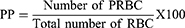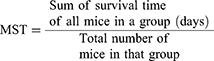Back to Journals » Infection and Drug Resistance » Volume 14
Evaluation of Antiplasmodial Activity of Hydroalcoholic Crude Extract and Solvent Fractions of Zehneria scabra Roots Against Plasmodium berghei in Swiss Albino Mice
Authors Nureye D , Tekalign E, Fisseha N , Tesfaye T, Woldeselassie Hammeso W
Received 29 April 2021
Accepted for publication 10 June 2021
Published 6 July 2021 Volume 2021:14 Pages 2583—2596
DOI https://doi.org/10.2147/IDR.S314262
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Dejen Nureye,1 Eyob Tekalign,2 Nebeyi Fisseha,1 Tarekegn Tesfaye,1 Workineh Woldeselassie Hammeso1
1Department of Pharmacology and Toxicology, School of Pharmacy, College of Medicine and Health Sciences, Mizan-Tepi University, Mizan-Aman, Southwest Ethiopia; 2Department of Medical Laboratory sciences, College of Medicine and Health Sciences, Mizan-Tepi University, Mizan-Aman, Southwest Ethiopia
Correspondence: Workineh Woldeselassie Hammeso Email [email protected]
Background: Since drug resistance makes controlling malaria parasites a major challenge, these pioneering researchers explore and discover new novel drugs from a variety of sources. As a result, this study aimed to assess the anti-plasmodial activity of hydroalcoholic crude extract and solvent fractions of Zehneria scabra roots in mice infected with Plasmodium berghei.
Methods: The antimalarial activity and safety profile of Zehneria scabra extracts were tested in a mouse model using four-day suppressive, prophylactic, and rane’s tests against chloroquine-sensitive Plasmodium berghei. Mice were divided into five groups at random: group I received distilled water (10 mL/kg), group II, III, and IV received 200, 400, and 600 mg/kg of the extract, respectively, and group V received chloroquine (25 mg/kg). The antimalarial activity of the extract was determined using parasitemia levels, survival time, rectal temperature, and weight variation.
Results: At all dose levels, the crude extract and solvent fractions of Zehneria scabra showed significant (p< 0.05 to p< 0.001) chemosuppression, with the crude extract and butanol fraction showing the highest chemosuppression (73.09% and 74.09%, respectively). Apart from suppressing parasitemia, the extract also increased survival time and secured packed cell volume reduction substantially (p< 0.05 to p< 0.001), while the crude extract had no significant impact on body weight or rectal temperature reduction in four-day suppressive and prophylactic models.
Conclusion: The result designated that Zehneria scabra is endowed with significant antimalarial activity. These results thus support the traditional use of Zehneria scabra, for the treatment of malaria.
Keywords: malaria, Plasmodium berghei, Zehneria scabra, Swiss albino mice
Introduction
Malaria is a life-threatening disease caused by Plasmodium parasites transmitted by female Anopheles mosquito bites. It affects over two hundred million people and kills over seven hundred thousand people per year. Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, Plasmodium malariae, and Plasmodium knowlesi are the five Plasmodium species known to cause malaria in humans. P. falciparum and P. vivax, among Plasmodium species, are the most virulent agents of human malaria, causing substantial global morbidity and mortality.1
Owing to the rising number of cases of resistance to the majority of currently available medications, controlling malaria parasites is becoming increasingly difficult. Despite decades of intensive research and development efforts, there is currently no commercially available malaria vaccine.2 As a result, chemotherapeutic agents are still in high demand for the complete treatment of malaria, and the rising rate of resistance means that finding new antimalarial drugs is a top priority.1
