms vanished, and IOP returned to normal 3 days after surgery.
Discussion
In our study, we found that retinal reattachment was successful and no keratophy, glaucoma, SO leakage, emulsification, or other complications occurred during this observation period. These results may demonstrate that FCVB is safe and effective in treating severe RD.
Retinal reattachment is vital for FCVB implementation in patients with severe RD. The vitreous substitute is necessary to tamponade the reattached retina after vitrectomy for severely damaged eyes.8 Retinal detachment is difficult to treat because each case is unique, varying with the location, size, and duration of the detachment, as well as patient age.9 There are several surgical approaches, such as scleral buckling (SB), PPV, and pneumatic retinopexy (PR); however, no consensus has been reached on which approach is ideal.10 Thus, we found that FCVB displayed better results in retinal reattachment, which is consistent with previous studies.7
IOP is an essential factor in sustaining the shape of the eye. Previous studies have shown that post-vitrectomy hypotony is a well-recognized postoperative complication in eyes after severe PVR and severe ocular trauma surgery.11 Hypotony may occur as a result of the increased absorption of intraocular fluid through the area of the bare retinal pigment epithelium. Scar tissue can cause dysfunction of the ciliary epithelium and/or mechanical detachment of the ciliary body (CB).12 In addition, IOP reduction was also a predictive factor in phacocanaloplasty with suprachoroidal drainage.13 Therefore, in our study, we used IOP as the main postoperative examination indicator. We found that the IOP was lower after surgery, which was consistent with previous studies. The change in IOP may be associated with transient hypotony.14 The possible mechanisms may be inflammatory response, reoperations, and length of the procedure. Inflammatory responses result from the surgical scleral incision and CB local injury, which could contribute to hypotony.15
Enucleation is one of the most difficult and final therapeutic decisions in ophthalmologists, which involves removing the entire globe with the separation of all connections from the orbit, including optic nerve transection.16 The three most common indications for enucleation are malignancy, trauma, and a blind eye with intolerable pain. Trauma is the leading indication of enucleation and the leading cause of blindness.17 Whenever possible, the traumatized eye should initially be adequately treated and dealt with by surgeons. Primary closure of the wound allows for planned secondary repair surgery.16 Angela et al found that primary enucleation or evisceration was performed in 27% of all eye removals, and enucleation was performed in 69.6% of all eye removals.18 In a case-control study, the extrusion of iris/lens, PVR-C, and choroid damage were the independent risk factors for unfavorable outcomes in explosion-related eye trauma.19 There are also many treatments in this process. However, in cases of severe infection, such as panophthalmitis with extraocular extension, it is reasonable to consider a two-stage approach to decrease the risk of infectious complications. One option illustrated by this case is enucleation with the insertion of an antimicrobial-eluting cement implant,20 followed by a secondary procedure to exchange the cement with a permanent orbital implant. In our study, we used FCVB to serve RD patients and found that FCVB can avoid the inconvenience caused by SO dependence and enucleation, which is consistent with previous studies.21
A number of studies have found that FCVB has good physical properties, optical properties, and biocompatibility in vivo, vitro, and animal studies.22,23 However, there is currently insufficient evidence in clinical studies to prove that it is safe and effective. Therefore, in our study, we summarized previous studies on FCVB and sorted out the effects of FCVB (Table 2). In such patients, the aim of treatment is globe preservation rather than vision restoration. Because such conditions are generally rare, this treatment has not been widely used in clinical practices.24 For severe retinal detachments, FCVB has high retinal reattachment and has been shown to be flexible, effective, and safe as a vitreous substitute over a 3-month implantation time.4 A 1-year exploratory analysis of SO-filled FCVB treatment for severe retinal detachment showed that FCVBs had good efficacy and safety.24 Li et al found that FCVB implantation was an effective and safe treatment in 20 patients with severe ocular trauma or SO dependent eyes.25
 |
Table 2 Article Review from PubMed About FCVB |
In addition, FCVB also plays an important role in drug delivery system (DDS). Due to numerous 300 nm tiny apertures on the surface of the capsule, FCVB can act as an intravitreal drug delivery system, and release drugs following associated pharmacokinetics synchronously. Moreover, FCVB cannot change the chemical property of drugs.26 For instance, Liu et al found that FCVB mechanically releases dexamethasone sodium phosphate (DexP) from its capsule, which can be detected up to 28 days. It has been proven that FCVB can sustainably and mechanically release DexP by capsule apertures in a time-dependent and dose-dependent manner in addition to serving as a vitreous substitute.27 Hence, the FCVB is a new potential approach to meet the demand for combining vitreous substitutes and drug treatments.
