Back to Journals » Drug Design, Development and Therapy » Volume 17
Evaluation of Chitosan-Oleuropein Nanoparticles on the Durability of Dentin Bonding
Authors Zhao S , Zhang Y, Chen Y, Xing X, Wang Y, Wu G
Received 22 September 2022
Accepted for publication 14 January 2023
Published 22 January 2023 Volume 2023:17 Pages 167—180
DOI https://doi.org/10.2147/DDDT.S390039
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Shuya Zhao,1 Yunyang Zhang,2 Yun Chen,3 Xianghui Xing,1 Yu Wang,1 Guofeng Wu4
1Department of Pediatric Dentistry, Nanjing Stomatological Hospital, Medical School of Nanjing University, Nanjing, People’s Republic of China; 2Center of Modem Analysis, Nanjing University, Nanjing, People’s Republic of China; 3Department of Pediatric Dentistry, Affiliated Hospital of Stomatology, Nanjing Medical University, Nanjing, People’s Republic of China; 4Department of Prosthodontics, Nanjing Stomatological Hospital, Medical School of Nanjing University, Nanjing, People’s Republic of China
Correspondence: Guofeng Wu; Xianghui Xing, Email [email protected]; [email protected]
Purpose: To evaluate the effects of dentin pretreatment with chitosan-loaded oleuropein nanoparticles (CONPs) on the durability of resin–dentin bonding interfaces.
Methods: Eighty freshly extracted non-carious human third molars were randomly divided into four groups (n = 20 each): a de-ionized water (DW) group, a chitosan (CS) group, a chlorhexidine (CHX) group and a CONP group. The dentin in the DW, CS, CHX, and CONP groups were pretreated with de-ionized water, 1.0 mg/L CS solution, 2% chlorhexidine solution, and CONP suspension (prepared with 100 mg/L oleuropein), respectively, followed by the universal adhesive and resin composites. The bonded teeth of each group were randomly divided into two subgroups: an immediate subgroup and an aged subgroup. The bonded teeth of each group were then cut into the bonded beams. We measured their microtensile bond strength (μTBS), observed the characteristics of bonding interface by atomic force microscope, calculated the percentage of silver particles in a selected area for interfacial nanoleakage analysis, and evaluated the endogenous gelatinase activity within the bonding interface for in-situ zymogram analysis. Data were analyzed with two-way ANOVA and LSD multiple comparison test (P < 0.05).
Results: Regardless of after 24 h or after thermocycling, CONP exhibited better μTBS (P < 0.05) than the other three groups except that there was not a statistical significance (P > 0.05) in the CONP and CHX groups after 24 h. Besides, the CONP group presented significantly higher modulus of elasticity in the hybrid layers (P < 0.05), lower expression of nanoleakage (P < 0.05), and better inhibitory effect of matrix metalloproteinases than the other three groups before and after thermocycling.
Conclusion: Altogether, the CONPs had the potential to act as a dentin primer, which could effectively improve the dentin-resin binding durability.
Keywords: atomic force microscopy, dentin, matrix metalloproteinases, microtensile bond strength, nanoleakage
Introduction
Various factors, such as caries, abrasion, and trauma, can lead to the defect of teeth. Due to a lack of cellular components, teeth cannot self-repair. Clinically, composite resin materials for bonding and filling are currently the most frequent treatment approach for repairing enamel and dentin defects. Dentin is a complex tissue composed of minerals, water, and organic components such as collagen. Because of the structural characteristics of dentin itself, the bonding effect between dentin and composite resin is not as good as that of enamel. The long-term effect of resin dentin bonding is inadequate and eventually leads to complications such restorative detachment and secondary caries.1–3 The stability and durability of the dentin bonding interface has always been an urgent problem to be solved in the field of adhesive dentistry. The hybrid layer has been considered as the main bonding and retention structure of dentin.4 Meanwhile, it also remains the most vulnerable area of the adhesive-resin bond.5 Dentin contains a variety of matrix metalloproteinases (MMPs) and cysteine cathepsins, which usually exist in the form of zymogen. When the tooth is stimulated by heat treatment, acid erosion, caries, and mechanical preparation, the zymogen will be activated to dissolve the dentin collagen matrix.6 The unprotected collagen fibrils within the hybrid layer are susceptible to degradation and disintegration due to these activated enzymes.7,8 Collagen degradation will lead to the loss of the anchoring function of the hybrid layer, with the consequent loss of bond strength.9 Therefore, the application of protease inhibitors in the bonding process can prevent the degradation of collagen fibers and improve the durability of dentin resin bonding.10,11
The use of synthetic MMP-inhibitors has been proposed to increase the durability of resin dentin bonds.12,13 Among these different approaches, the use of natural crosslinking agents, which have good biocompatibility, low biological toxicity, good compatibility, and moderate reaction speed, has recently attracted the interest of investigators. Biomodification of dentin collagen by natural crosslinking agents can effectively enhance the mechanical properties of dentin, reduce collagen degradation by inhibiting proteases, and prevent dental caries and promote the remineralization of dentin. Natural polyphenols, such as proanthocyanidin (PA),14 epigallocatechin-3-gallate (EGCG),15 and curcumin,16 have been proved to enhance the bonding properties of dentin. Their chemical structure contains more phenolic hydroxyl groups, which can form covalent bonds, ionic bonds, hydrogen bonds, and other combinations with amino groups of collagen peptide chain residues. The improvement of their crosslinking degree is the key to keep the collagen fiber web in a state of no collapse, which is conducive to the infiltration of bonding resin, and can improve the mechanical properties of dentin collagen, and the three-dimensional structure is not changed. However, the pigmentation of these plant extracts leads to dentin surface staining, which is a clinical problem to be considered. It is important to modify the polyphenols to increase stability and biocompatibility.
Chitosan (CS) is a linear and semicrystalline natural polysaccharide from chitin. Because of its biodegradable, biocompatible quality, moderate or nontoxic effects on animals and people, antibacterial and antifungal activity, CS has emerged as one of the most promising polymers for forming nanoparticles.17–19 In the field of prevention and treatment of oral diseases, many studies have confirmed that CS can promote wound healing,20 regulate mineralization balance,21,22 resist bacteria,23 and guide the regeneration of alveolar bone and jaw bone.24 A study has shown that chelating dentin with CS for 60s prior to bonding, instead of phosphoric acid etching, can reduce endogenous MMP-initiated collagen degradation, prevent water permeation within hybrid layers, and kill bacteria on dentin surfaces to enhance resin–dentin bond durability.25 The CS can also be added to resin filling or adhesive materials to enhance the antibacterial property and edge tightness of the resin matrix or adhesive, and prevent secondary caries after filling treatment.