Aside from clinically approved medications, various plants have long been used to prevent, treat, and/or relieve the symptoms of malaria. Medicinal plants were used by more than 80% of Africans, including Ethiopians, to meet their basic healthcare needs. The majority of Ethiopian land is infected with malaria, and 68% of the population lives in such areas. Ethiopia is a malaria-endemic and impoverished region. Medicinal plants are the key sources of the drug for different health ailments in Ethiopian traditional medical practices, including malaria, due to cultural acceptability, low cost, and availability. In Ethiopia, more than 200 medicinal plants are used to treat malaria. However, little is known about the purity, consistency, and efficacy of these medicinal plants.3 Zehneria Scabra L.F. Sond is an essential medicinal plant that belongs to the Cucurbitaceae family and is well known for its medicinal value in herbal folklore. It is known in Amharic as Areg-resa, in Ge’ez as Etse-sabieq, in Afaan Oromo as Daaymii, in Tigrigna as Hafaflo, and in Gedeo as Kiete.4–7
The antimicrobial activity of the ethanol and ethyl acetate root extracts of Z. scabra against the most common bacterial pathogens, Staphylococcus aureus and Escherichia coli, has been confirmed by a recent report.8 The antifungal properties of the ethanolic tuber extract were also demonstrated against five forms of fungi, including Aspergillus fumigatus, Aspergillus niger, Aspergillus flavus, Mucor indicus, and Candida albicans.9 Furthermore, the antidiarrheal, antisecretory,10 antimalarial,8 anti-nociceptive, and anti-inflammatory properties of the 80% methanolic leaves extract have been demonstrated.11 Furthermore, the plant is extremely valuable ethnobotanically. It is used to treat fever and stomach pain in the Tamil people of southern India and Sri Lanka, for example, using root and leaf extracts.12 The leaves are crushed and treated for common colds by the Marakwet Community in Kenya, and the whole plant is boiled with other herbs to treat cancer.13
In Ethiopia, Z. scabra is one of the traditional medicinal plants commonly used for the treatment of a number of illnesses. By Amhara ethinic group of Bahir Dar zuria district for example, the leave juice is used to treat fever and headache.14 Peoples of Kilte Awulaelo district of Tigray use the leaves for treatment of paralysis, michi, and external wounds, while the root is used for abdominal pain and ascariasis5. Its flowers have been combined with other herbals for topical treatment of alopecia, wounds, and eczema.15 People in the Guangua Sub district of the Agew-Awi zone of the Amhara Regional State use the root to treat anemia.16 Traditional healers in Southwestern Ethiopia said that the root part of the plant had antimalarial properties, but this has not been proved scientifically.15
Nevertheless, the effectiveness, consistency, and efficacy of Z. scabra for malaria treatment have not been scientifically proven. To promote its traditional use in the treatment of malaria, scientific evaluation of its anti-malarial operation in a rodent malarial model is justified. The aim of this study was to determine the anti-malarial activity of Z. scabra root hydroalcoholic crude extract and solvent fractions against Plasmodium berghei infection in mice.
Materials and Methods
Distilled water, 2% tween 80, absolute methanol, 80% methanol, chloroform, regular isotonic saline, trisodium citrate, oil immersion, geimsa stain, and chloroquine were among the chemicals used. Many of the reagents were of analytical quality. In addition, 1mL insulin syringes with needles, scissor, feeding tube, electronic balance, gloves, and a light microscope were used in this research.
Plant Material
Z. scabra root was collected in its natural habitat near Sheko woreda, Bench-Sheko zone, South West Ethiopia, about 581 kilometers from Addis Ababa. A taxonomist at the National Herbarium, College of Natural and Computational Sciences, Addis Ababa University, identified and authenticated the plant specimens, and a voucher specimen was held there for future reference with the voucher number TT 001/2019.