There are some limitations to our study. First, the sample size of this study is small, and there is a lack of long-term follow-up results. At the same time, follow-up time can be up to 1 year or longer, which leads to the loss of follow-up. Second, this study was not a randomized controlled study, and the persuasiveness of the clinical evidence was somewhat diminished. To ensure the superiority and safety of FCVB among all vitreous substitutes, it is worth conducting large-scale comparative clinical trials in the future.
Conclusion
The current vitreous substitute FCVB is an alternative treatment for enucleation in eyes with end stage complex retinal detachments that failed repeated traditional retinal surgeries in ophthalmic applications. Further clinical design of FCVB should focus on complications during the long-term follow-up period.
Acknowledgments
We would like to thank all the participants and our hospital.
Funding
This project was qualified by the Research Fund of Shanghai Tongren Hospital, Shanghai Jiaotong University School of Medicine (No: TRYJ2021JC02) and Changning District Science and Technology Committee scientific research projects (No: CNKW2020Y15).
Disclosure
The authors declare no conflicts of interest in relation to this work.
References
1. Grzybowski A, Kanclerz P. Early descriptions of vitreous surgery. Retina. 2021;41(7):1364–1372. doi:10.1097/iae.0000000000003149
2. Ryan EH. Current treatment strategies for symptomatic vitreous opacities. Curr Opin Ophthalmol. 2021;32(3):198–202. doi:10.1097/icu.0000000000000752
3. Nicolai M, Lassandro N, Franceschi A, et al. Intraocular pressure rise linked to silicone oil in retinal surgery: a review. Vision. 2020;4(3). doi:10.3390/vision4030036
4. Lin X, Ge J, Gao Q, et al. Evaluation of the flexibility, efficacy, and safety of a foldable capsular vitreous body in the treatment of severe retinal detachment. Invest Ophthalmol Vis Sci. 2011;52(1):374–381. doi:10.1167/iovs.10-5869
5. Xu X, Ge H, Li J, et al. Outcomes of a foldable capsular vitreous body implantation: a retrospective study. Dis Markers. 2021;2021:6575195. doi:10.1155/2021/6575195
6. Liu Y, Jiang Z, Gao Q, et al. Technical standards of a foldable capsular vitreous body in terms of mechanical, optical, and biocompatible properties. Artif Organs. 2010;34(10):836–845. doi:10.1111/j.1525-1594.2010.01006.x
7. Zhang Z, Liu S, Xie F, Jiang B, Sun M, Sun D. Comparison of viscoelastic substance injection versus air filling in the anterior chamber during Foldable Capsular Vitreous Body (FCVB) implant surgery: a prospective randomized controlled trial. Adv Ther. 2021;38(9):4859–4871. doi:10.1007/s12325-021-01840-5
8. Alovisi C, Panico C, de Sanctis U, Eandi CM. Vitreous substitutes: old and new materials in vitreoretinal surgery. J Ophthalmol. 2017;2017:3172138. doi:10.1155/2017/3172138
9. Kunikata H, Abe T, Nakazawa T. Historical, current and future approaches to surgery for rhegmatogenous retinal detachment. Tohoku J Exp Med. 2019;248(3):159–168. doi:10.1620/tjem.248.159
10. Xiao J, Jiang C, Jiang H. Short-term external buckling with pneumatic retinopexy for retinal detachment with inferior retinal breaks. Am J Ophthalmol. 2013;156(3):624–625. doi:10.1016/j.ajo.2013.05.015
11. Tsui I, Schubert HD. Retinotomy and silicone oil for detachments complicated by anterior inferior proliferative vitreoretinopathy. Br J Ophthalmol. 2009;93(9):1228–1233. doi:10.1136/bjo.2008.140988
12. Kolomeyer AM, Grigorian RA, Mostafavi D, Bhagat N, Zarbin MA. 360 degrees retinectomy for the treatment of complex retinal detachment. Retina. 2011;31(2):266–274. doi:10.1097/IAE.0b013e3181eef2c7
13. Seuthe AM, Jung S, Januschowski K, Szurman P. Predictive factors for the IOP reduction in phacocanaloplasty with suprachoroidal drainage. Int Ophthalmol. 2020;40(8):1897–1903. doi:10.1007/s10792-020-01361-0
14. Nishino M, Eguchi H, Iwata A, Shiota H, Tanaka M, Tanaka T. Are topical steroids essential after an uneventful cataract surgery? J Med Invest. 2009;56(1–2):11–15. doi:10.2152/jmi.56.11
15. Lubinski W, Goslawski W, Podboraczynska-Jodko K, Mularczyk M, Post M. Comparison of 27-gauge versus 25-gauge vitrectomy results in patients with epiretinal membrane: 6-month follow-up. Int Ophthalmol. 2020;40(4):867–875. doi:10.1007/s10792-019-01250-1