Olive is a plant with abundant polyphenols. Olive trees are a common plant species in the Mediterranean region and one of the most important farmed crops.26 The primary phenolic compound of olive is oleuropein.27 The biological properties of oleuropein have been documented to contain antidiabetic, antioxidant, antiatherogenic, antihypertensive, and antiinflammatory effects.28–34 In oral research, it can inhibit the growth of oral microorganisms.35 There are few studies on the crosslinking effect of oleuropein to improve the durability of the resin–dentin bond. Because of its biocompatibility, biodegradability, high permeability, cost-effectiveness, and lack of toxicity, CS nanoparticles have grown in popularity as carriers for active ingredient delivery in a variety of applications.36 The CS-loaded oleuropein nanoparticles (CONPs) have been found to be fungicides to control plant diseases.37
Inspired by the above research, we used the CONPs in dentin bonding to enhance the integration of the hybrid layer and improve the long-term adhesion between resin and dentin. In this study, the microtensile bonding strength of the bonding interface was examined, the image and the elastic modulus of resin–dentin interface were obtained by atomic force microscope, the nanoleakage expression was examined by a field emission scanning electron microscope, and the endogenous gelatinase activity within the bonding interface was evaluated for in-situ zymogram analysis. By the above analysis experiments, we would complete the purpose of our test. The objective of this study was to evaluate the effects of dentin pretreatment with CONPs on the durability of resin–dentin bonding interfaces, providing a new drug for strengthening dentin bonding for clinical use. The null hypotheses are as follows: 1) no significant differences will be observed in mean μTBS value among the four groups, 2) no significant differences will be found in the modulus of elasticity of the four groups by atomic force microscope and 3) no significant differences will be noted in the perinterfacial nanoleakage expression among the four groups.
Materials and Methods
Tooth Preparation
The study complied with the Declaration of Helsinki. Eighty freshly extracted non-carious human third molars were used for in vitro research based on a protocol approved by the Ethical Committee of the Nanjing Stomatological Hospital, Medical School of Nanjing University, China. Eighty teeth were taken from 80 distinct patients. All patients gave written informed consent and agreed to participate in the study. The teeth were obtained with no associated patient identifiable information and collected according to relevant guidelines and regulations. The initial clinical indication of tooth extraction has nothing to do with this study. The teeth were cleaned by removing the remaining soft tissues and debris, and then immediately stored in Hanks’ solution at 4 °C to reduce demineralization. The crown enamel and superficial dentin (approximately 3 mm thick) were removed perpendicular to the long axis of each tooth with a low-speed cutting saw (Isomet, Buehler, USA) under water cooling, which were then wet-polished with 400-grit silicon carbide paper to create a standardized smear layer. The dentin surfaces were carefully examined using a dental microscope (OMS2350 Dental Microscope, Zumax, China) to ensure that there was no residual enamel or pulp exposure. Afterwards, the exposed dentin was wet polished with 400-grit and 600-grit silicon carbide sandpaper in turn for 1 min, and treated in an ultrasonic bath containing deionized water for 5 min to obtain a standardized smear layer. Each tooth was etched with 35% phosphoric acid etchant (Gluma Etch 35 Gel, Germany) before being thoroughly rinsed with distilled water for 30s and then gently dried with a cotton pellet. All experimental procedures were carried out in accordance with relevant guidelines and regulations and performed by the same one trained operator.
Preparation of the CONP
The CONPs were synthesized using a modified ionotropic gelation.37 First, 1 mg/mL chitosan solutions were prepared by dissolving the medium molecular weight CS (50,000–190,000 Da, 75–85% deacetylated, Aladdin Biochemical Technology Co. Ltd, USA) in a solution of hydrochloric acid to 0.04% (v/v). The CS solution was stirred for 1 h and adjusted to pH = 5.5 by NaOH. The oleuropein solutions were prepared by dissolving 0.72 mg oleuropein (Jiangsu Caiwei Biology Science and Technology Co. Ltd, China) in 1mL water. Next, add 1 mL of oleuropein solution to 5 mL of CS solution, stir for a few minutes, and adjust the pH to 5.5. 2 mg/mL tripolyphosphate solution was obtained by dissolving tripolyphosphate (Sigma Aldrich, USA) in distilled water and added dropwise to the CS solution with a volume ratio of 1:5. Finally, the suspensions were stirred for 30 min at room temperature. The concentration of the oleuropein solution used for preparing CONPs was 100 mg/L. Five drops of the suspension were prepared by allowing a single drop of nanoparticle suspension to dry overnight at room temperature on a 200-mesh copper grid. Transmission electron microscope (TEM, HT7700, Hitachi, Japan) was used to observe the morphology of the synthesized nanoparticles (Figure 1).
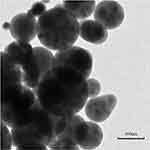 |
Figure 1 Transmission electron microscopy (TEM) image of CONPs (scale bar = 100 nm). |
Treatment of Dentin Samples
The specimens were randomly divided into four groups (n = 20 each): a DW group, a CS group, a CHX group, and a CONP group. The exposed dentin in the DW, CS, CHX, and CONP groups were coated with deionized water, 1.0 mg/L CS solution, 2% chlorhexidine solution, and CONP suspension, respectively, for 10s. Then, the excess solutions in the dentin of the four groups were dipped with cotton balls. The adhesive (Single Bond Universal Adhesive, 3M ESPE, USA) was applied to the dentin surface, and gently air-dried for 5 s. The specimens were light-cured vertically for 20s at a position 1mm away from the adhesive surface with a Demiplus L.E.D. (Light Emitting Diode) curing light (Kerr, Orange, CA, USA) delivering 1100 mW/cm2. A 4-mm-thick resin composite buildup (Filtek Z350, 3M ESPE, USA) was performed using a layering technique and then light-cured. The bonded teeth of each group were randomly divided into two subgroups (an immediate subgroup and an aged subgroup). In the immediate subgroup, the bonded teeth were stored in artificial saliva (pH = 7.0) at 37 °C for 24 h, and then the following tests would be carried out. The composition of the artificial saliva was 125.6 mg/L NaCl, 963.9 mg/L KCl, 189.2 mg/L KSCN, 654.5mg/l KH2PO4, 200.0mg/L Urea, 763.2mg/L NaSO4C10H2O, 178mg/L NH4Cl(), 227.8mg/L CaCl2·2H2O, and 630.8mg/L NaHCO3. In the aged subgroup, the bonded teeth were submitted to thermocycling (5 °C for 1 min and 55 °C for 1 min, 10,000 times) to simulate clinical use for one year. Each tooth was serially sectioned perpendicular to the bonding interface to obtain 1-mm-thick beams. The beams were embedded with wax and the self-setting resin into a whole and then cut into 1-mm × 1-mm rectangular sections (Figure 2).