Experimental Animals and Rodent Parasite
P. berghei (ANKA strain) sensitive to chloroquine was obtained from Ethiopian Public Health Institute. Then, until 30–37% of parasitemia level was attained, the parasites were maintained by Serial passage of blood from infected mice to non-infected ones every week.20
Female Swiss albino mice (23−32g), obtained from the Department of Pharmacology and Toxicology Animal House, Mizan-Tepi University, Female mice were used for the acute oral toxicity and male mice were used to antiplasmodial activity testing. All experimental animals were housed under standard environmental conditions of temperature at 22–24°C under a 12 hour dark–light cycle and allowed free access to drinking water and standard pellet diet. Animal attendants maintained hygiene by constant cleaning and removal of feces from cages every 4 days. Before the actual experiment, animals were acclimatized for 1 week to the experimental environment. The care and handling of the experimental animals was according to the national research council guide for the care and use of laboratory animals.17
Preparation of Plant Extract
To clear dirt and soil from Z. scabra roots, tap water was used and gauze was used to clean them. The sample was then air-dried in the shade before being pounded with a sterile pestle and mortar into a coarse powder. The roots of Z. scabra were then extracted using a cold maceration technique in an Erlenmeyer flask for three days at room temperature using an 80% methanol solution. The extraction process was aided by shaking at 120 revolutions per minute with an orbital shaker (Bibby Scientific Limited Stone Staffo Reshire, UK). Gauze was used to filter the extract, and then Whatman filters paper No. 1 was used to filter it (Maidstone, UK). To improve the yield, the residue was re-macerated for the second and third time with the same amount of fresh solvent. Under reduced pressure, the filtrates were mixed and concentrated using a Rotary evaporator (Buchi Rotavapor R 200, Flawil, Switzerland). After that, all extracts were dried and concentrated in a dry oven (Okhla industrial region, India) at a temperature of not more than 40°C. Finally, the extract was moved to an amber glass bottle and stored at −20°C until the experiment was completed. Fractionation of the crude hydroalcoholic extract was performed using solvents of varying polarity. The chloroform, n-butanol, and aqueous fractions’ percentage yields were transferred to an amber glass bottle and stored at −20°C before usage.
Acute Oral Toxicity Test
The acute oral toxicity test of Z. scabra root was done based on the limit test recommendations of Organization for Economic Co-operation and Development (OECD) No 425 Guideline18 Healthy young adult female Swiss albino mice (age of 8–12 weeks, weighting 20–30 g) were employed for this test. On day one, a Swiss albino mouse fasted for 3–4 h was given 2000 mg/kg of the extract orally. The mouse was then kept under strict observation of physical or behavioral changes for 24 h, with special attention during the first 4 h. Following the results from the first mouse, the other four mice were recruited and fasted for 3–4 h and administered a single dose of 2000 mg/kg and were observed in the same manner. These observations continued for further 14 days for any signs of overt toxicity.
Grouping and Dosing of Animals
A total of thirty mice were divided into five groups, each with six mice. Group I mice were given the vehicle (distilled water, 10 mL/kg), group II mice were given the regular medication (chloroquine (Addis Pharmaceuticals Factory, Ethiopia), 25 mg/kg, which acted as a positive control), and group III, IV, and V mice were given 200, 400, and 600 mg/kg crude extract or solvent fractions, respectively.
Inoculum Preparation
The parasitaemia levels of donor mice were first determined by cutting a 0.5 to 1mm segment from the mice’s tail with scissor.19 To infect the mice, a donor mouse with a parasitaemia of 30–37% was sacrificed by head blow, and blood was collected through the jugular vein incisions into a test tube containing 3.8% trisodium citrate (BDH chemicals, England) added as an anticoagulant.20
The blood was diluted in normal saline until each 0.2 mL suspension contained approximately 1×107 infected red blood cells (IRBCs).21 The dilution was calculated based on the donor mice’s parasitaemia and the standard mice’s RBC count, with 1mL blood containing 5×107 infected erythrocytes.19,22 As a result, each mouse was inoculated intraperitoneally with 0.2 mL infected blood containing around 1×107 parasitized RBCs from P. berghei.
Anti-Malarial Activity Test
Four-Day Suppressive Test
The crude extract and solvent fractions were tested for schizonticidal activity on early infection using the method defined by Peters 1975.23 On the first day (D0) 2 hours after infection, three treatment groups were given different concentrations of the extract, one with the vehicle used for reconstitution of the extract, and the other with the normal medication chloroquine orally.
For the next four days, the treatment was repeated every day. Blood was extracted from each mouse’s tail on the fifth day (D4) using clean, non-greasy slides and thin films made as required, which were then allowed to air-dry. The films were fixed with a few drops of methanol, air-dried for 15 to 20 minutes, and stained for 15 minutes with 10% Giemsa at pH 7.2. The stain was removed and the slides were allowed to air dry before being examined under a light microscope with the oil immersion objective for blood parasite suppression and parasitaemia microscopically with the X100 objective for parasitaemia.