16. Fu L, Patel BC. Enucleation. In: StatPearls. StatPearls Publishing; 2022.
17. Wolf A, Schrader W, Agostini H, Gabel-Pfisterer A. [Fireworks injuries of the eye: an overview of current diagnostic and treatment options] Diagnostik und Akuttherapie von Augenverletzungen durch Feuerwerkskorper. Ophthalmologe. 2019;116(12):1152–1161. doi:10.1007/s00347-019-01000-9
18. Gauthier AC, Oduyale OK, Fliotsos MJ, et al. Clinical characteristics and outcomes in patients undergoing primary or secondary enucleation or evisceration after ocular trauma. Clin Ophthalmol. 2020;116:3499–3506. doi:10.2147/OPTH.S273760
19. Feng K, Yao Y, Wang ZJ, et al. Mechanism and prognostic indicators for explosion-related eye trauma: eye injury vitrectomy study. Acta Ophthalmol. 2021;99(6):e956–e962. doi:10.1111/aos.14713
20. Clauss KD, Chen Y, Shoji MK, Johnson TE, Turbin RE. Antimicrobial-eluting cement orbital implant: a case report of staged enucleation for infectious panophthalmitis with extraocular extension. Orbit. 2021;1–5. doi:10.1080/01676830.2021.1980893
21. Zeng B, Wang Q, Sui G, Wang M, Xie W, Fu J. Foldable capsular vitreous body implantation for treatment of traumatic retinal detachment: two case reports. J Int Med Res. 2021;49(2):300060521990257. doi:10.1177/0300060521990257
22. Chen J, Gao Q, Liu Y, et al. Clinical device-related article evaluation of morphology and functions of a foldable capsular vitreous body in the rabbit eye. J Biomed Mater Res B Appl Biomater. 2011;97(2):396–404. doi:10.1002/jbm.b.31812
23. Chen H, Feng S, Liu Y, et al. Functional evaluation of a novel vitreous substitute using polyethylene glycol sols injected into a foldable capsular vitreous body. J Biomed Mater Res A. 2013;101(9):2538–2547. doi:10.1002/jbm.a.34560
24. Lin X, Wang Z, Jiang Z, et al. Preliminary efficacy and safety of a silicone oil-filled foldable capsular vitreous body in the treatment of severe retinal detachment. Retina. 2012;32(4):729–741. doi:10.1097/IAE.0b013e31822b1f80
25. Zhang X, Tian X, Zhang B, Guo L, Li X, Jia Y. Study on the effectiveness and safety of foldable capsular vitreous body implantation. BMC Ophthalmol. 2019;19(1):260. doi:10.1186/s12886-019-1268-x
26. Zheng H, Wang Z, Wang P, Liu Y, Jiang Z, Gao Q. Evaluation of 5-fluorouracil released from a foldable capsular vitreous body in vitro and in vivo. Graefes Arch Clin Exp Ophthalmol. 2012;250(5):751–759. doi:10.1007/s00417-011-1862-y
27. Liu Y, Ke Q, Chen J, et al. Sustained mechanical release of dexamethasone sodium phosphate from a foldable capsular vitreous body. Invest Ophthalmol Vis Sci. 2010;51(3):1636–1642. doi:10.1167/iovs.09-4134
28. Zhang R, Wang T, Xie C, et al. Evaluation of supporting role of a foldable capsular vitreous body with magnetic resonance imaging in the treatment of severe retinal detachment in human eyes. Eye (Lond). 2011;25(6):794–802. doi:10.1038/eye.2011.61
29. Chen S, Tian M, Zhang L, et al. Reattachment after foldable capsular vitreous body implantation in severe retinal detachment eyes. Transl Vis Sci Technol. 2021;10(11):8. doi:10.1167/tvst.10.11.8
30. Deng J, Song HP, Zhou RL, Chen T, Wang JZ, Zhu ZL. Evaluation of the long-term effect of foldable capsular vitreous bodies in severe ocular rupture. Int J Ophthalmol. 2021;14(12):1935–1940. doi:10.18240/ijo.2021.12.19
31. Liu N, Kang L, Yu X, et al. Preliminary clinical application of foldable capsular vitreous body in severe silicone oil-dependent eyes. Ann Palliat Med. 2021;10(10):10922–10929. doi:10.21037/apm-21-2554
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.