 |
Figure 2 Schematic diagram of preparation method of the resin–dentin beams. |
μTBS Test
Ten bonded teeth of different subgroups in each group were chosen, and bonded beams (1mm × 1mm × 8mm) adjacent to the center of each tooth were chosen for microtensile testing. The cross-sectional areas of the dentin samples were measured and recorded by an electronic vernier caliper (MNT-150, Meinaite, China), with the accuracy of 0.01 mm. The dentin bond strengths in these sections were evaluated using a simplified universal testing machine (Bisco; Schaumburg, IL, USA) at a crosshead speed of 1 mm/min until failure. The tensile force F (N) was recorded. The measurement result is expressed in MPa, which is obtained by dividing the force F (N) by the cross-sectional area of a single sample. The mean bond strength value obtained from the 4 bonded beams was used to represent the bond strength of the tooth. The microtensile bond strength of an immediate subgroup and an aged subgroup in each group was recorded, and the average value and standard deviation were calculated.
Atomic Force Microscopy
A Dimension® IconTM atomic force microscope (AFM, Bruker Dimension Icon, Bruker technology Co., Ltd., USA) was employed in this study. The bonded beams of different subgroups (n = 10) in each group were polished successively through SiC abrasive paper from 320 up to 4000 grits (1 min for each grid) under water cooling. At each polishing step, the beams were treated in an ultrasonic bath containing deionized water for 5 min. After vacuum drying, the specimens were examined using AFM in contact mode with a silicon tip of the Bruker AFM probe at a 70-kHz resonance frequency, 0.4-N/m spring constant, and a scan rate of 2 Hz. The image of the bonding interface (80 × 80 µm) of each group was acquired. The modulus of elasticity of the specimens in the bonding interface was evaluated by AFM. The standard sample was first measured, and then the bonded samples were measured. Two indentations in a straight line 3 µm apart were recorded, and the modulus of elasticity of each position at an indentation depth of 300 nm was quantified. Finally, the elastic modulus of the three separated positions of a specimen was averaged to obtain the elastic modulus of the bonded sample.
Nanoleakage Evaluation
Twenty 1-mm-thick resin dentin slabs of each group were used for qualitative and quantitative nanoleakage expression. They were randomly divided into two subgroups: an immediate subgroup (n = 10) and an aged subgroup (n = 10). The slabs were coated with two layers of nail varnish in the 1-mm region away from the bonding interface. Afterwards, the specimens were immersed in a 50 wt% ammoniacal silver nitrate solution (pH 9.5) protected from light for 24 h. After rinsed thoroughly in distilled water, all specimens were processed with a photo-developing solution for 8 h under fluorescent light to reduce silver ions to metallic silver grains, and subsequently immersed in a fixed solution for 8 h. All slabs were sequentially wet-polished with 600-, 1200-, 2000-and 5000-grit SiC paper. After ultrasonic cleaning for 5 minutes, they were dehydrated for 24 h in an incubator with silica gel at 37 °C. Finally, the specimens were sputter-coated with gold and observed with a field emission scanning electron microscopy (FE-SEM, JSM-7800F Prime, JEOL, Japan) in backscattered electron mode with 10 kV and 10mm working distance. Three bonding interface micrographs of each disk were captured randomly (n = 10 for each subgroup) for nanoleakage analysis. Image-analysis software (NIH ImageJ 1.60, Scion, Frederick, MD, USA) was used to quantitatively calculate the percentage of metallic silver particles (%) on the interface in a previously selected area (400 × magnification; height × width = 20 × 250 µm).
In situ Zymography
Specimens from each group were divided into 2 subgroups. One subgroup was stored in deionized water at 37 °C for 24 h. The other subgroup was thermomechanically challenged. The 2-mm-thick dentin slices from each tooth containing the bonding interface were polished with 320-, 600-, 1200-, 2000-, 3200-, and 4000-grit silicon carbide paper for 1 min each, with water flowing. We evaluated the endogenous gelatinase activity within the bonding interface using an EnzChek collagenase/gelatinase kit (Thermo Fisher Scientific, USA). The DQ-gelatin (dye-quenched-gelatin) was used as MMP substrate for in-situ zymogram analysis. The 1.0 mg/mL stock solutions of DQ gelatin were prepared by adding 1.0 mL deionized water directly to one of the five vials containing the lyophilized substrate. The solution was agitated in an ultrasonic water bath in 50 °C for 5 minutes to facilitate dissolution. The 1X Reaction Buffers were prepared by diluting 2 mL of the 10X Reaction Buffer in 18 mL deionized water. The final substrate concentration of 100 ug/mL DQ gelatin was achieved by adding 20 uL of the 1.0 mg/mL stock solution to 80 uL of 1X Reaction Buffer. The dentin slices were fully soaked in 100 uL gelatin mixture, kept away from light, and incubated in a 37 °C incubator for 24 h. We used the confocal laser scanning microscopy (Nikon-Eclipse-Ti, Japan) to evaluate the activity of endogenous gelatinase in dentin.
Statistical Analysis
Quantitative data were analyzed with SPSS 26.0 software (SPSS Inc., Chicago, IL, USA) with a level of significance of α = 0.05. All experimental data were presented as the mean ± standard deviation for each group. Analysis was performed with two-way analysis of variance (ANOVA) to characterize the effects of “agent” and “aging” on μTBS, the elasticity modulus of the bonding interface, and nanoleakage data (%); we used LSD multiple comparison test for pairwise comparisons. The Shapiro–Wilk test was performed to confirm the normal distribution of the data before the ANOVA.
Results
Microtensile Bond Strength (μTBS) Test
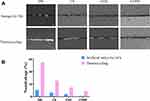
The resin–dentin bond strength of specimens stored in artificial saliva at 37 °C for 24 h and submitted to thermocycling from the four groups is shown in Figure 3. In the immediate subgroups, the mean μTBS values of the CS, chlorhexidine (CHX), and CONP groups were significantly higher than that of the deionized water (DW) group (P < 0.05). The mean μTBS value of the CHX and CONP groups was significantly higher than that of the CS group (P < 0.05). The mean μTBS of CONP (42.5 MPa) was higher than that of the CHX group (41.7 MPa), but there was not a statistical significance (P > 0.05). After 10,000 thermocycles, we observed a sharp decrease in the bond strength of all groups compared with that after storage for 24 h (P < 0.05). CS, CHX, and CONP showed higher tensile bond strength values than DW after thermocycling (P < 0.05 for each comparison). The mean μTBS value of the CHX and CONP groups was significantly higher than that of the CS group (P < 0.05). Besides, the mean μTBS of CONP was higher than that of the CHX group (P < 0.05).