Each mouse's percentage parasitaemia was determined on day 5 (D4),while body weight, rectal temperature and packed cell volume (PCV) were reported just before infection and at day 5 (D4) of the experiment..
Rane’s (Curative) Test
The crude extract’s curative capacity was assessed using the method defined by Ryley and Peters 1970.24 On the first day, thirty mice were chosen, grouped, and intraperitoneally injected with standard inoculums of 1×107 P.berghei infected red cells. The groups were treated with their respective doses three days (72 hours) after infection, as defined in grouping and dosing of animals. The treatment was continued every day until the seventh day, when blood was drawn from each mouse’s tail and thin films were made. Each mouse’s weight, temperature, and parasitemia level were reported just before the first dose and daily until day 8 (D7), while PCV was recorded just before the first extract dose and at the end of the experiment.
prophylactic test
The residual infection technique described by Peters was used to assess the crude extract’s prophylactic activity 1965.25 Weighing adult male mice, they were divided into five classes of six mice each. For four days, three groups were given different concentrations of the extract, one with only the vehicle used to reconstitute the extract, and the other with the standard drug chloroquine (D0-D3). Both mice were infected with Plasmodium on the fifth day (D4) and monitored for 72 hours. Each mouse’s weight, temperature, and parasitemia level were measured just before inoculation and throughout the experiment until day 8 (D7), while PCV was measured just before inoculation and at the end.
Packed Cell Volume Measurement
PCV was used to predict the efficacy of crude extracts and solvent fractions in preventing hemolysis caused by parasitemia progression in malaria patients. It is a measurement of the proportion of RBCs to plasma in whole blood, and it is calculated using the formula below:26
Parasitemia and Survival Time Measurement
Counting the number of parasitized red blood cells (PRBC) out of the total erythrocytes in random fields of the microscope yielded the percentage parasitemia. For each mouse, two stained slides were examined.27 For each slide, six fields were counted, an average was taken, and percentage parasitemia (PP) was calculated using the formula below.28
The crude extract and solvent fractions were compared to the controls in terms of parasitemia suppression. The formula below was used to measure the percent parasitemia suppression:28
Throughout the follow-up period (28 days), the mice were observed daily, and the number of days from the time of inoculation to death was reported for each mouse in the treatment and control groups. The mean survival time (MST) for each category was calculated using the formula below:29
Body Weight and Rectal Temperature Measurement
A sensitive digital weighing balance was used to weigh each mouse in a group, and a digital rectal thermometer was used to measure rectal temperature. After that, the percentage differences in their mean values before and after treatment were measured.
Phytochemical Screening
Standard protocols were used to test 80% methanolic root extract and solvent fractions of Z.scabra for the presence or absence of secondary metabolites such as alkaloids, steroidal compounds, phenolic compounds, tannins, saponins, flavonoids, cardiac glycosides, and anthraquinones.30,31
Statistical Analysis
For each treatment group, the study’s results were expressed as mean ± SEM (standard error of mean). Using Windows SPSS version 22, data on parasitaemia levels, changes in body weight, PCV, and survival time were analyzed. The discrepancies between the control and treatment groups were analyzed using a one-way ANOVA followed by a Tukey post hoc examination. If P values at 95% confidence intervals were less than 0.05, the difference was deemed statistically important.
Results
Phytochemical Characterization
The percentage yield of Z.scabra and the chloroform, n-butanol, and aqueous fractions was 48 gram (9.6%), 8.61 gram (26.91%), 10.89 gram (34.03%), and 12.50 gram (39.06%), respectively. The existence of diverse secondary metabolites such as tannins, flavonoids, terpenoids, and alkaloids was discovered in an 80% methanolic extract of Z. scabra’s root (Table 1). Tannins were found in any fraction, but flavonoids and steroids were only found in the chloroform fraction. Tannins, terpenoids, and alkaloids were found in both the n-butanol and aqueous fractions, while saponins were only found in the aqueous fraction.
 |
Table 1 Preliminary Phytochemical Screenings of the Hydroalcoholic Extract and solvent fractions of Zehneria scabra Roots |
Acute Oral Toxicity Test
There were no signs of toxicity in the 80% methanolic extract of Z. scabra root, such as convulsions, hair erection, diarrhea, or restlessness. Furthermore, no deaths were observed in all mice given the full dose of the extract, up to 2000 mg/kg, during the 14-day toxicity test. As a result, the extract’s median lethal dose (LD50) may be greater than 2000 mg/kg.