Atomic Force Microscopy
Three-dimensional examination of the resin–dentin interface by AFM revealed that there is an abrupt color change between the adhesive and the underlying dentin in the DW and CS groups. However, the resin–dentin interface in the CHX and CONP groups presented a relatively constant color gradient between the adhesive and the underlying dentin (Figure 4A). This meant that the interface in the CHX and CONP groups were relatively high-quality compared with the other two groups. The modulus of elasticity in the hybrid layers of the CHX, CS, and CONP groups was significantly higher than that of the DW group (P < 0.05) (Figure 4B). The modulus of elasticity of the CHX and CONP groups was also significantly higher than that of the CS group (P < 0.05). In addition, the modulus of elasticity of CONP was higher than that of the CHX group (P < 0.05). After thermocycling, the elastic modulus of the hybrid layers in the DW, CHX, CS, and CONP groups have decreased dramatically (all P < 0.05). Besides, the hybrid layers in the CONP exhibited the highest modulus of elasticity among the groups after thermocycling (P < 0.05).
Nanoleakage Evaluation
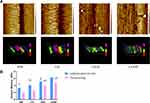
Representative FE-SEM images of nanoleakage in the bonding interface from the four groups are illustrated in Figure 5A. The evidence of spotted silver deposits that are randomly distributed through the bonding interface can be seen. The extent of leakage within the bonding interface differed among the groups. We observed only spots of silver deposition within the hybrid layer in the CHX and CONP groups after 24 h of artificial saliva storage and more silver deposition in the hybrid layer after thermocycling. In contrast, banded silver deposition and leakage were present in the DW and CS groups. The images in which the total percentage distribution of silver particle penetration at the resin–dentin interface was calculated are visualized in Figure 5B. No group completely eliminated nanoleakage. The percentage of silver particles (%) in a selected area of the CHX and CONP groups was significantly lower than that of the DW and CS groups (P < 0.05). The percentage of silver particles in the CONP group was significantly lower than that in the CHX group (P < 0.05). After thermocycling, there was a distinct increase in nanoleakage for the four groups. The CHX and CONP groups showed significantly less silver particle uptake than the other two groups (P < 0.05). Besides, the percentage of silver particles (%) in a selected area of the CONP group was lower than that of the CHX group (P < 0.05).
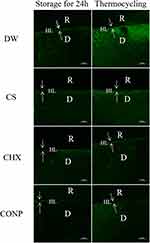
In situ Zymography
Figure 6 shows the representative images of in situ zymography of the resin–dentin bonding interface from each group before and after thermocycling. The activity of endogenous gelatinase, created by the breakdown of fluorescein-quenched gelatin in the hybrid layer and dentinal tubules, appears as green fluorescence. After storage for 24 h, strong green fluorescence in the DW group can be seen in the hybrid layer and dentinal tubules, which indicated extensive gelatinolytic activity. In the CONP group, we observed almost no green fluorescence within the bonding interface, indicating that endogenous enzymes within the hybridized dentin were inactive, resulting in the absence of obvious enzymatic hydrolysis. Compared with the DW group, the fluorescence decreased slightly in the CS group. The fluorescence intensity in the CHX group decreased clearly, but the CONP groups had lower green fluorescence than the CHX group. We observed similar results in the specimens challenged by thermocycling. However, we observed stronger green fluorescence in the hybrid layer and dentinal tubules of all groups after 10,000 thermocycles compared with that after storage for 24 h.
Discussion
The micromechanical locking action between resin and dentin in the hybrid layer is primarily responsible for the adhesive force of the resin–dentin interface.38,39 Collagen fibers eventually lose their internal and exterior minerals during dentin etching, forming a loose collagen fiber network into which resin monomers infiltrate to form a dense hybrid layer. However, the penetration depth of the resin monomer cannot completely match the demineralization depth of dentin,40 resulting in collagen fiber exposure and forming nanoscale collagen exposed areas. The exposed collagen fibrils in the dentin matrix are susceptible to proteases from bacteria and saliva, as well as host-derived matrix metalloproteinases, which degrade and destroy dentin bonding interface components, reducing the clinical bonding effects.41,42 In the previous study, we found that CONP could stabilize the collagen fiber,43 but whether it can effectively stabilize the resin–dentin interface of the bonded teeth needs further study, so as to provide a certain value for clinical application of dentin restoration and bonding. Thus, the present study was designed to evaluate the effectiveness of CONPs on the durability of dentin bonding. In the experiment, the immediate and thermally aged μTBS, atomic force microscopy, nano-leakage evaluation, and in situ zymography of the resin–dentin interface of the bonded teeth treated with four different solutions (DW, CS, CHX and CONP) immediately and after aging were examined. The bonded teeth in the CONP group performed better in these tests, indicating that CONPs would be a better choice for an effective collagen crosslinking treatment to improve dentin bonding durability.
Oleuropein is a phenolic ingredient of olive oil, which is a secoiridoid phenolic chemical made up of hydroxytyrosol (polyphenol), elenolic acid (secoiridoid), and a glucose molecule. A large number of research results show that oleuropein has good pharmacological activities in antibacterial, anti-inflammatory, and antioxidant.44,45 However, there has been relatively little research on oleuropein in stomatology. When compared to other well-known chemicals, natural polyphenols offer greater bioactivity, biocompatibility, and application. Many plant-derived polyphenols14,15,46 can function as MMP inhibitors to stabilize dentin collagen and improve the anti-enzymatic properties of collagen. Oleuropeins are rich in polyphenols, which have multiple hydroxyl structures. Hydroxyl groups can generate hydrogen bonds with collagen’s amide NH groups, and hydrogen bonds are the primary force of crosslinking.47 As a result, we want to verify whether oleuropein can also stabilize collagen. To boost the stability of easily oxidized oleuropeins, we coated them with CS to prepare nanoparticles. In the preliminary experiment, CONP suspensions were prepared with 10mg/L, 50 mg/L, 100 mg/L, 200 mg/L, and 300 mg/L oleuropein solution. A scanning electron microscope revealed that one nanoparticle in CONP suspensions prepared with 100 mg/L oleuropein contained more oleuropein inside, which are not easy to deposit and have better stability at the same time. It was found that the higher the concentration of oleuropein, the better the crosslinking effect was. However, the nanoparticles prepared with 200 mg/L and 300 mg/L concentrations contained more oleuropeins, which were easy to deposit and had poor stability, so we finally chose 100 mg/L oleuropein to prepare CONPs. Consistent with this, olive leaf extracts could be used as fungi to control plant diseases, and the concentration of oleuropein in preparing CONPs was also 100 mg/L.37
Before being used in further clinical investigations, new agents must be thoroughly tested for their bond properties. Dentin bonding can be efficiently characterized by μTBS tests.48 As can be seen from Figure 3, after water storage for 24h, the mean μTBS values of the CHX, CS, and CONP groups were significantly higher than that of the DW group (P < 0.05). The mean μTBS value of the CHX and CONP groups was significantly higher than that of the CS group (P < 0.05). The mean μTBS of CONP (42.5 MPa) was higher than that of the CHX group (41.8MPa), but there was not a statistical significance (P > 0.05). In general, CS, CHX and CONP could all increase the immediate bonding strength, on the other hand, the CHX and CONP group demonstrated favourable immediate bond strength. After 10,000 thermocycles, there was a sharp decrease in bond strength of all groups. The CS, CHX, and CONP groups showed higher tensile bond strength values than the DW group after thermocycling (P < 0.05 for each comparison). The mean μTBS value of the CHX and CONP groups was significantly higher than that of the CS group (P < 0.05). Consistent with the findings of previous studies, CS can improve resin–dentin bonding strength by preserving intrafibrillar minerals, minimizing endogenous protease-initiated collagen degradation, and inhibiting water permeability within hybrid layers.25 Similarly, the CHX group had a lower reduction, which was in line with earlier research.49,50 CHX has been reported to diminish the loss of bond strength over time because it could inhibit endogenous collagenolytic activity through a chelating mechanism.51 Because CHX cannot co-polymerize with methacrylate resin monomers, it has been found to diffuse out of the polymerized resin network.52 Furthermore, because water acts as a desorption medium, CHX’s long-term anti-MMP effectiveness is harmed by its electrostatic bonding properties.53,54 When 2.0% CHX was applied to the dentin surface, precipitates formed55 and would change the morphology and chemical properties of the dentin.56 In our research, we also discovered that after thermocycling, the mean TBS of the CONP group was higher than that of the CHX group (P < 0.05), despite the fact that there was no statistical significance in the immediate CHX and CONP groups. This revealed that CONP showed good binding strength after 24 hours water storage and after thermocycling. CONP could maintain its bond durability in this study, and its effectiveness was less susceptible to aging. Previously, some scholars combined CS nanoparticles with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide, proving that they reduced the degradation of dentin collagen and improved the stability of the bonding interface without affecting the bonding polymerization.57 Therefore, the joint effect of oleuropein and CS contributed to the reduction of bonding strength in the CONP group of this study.
AFM can be used to observe the morphology of demineralized dentin collagen fibers protected by crosslinking agents and resistant to the degradation of proteolytic enzymes. In three-dimensional AFM imaging of the resin–dentin interface, there is an abrupt color change in the DW and CS groups, but the interface in the CHX and CONP groups presented a relatively constant color gradient. The modulus of elasticity in the hybrid layers of the CHX, CS, and CONP groups are consistent with the previous μTBS results, except that the modulus of elasticity of CONP was higher (P < 0.05) than that of the CHX group before thermocycling. This shows that the microscopic modulus of elasticity in the hybrid layers and the macroscopic tensile bond strength are consistent in terms of mechanical properties of the bonding interface, and the microscopic modulus of elasticity may be a bit more sensitive. The AFM results showed a high-quality hybrid layer in the CONP group. Thermocycling aging produced only mild adverse effects on the CONP group compared to the other three groups. This further shows that CONP can make the mixed layer structure more stable, enhance the mechanical properties of the interface, and is not easily affected by various external factors.
Sano et al discovered that special substances infiltrated into the mixed layer even without edge cracks by the electron microscope.58 To distinguish it from micro-leakage, the concept of nanoleakage was later proposed.59,60 Between the mixed layer and the non-demineralized dentin matrix, there exist certain porous structures with a diameter of less than 50 nm, which could allow tiny molecules such as water and bacterial products to penetrate the bonding interface and cause damage. Furthermore, the aging of the bonding surface may be hastened by the introduction of water and bacterial metabolites, compromising the long-term bonding effect. Many methods have been tried to quantify the degree of nanoleakage and then evaluate the relationship between nanoleakage and adhesive strength.61,62 In this study, the silver nanoleakage of dental–resin interface was observed by scanning electron microscope in backscattered electron mode. The operation is straightforward, and crisper images can be obtained for quantitative analysis. Our nanoleakage observations revealed that the CONP group had less silver deposition than the other three groups before and after thermocycling, which indicated lower nanoleakage in the CONP group. The SEM results also suggest the reason for the CONP group’s higher bonding strength after 24 hours water storage and thermocycling aging. An optimal resin–dentin bond should seal tooth–restoration interfaces efficiently and durably, as well as survive all oral environmental stresses. CONP, as a crosslinking agent, can form hydrogen bonds with dentin collagen because of its multiple hydroxyl groups, thus achieving a higher degree of polymerization and creating a more complete bonding interface. This effect can explain the uniform appearance of the resin-collagen mixed layer produced by CONP, which shows the highest elastic modulus and the lowest nanoleakage.
The hydrolysis of MMPs can only be exerted after being activated, and MMPs are easily activated during acid etching.63 After being activated, MMPs can degrade the exposed collagen fibers in the mixed layer of the bonding interface, leading to the hydrolysis of the mixed layer, thus reducing the bonding strength.7 Inhibiting the enzymatic degradation of demineralized dentin matrices induced by MMPs has been a strategy to preserve the integrity of collagen in the hybrid layer and improve the longevity of adhesive restorations.64 In our study, CONP was found to have an MMP inhibitory effect comparable to that of chlorhexidine. In situ zymography showed almost no green fluorescence in the bonding interface of the CONP group, indicating that endogenous enzymes within the hybridized dentin were inactive and collagen hydrolysis in the CONP decreased relative to that in the other three groups. Although we observed stronger green fluorescence after 10,000 thermal cycles, the green fluorescence of the CONP group was still lower than that of the other three groups. Therefore, the acid-etched dentin treated with CONP could inhibit the activity of MMPs and reduce the solubility of collagen. Compared with the DW group, the fluorescence decreased slightly in the CS group. This is because CS chelation contributes to the stability of the resin–dentin interface, as demonstrated by in situ zymography before and after thermomechanical cycling.25,65 CHX is a nonspecific MMPs inhibitor, which can inhibit MMP-2, MMP-8, and MMP-9.66 Pretreatment of dentin with chlorhexidine can protect collagen in the mixed layer from degradation by MMPs and maintain the stability of the dentin bonding interface.67,68 In our research, through in situ zymography, μTBS, and nanoleakage evaluation, it is confirmed that chlorhexidine can stabilize the adhesive interface by inhibiting gelatinase activity in the adhesive interface, in agreement with previous findings and reports.12,49,69,70 The CONP group had lower green fluorescence than the CHX group after storage for 24 h and after 10,000 thermocycles, which means that the application of CONP could be a valid strategy to improve the durability of dentin-resin bonding.