Effect of Crude Extract and Solvent Fractions in the 4-Day Suppressive Test
Table 2 shows the results of an 80% methanolic extract of Z. scabra root. In comparison to the CON, treatment with crude extract and its fractions with all doses of the root extract resulted in substantial (p<0.05 to p<0.001) parasitemia suppression. At a dosage of 600mg/kg, the n-butanol fraction displayed the greatest suppression (74.33%, p< 0.001). Similarly, the crude extract at 600 mg/kg demonstrated substantial chemosuppression (73.08%, P <0.001). The lowest dose of the aqueous fraction, on the other hand, demonstrated the least chemosuppression (16.43%, p<0.001). At 600 mg/kg, n-butanol (74.33%, p<0.001) > chloroform (68.50%, p<0.001) > aqueous (37.72%, p<0.001) were the solvent fractions with the greatest percent parasitemia suppression. When the effects of the crude extract and its solvent fractions were compared, the parasitemia suppression induced by the lowest dose (200 mg/kg) and middle dose (400 mg/kg) were significantly (p<0.001) lower than the effect produced by the 600 mg/kg dose of the crude extract and its solvent fractions.
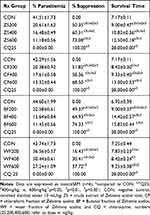 |
Table 2 Antimalarial Activity Test of the Hydroalcoholic Crude Extract and Solvent Fractions of Zehneria Scabra Roots, Against P. Berghei in the Four-Day Suppressive Test |
The effect of the crude extract on survival time showed that as the dose of the extract increased, there was a pattern of substantial (p<0.01 to p<0.001) extension in survival day. The n-butanol fraction showed substantial extension in MST at all doses (p<0.01 to p<0.001), as did the chloroform fraction at middle (p<0.01) and high dose (p<0.001), as well as the aqueous fraction at higher dose (p<0.001).
Furthermore, when the doses of crude extract were compared, it was discovered that ZS400 and ZS600 significantly increased the survival time of mice (p<0.01 and p<0.001) when compared to the ZS200 treated community. Similarly, mice given 600 mg/kg doses lived longer than mice given 200 mg/kg doses in all fraction-treated groups (p<0.001). The crude extract and chloroform fraction, on the other hand, failed to defend against body weight loss and rectal temperature loss at all doses as compared to their respective CON. In comparison to their respective control group, mice treated with ZS600 of both n-butanol and aqueous fraction significantly (p<0.05 to p<0.001) avoid loss of body weight and rectal temperature (Table 3). In comparison to the negative control, the crude extract at 400 and 600 mg/kg doses significantly (p<0.05) prevents the decrease in PCV (Figure 1). Similarly, the n-butanol fraction at 600 mg/kg dose prevented the decrease in PCV substantially (p<0.05) as compared to CON.
 |
Table 3 Body weight and Rectal Temperature P. Berghei Infected Swiss Albino Mice treated with Hydroalcoholic Crude Extract and Solvent Fraction of Zehneria scabra Roots in the 4-day suppressive Test |
Effect of Crude Extract in the Curative Test
The parasitemia level was increased on the 4th and 5th days with a 200 mg/kg dose of the crude extract, as shown in (Table 4), despite the fact that the first dose was given on the 3rd day. The parasitemia level started to decline on the 6th day for all three doses of the crude extract and continued to decrease during the treatment days until the third dose was administered on the 5th day. The parasitemia in the negative control group, on the other hand, increased daily from day 3 to day 7. On the 6th and 7th days, all doses of the crude extract had a substantial (p<0.001) curative effect as compared to the negative control. As compared to the 200 mg/kg doses of crude extract (p<0.001), the 600 mg/kg dose of crude extract demonstrated substantial chemosuppression. The crude extract significantly (p<0.05 to p<0.001) improved survival time as compared to the negative control at all doses, according to survival time study. In addition, the crude extract at 600 mg/kg dose showed preventive effect in weight reduction in infected mice compared to negative control. However, as opposed to the negative regulation, all doses of crude extract substantially (p<0.05 to p<0.001) protect the decrease in rectal temperature (Figure 2). As compared to the negative regulation, the crude extract doses of 400 and 600 mg/kg significantly protected the decrease in PCV (p< 0.05 and p<0.01). Except between 200 mg/kg and 600 mg/kg of crude extract, there were no significant differences in preventing PCV reduction among the test groups (Figure 2).