Consistent with many previous reports, the results of the current experiment indicate that modern bonding systems produce an initial satisfactory bonding that deteriorates with aging.1,71,72 Our observations confirmed that regardless of any agent, aging via thermocycling deteriorates the effectiveness of interface attachment complexes as evidenced by µTBS, atomic force microscopy, nanoleakage evaluation and in-situ zymography of the resin–dentin interface. Furthermore, our study found that the application of CONP as the primer of dentin bonding for one minute could amend the above experiments. Therefore, the CONP might have a positive effect on improving the dentin resin binding durability. However, there are certain issues that need to be resolved right now. Although the dyeing problem of natural crosslinking agents was handled in this study by synthesizing nanoparticles, the solution’s preservation and subsequent stability should be considered as well. Moreover, excessive crosslinking can compromise the integrity of the dentin matrix and reduce the dentin’s ultimate tensile strength. The in vitro model cannot entirely replicate the complex oral environment, so there is a long way of implementing these findings in the clinic. As a result, more research is needed into the exact mechanism of CONP in dentin bonding.
Conclusion
This study showed that pretreatment of dentin surface with CONP reduced both the activation of MMPs and nanoleakage and maintained the bond strength. Therefore, within the limitations of this in vitro study, it may be concluded that CONP has the potential to act as a dentin primer to improve the dentin-resin binding durability.
Data Sharing Statement
The data used to support the findings of this study are included in the article. Should further data or information be required, these are available from the corresponding authors.
Ethical Statement
The experiments in this study were approved by the Ethical Committee of the Nanjing Stomatological Hospital, Medical School of Nanjing University (Nanjing, China), with an approval number of 2016NL-037(KS).
Acknowledgments
The authors would like to acknowledge the support from Jiangsu Provincial Key Research and Development Program (BE2019622) and “3456” Cultivation Program for Junior Talents of Nanjing Stomatological School, Medical School of Nanjing University (0222C114) for this work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. De Munck J, Van Landuyt K, Peumans M, et al. A critical review of the durability of adhesion to tooth tissue: methods and results. J Dent Res. 2005;84(2):118–132. doi:10.1177/154405910508400204
2. De Munck J, Mine A, Poitevin A, et al. Meta-analytical review of parameters involved in dentin bonding. J Dent Res. 2012;91(4):351–357. doi:10.1177/0022034511431251
3. Drummond JL. Degradation, fatigue, and failure of resin dental composite materials. J Dent Res. 2008;87(8):710–719. doi:10.1177/154405910808700802
4. Pashley DH, Tay FR, Breschi L, et al. State of the art etch-and-rinse adhesives. Dent Mater. 2011;27(1):1–16. doi:10.1016/j.dental.2010.10.016
5. Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982;16(3):265–273. doi:10.1002/jbm.820160307
6. Nishitani Y, Yoshiyama M, Donnelly AM, et al. Effects of resin hydrophilicity on dentin bond strength. J Dent Res. 2006;85(11):1016–1021. doi:10.1177/154405910608501108
7. Breschi L, Maravic T, Cunha SR, et al. Dentin bonding systems: from dentin collagen structure to bond preservation and clinical applications. Dent Mater. 2018;34(1):78–96. doi:10.1016/j.dental.2017.11.005
8. Huang B, Cvitkovitch DG, Santerre JP, Finer Y. Biodegradation of resin-dentin interfaces is dependent on the restorative material, mode of adhesion, esterase or MMP inhibition. Dent Mater. 2018;34(9):1253–1262. doi:10.1016/j.dental.2018.05.008
9. Feitosa VP, Sauro S, Zenobi W, et al. Degradation of adhesive-dentin interfaces created using different bonding strategies after five-year simulated pulpal pressure. J Adhes Dent. 2019;21(3):199–207. doi:10.3290/j.jad.a42510
10. Tjaderhane L. Dentin bonding: can we make it last? Oper Dent. 2015;40(1):4–18. doi:10.2341/14-095-BL
11. Zhou W, Liu S, Zhou X, et al. Modifying adhesive materials to improve the longevity of resinous restorations. Int J Mol Sci. 2019;20(3):723. doi:10.3390/ijms20030723
12. Breschi L, Mazzoni A, Nato F, et al. Chlorhexidine stabilizes the adhesive interface: a 2-year in vitro study. Dent Mater. 2010;26(4):320–325. doi:10.1016/j.dental.2009.11.153
13. Sabatini C, Scheffel DL, Scheffel RH, et al. Inhibition of endogenous human dentin MMPs by Gluma. Dent Mater. 2014;30(7):752–758. doi:10.1016/j.dental.2014.04.006
14. Vidal CM, Zhu W, Manohar S, et al. Collagen-collagen interactions mediated by plant-derived proanthocyanidins: a spectroscopic and atomic force microscopy study. Acta Biomater. 2016;41:110–118. doi:10.1016/j.actbio.2016.05.026
15. Yu J, Zhang Z, Guo R, Peng W, Yang H, Huang C. Epigallocatechin-3-gallate/nanohydroxyapatite platform delivery approach to adhesive-dentin interface stability. Mater Sci Eng C Mater Biol Appl. 2021;122:111918. doi:10.1016/j.msec.2021.111918
16. Seseogullari-Dirihan R, Tekbas Atay M, Pashley DH, Tezvergil-Mutluay A. Inhibitory effect of curcuminoid pretreatments on endogenous dentin proteases. Dent Mater J. 2018;37(3):445–452. doi:10.4012/dmj.2017-116
17. Lopez-Leon T, Carvalho EL, Seijo B, Ortega-Vinuesa JL, Bastos-Gonzalez D. Physicochemical characterization of chitosan nanoparticles: electrokinetic and stability behavior. J Colloid Interface Sci. 2005;283(2):344–351. doi:10.1016/j.jcis.2004.08.186
18. Akamatsu K, Chen W, Suzuki Y, et al. Preparation of monodisperse chitosan microcapsules with hollow structures using the SPG membrane emulsification technique. Langmuir. 2010;26(18):14854–14860. doi:10.1021/la101967u
19. Kashyap PL, Xiang X, Heiden P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int J Biol Macromol. 2015;77:36–51. doi:10.1016/j.ijbiomac.2015.02.039
20. Shao J, Wang B, Li J, Jansen JA, Walboomers XF, Yang F. Antibacterial effect and wound healing ability of silver nanoparticles incorporation into chitosan-based nanofibrous membranes. Mater Sci Eng C Mater Biol Appl. 2019;98:1053–1063. doi:10.1016/j.msec.2019.01.073
21. Arnaud TM, de Barros Neto B, Diniz FB. Chitosan effect on dental enamel de-remineralization: an in vitro evaluation. J Dent. 2010;38(11):848–852. doi:10.1016/j.jdent.2010.06.004
22. Ruan Q, Zhang Y, Yang X, Nutt S, Moradian-Oldak J. An amelogenin-chitosan matrix promotes assembly of an enamel-like layer with a dense interface. Acta Biomater. 2013;9(7):7289–7297. doi:10.1016/j.actbio.2013.04.004
23. Verlee A, Mincke S, Stevens CV. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr Polym. 2017;164:268–283. doi:10.1016/j.carbpol.2017.02.001
24. Abinaya B, Prasith TP, Ashwin B, Viji Chandran S, Selvamurugan N. Chitosan in surface modification for bone tissue engineering applications. Biotechnol J. 2019;14(12):e1900171. doi:10.1002/biot.201900171
25. Gu LS, Cai X, Guo JM, et al. Chitosan-based extrafibrillar demineralization for dentin bonding. J Dent Res. 2019;98(2):186–193. doi:10.1177/0022034518805419
26. Abaza L, Taamalli A, Nsir H, Zarrouk M. Olive tree (Olea europeae L.) leaves: importance and advances in the analysis of phenolic compounds. Antioxidants. 2015;4(4):682–698. doi:10.3390/antiox4040682
27. Goldsmith CD, Vuong QV, Sadeqzadeh E, Stathopoulos CE, Roach PD, Scarlett CJ. Phytochemical properties and anti-proliferative activity of Olea europaea L. leaf extracts against pancreatic cancer cells. Molecules. 2015;20(7):12992–13004. doi:10.3390/molecules200712992
28. Hadrich F, Mahmoudi A, Bouallagui Z, et al. Evaluation of hypocholesterolemic effect of oleuropein in cholesterol-fed rats. Chem Biol Interact. 2016;252:54–60. doi:10.1016/j.cbi.2016.03.026
29. Janahmadi Z, Nekooeian AA, Moaref AR, Emamghoreishi M. Oleuropein offers cardioprotection in rats with acute myocardial infarction. Cardiovasc Toxicol. 2015;15(1):61–68. doi:10.1007/s12012-014-9271-1
30. Hadrich F, Bouallagui Z, Junkyu H, Isoda H, Sayadi S. The alpha-glucosidase and alpha-amylase enzyme inhibitory of hydroxytyrosol and oleuropein. J Oleo Sci. 2015;64(8):835–843. doi:10.5650/jos.ess15026
31. Hadrich F, Garcia M, Maalej A, et al. Oleuropein activated AMPK and induced insulin sensitivity in C2C12 muscle cells. Life Sci. 2016;151:167–173. doi:10.1016/j.lfs.2016.02.027
32. Andreadou I, Iliodromitis EK, Mikros E, et al. The olive constituent oleuropein exhibits anti-ischemic, antioxidative, and hypolipidemic effects in anesthetized rabbits. J Nutr. 2006;136(8):2213–2219. doi:10.1093/jn/136.8.2213
33. De Marino S, Festa C, Zollo F, et al. Antioxidant activity and chemical components as potential anticancer agents in the olive leaf (Olea europaea L. cv Leccino.) decoction. Anticancer Agents Med Chem. 2014;14(10):1376–1385. doi:10.2174/1871520614666140804153936
34. Barbaro B, Toietta G, Maggio R, et al. Effects of the olive-derived polyphenol oleuropein on human health. Int J Mol Sci. 2014;15(10):18508–18524. doi:10.3390/ijms151018508
35. Karygianni L, Cecere M, Argyropoulou A, et al. Compounds from Olea europaea and Pistacia lentiscus inhibit oral microbial growth. BMC Complement Altern Med. 2019;19(1):51. doi:10.1186/s12906-019-2461-4
36. Shukla SK, Mishra AK, Arotiba OA, Mamba BB. Chitosan-based nanomaterials: a state-of-The-art review. Int J Biol Macromol. 2013;59:46–58. doi:10.1016/j.ijbiomac.2013.04.043
37. Muzzalupo I, Badolati G, Chiappetta A, Picci N, Muzzalupo R. In vitro antifungal activity of olive (Olea europaea) leaf extracts loaded in chitosan nanoparticles. Front Bioeng Biotechnol. 2020;8:151. doi:10.3389/fbioe.2020.00151
38. Shahravan A, Haghdoost AA, Adl A, Rahimi H, Shadifar F. Effect of smear layer on sealing ability of canal obturation: a systematic review and meta-analysis. J Endod. 2007;33(2):96–105. doi:10.1016/j.joen.2006.10.007
39. Marshall SJ, Bayne SC, Baier R, Tomsia AP, Marshall GW. A review of adhesion science. Dent Mater. 2010;26(2):e11–16. doi:10.1016/j.dental.2009.11.157
40. Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R. Dental adhesion review: aging and stability of the bonded interface. Dent Mater. 2008;24(1):90–101. doi:10.1016/j.dental.2007.02.009
41. Carrilho MR, Geraldeli S, Tay F, et al. In vivo preservation of the hybrid layer by chlorhexidine. J Dent Res. 2007;86(6):529–533. doi:10.1177/154405910708600608
42. Liu R, Fang M, Xiao Y, et al. The effect of transient proanthocyanidins preconditioning on the cross-linking and mechanical properties of demineralized dentin. J Mater Sci Mater Med. 2011;22(11):2403–2411. doi:10.1007/s10856-011-4430-4
43. Wang Y, Mei L, Zhao S, Xing X, Wu G. Effect of chitosan-oleuropein nanoparticles on dentin collagen cross-linking. Technol Health Care. 2022. doi:10.3233/THC-220195
44. Coban J, Oztezcan S, Dogru-Abbasoglu S, Bingul I, Yesil-Mizrak K, Uysal M. Olive leaf extract decreases age-induced oxidative stress in major organs of aged rats. Geriatr Gerontol Int. 2014;14(4):996–1002. doi:10.1111/ggi.12192
45. Rosillo MA, Sanchez-Hidalgo M, Gonzalez-Benjumea A, Fernandez-Bolanos JG, Lubberts E, Alarcon-de-la-lastra C. Preventive effects of dietary hydroxytyrosol acetate, an extra virgin olive oil polyphenol in murine collagen-induced arthritis. Mol Nutr Food Res. 2015;59(12):2537–2546. doi:10.1002/mnfr.201500304
46. Demeule M, Brossard M, Page M, Gingras D, Beliveau R. Matrix metalloproteinase inhibition by green tea catechins. Biochim Biophys Acta. 2000;1478(1):51–60. doi:10.1016/S0167-4838(00)00009-1
47. Bedran-Russo AK, Pauli GF, Chen SN, et al. Dentin biomodification: strategies, renewable resources and clinical applications. Dent Mater. 2014;30(1):62–76. doi:10.1016/j.dental.2013.10.012
48. Yesilyurt C, Bulucu B. Bond strength of total-etch and self-etch dentin adhesive systems on peripheral and central dentinal tissue: a microtensile bond strength test. J Contemp Dent Pract. 2006;7(2):26–36. doi:10.5005/jcdp-7-2-26
49. Favetti M, Schroeder T, Montagner AF, Correa MB, Pereira-Cenci T, Cenci MS. Effectiveness of pre-treatment with chlorhexidine in restoration retention: a 36-month follow-up randomized clinical trial. J Dent. 2017;60:44–49. doi:10.1016/j.jdent.2017.02.014
50. Collares FM, Rodrigues SB, Leitune VC, Celeste RK, Borba de Araujo F, Samuel SM. Chlorhexidine application in adhesive procedures: a meta-regression analysis. J Adhes Dent. 2013;15(1):11–18. doi:10.3290/j.jad.a28732
51. Tezvergil-Mutluay A, Agee KA, Hoshika T, et al. The requirement of zinc and calcium ions for functional MMP activity in demineralized dentin matrices. Dent Mater. 2010;26(11):1059–1067. doi:10.1016/j.dental.2010.07.006
52. Sadek FT, Braga RR, Muench A, Liu Y, Pashley DH, Tay FR. Ethanol wet-bonding challenges current anti-degradation strategy. J Dent Res. 2010;89(12):1499–1504. doi:10.1177/0022034510385240
53. Blackburn RS, Harvey A, Kettle LL, Manian AP, Payne JD, Russell SJ. Sorption of chlorhexidine on cellulose: mechanism of binding and molecular recognition. J Phys Chem B. 2007;111(30):8775–8784. doi:10.1021/jp070856r
54. Tezvergil-Mutluay A, Agee KA, Uchiyama T, et al. The inhibitory effects of quaternary ammonium methacrylates on soluble and matrix-bound MMPs. J Dent Res. 2011;90(4):535–540. doi:10.1177/0022034510389472
55. Misra DN. Interaction of chlorhexidine digluconate with and adsorption of chlorhexidine on hydroxyapatite. J Biomed Mater Res. 1994;28(11):1375–1381. doi:10.1002/jbm.820281116
56. Di Hipolito V, Rodrigues FP, Piveta FB, et al. Effectiveness of self-adhesive luting cements in bonding to chlorhexidine-treated dentin. Dent Mater. 2012;28(5):495–501. doi:10.1016/j.dental.2011.11.027
57. Xiong J, Shen L, Jiang Q, Kishen A. Effect of crosslinked chitosan nanoparticles on the bonding quality of fiber posts in root canals. J Adhes Dent. 2020;22(3):321–330. doi:10.3290/j.jad.a44555
58. Sano H, Shono T, Takatsu T, Hosoda H. Microporous dentin zone beneath resin-impregnated layer. Oper Dent. 1994;19(2):59–64.
59. Sano H, Takatsu T, Ciucchi B, Horner JA, Matthews WG, Pashley DH. Nanoleakage: leakage within the hybrid layer. Oper Dent. 1995;20(1):18–25.
60. Sano H, Yoshiyama M, Ebisu S, et al. Comparative SEM and TEM observations of nanoleakage within the hybrid layer. Oper Dent. 1995;20(4):160–167.
61. Okuda M, Pereira PN, Nakajima M, Tagami J. Relationship between nanoleakage and long-term durability of dentin bonds. Oper Dent. 2001;26(5):482–490.
62. Bertolo MVL, Guarda MB, Fronza BM, et al. Electric current effects on bond strength, nanoleakage, degree of conversion and dentinal infiltration of adhesive systems. J Mech Behav Biomed Mater. 2021;119:104529. doi:10.1016/j.jmbbm.2021.104529
63. Cui N, Hu M, Khalil RA. Biochemical and biological attributes of matrix metalloproteinases. Prog Mol Biol Transl Sci. 2017;147:1–73.
64. Bertassoni LE, Orgel JP, Antipova O, Swain MV. The dentin organic matrix - limitations of restorative dentistry hidden on the nanometer scale. Acta Biomater. 2012;8(7):2419–2433. doi:10.1016/j.actbio.2012.02.022
65. Gu L, Mazzoni A, Gou Y, et al. Zymography of hybrid layers created using extrafibrillar demineralization. J Dent Res. 2018;97(4):409–415. doi:10.1177/0022034517747264
66. Gendron R, Grenier D, Sorsa T, Mayrand D. Inhibition of the activities of matrix metalloproteinases 2, 8, and 9 by chlorhexidine. Clin Diagn Lab Immunol. 1999;6(3):437–439. doi:10.1128/CDLI.6.3.437-439.1999
67. Hebling J, Pashley DH, Tjaderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res. 2005;84(8):741–746. doi:10.1177/154405910508400811
68. Manfro AR, Reis A, Loguercio AD, Imparato JC, Raggio DP. Effect of different concentrations of chlorhexidine on bond strength of primary dentin. Pediatr Dent. 2012;34(2):e11–15.
69. Bravo C, Sampaio CS, Hirata R, Puppin-Rontani RM, Mayoral JR, Giner L. Effect of 2 % Chlorhexidine on dentin shear bond strength of different adhesive systems: a 6 months evaluation. Int J Morphol. 2017;35(3):1140–1146. doi:10.4067/S0717-95022017000300052
70. Jowkar Z, Shafiei F, Asadmanesh E, Koohpeima F. Influence of silver nanoparticles on resin-dentin bond strength durability in a self-etch and an etch-and-rinse adhesive system. Restor Dent Endod. 2019;44(2):e13. doi:10.5395/rde.2019.44.e13
71. De Munck J, Luehrs AK, Poitevin A, Van Ende A, Van Meerbeek B. Fracture toughness versus micro-tensile bond strength testing of adhesive-dentin interfaces. Dent Mater. 2013;29(6):635–644. doi:10.1016/j.dental.2013.03.010
72. El Gezawi M, Haridy R, Abo Elazm E, Al-Harbi F, Zouch M, Kaisarly D. Microtensile bond strength, 4-point bending and nanoleakage of resin-dentin interfaces: effects of two matrix metalloproteinase inhibitors. J Mech Behav Biomed Mater. 2018;78:206–213. doi:10.1016/j.jmbbm.2017.11.024
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.