 |
Table 4 Antimalarial Activity Test of the Hydroalcoholic Crude Extract of Zehneria Scabra Roots Against P. Berghei in the Curative Test |
Effect of Crude Extract in the Prophylactic Test
Prophylactic testing found that all doses of crude extract had dose-dependent chemoprophylactic activity against P. berghei-infected mice (Table-5). When compared to the negative control, the crude extract significantly reduced parasitemia (p<0.001) at all doses (200mg/kg, 400mg/kg, and 600mg/kg). When the amount of chemosuppression among the extract doses was compared, ZS600 had significantly more suppression (p<0.001) than ZS200. Furthermore, when compared to all doses of the crude extract, thestandard drug displayed a strong chemosuppression (p< 0.001). Moreover, the normal drug showed a heavy chemosuppression as compared to all doses of the crude extract (p< 0.001). Furthermore, as opposed to the negative control, the middle and higher doses of the extract significantly (p<0.01 and p<0.001) extended survival time, while the lower dose of extract had no meaningful difference. The extract, on the other hand, was unable to prevent the reduction of all parameters (weight, rectal temperature, and PCV) at any dose, with the exception of ZS600, which significantly (p<0.5) reduced only rectal temperature when compared to the negative control. There was no substantial difference between the doses in terms of preventing temperature, weight, or PCV reductions, according to several comparisons (Figure 3).
 |
Table 5 Antimalarial Activity Test of the Hydroalcoholic Crude Extract of Zehneria Scabra Roots Against P. Berghei in the Prophylactic Test |
Discussion
Since malaria is a major public health threat in developing countries, it is essential to do extensive researches to be directed towards the search for new anti-malarial drugs.32 Therefore, it is reasonable that such studies should be done in screening claimed plants to provide a potential lead for novel antimalarial drug development. The use of chloroquine in invivo studies is highly recommended, and P. berghei is recognized as a well-known parasite for in vivo research.19 The four-day suppressive test is standard and widely utilized rodent model for screening antimalarial activities.33 Four-day suppressive test using P. berghei infected mice provides a pre-clinical indication of potential bioactivity of the test sample.23 In both rane’s and four-day suppressive test, determination of percent inhibition of parasitemia is the most reliable parameter.27
Methanol is a universal solvent in solvent extraction for phytochemical investigation.34 For the purpose of extracting components, which are soluble in water such as polysaccharides, polypeptides, tannins, mixtures of solvents are quite common; the most common one being alcohol and water. These mixtures of solvents not only extract the above mentioned constituents but also most of the polar and non-polar components of the plant. Therefore, in this work, 80% methanol was chosen to serve as solvent mixture. It has been reported that alcoholic extracts of Z. scabra exhibited antibacterial and in vitro antimalarial activities.11 Oral dosing of the extracts and fractions was used, to replicate the ethno-medical method of administration and the likely route during clinical evaluation.35
The antimalarial ability of hydroalcoholic crude extract and solvent fractions of Z. scabra roots against P. berghei in Swiss albino mice was investigated in this research. The root of Z. scabra was administered at all tested doses of crude extract and solvent fractions and resulted in substantial parasite suppression in the current sample. For ZS600, the maximum suppression (73.08%) was reported. The results of this analysis agree with those of a study performed with crude extract of Aloe sp.36 which found 73.09% chemosuppression, but are higher than those found in crude extracts of Gardenia ternifolia37 and A. pirottae,38 which found 59.25 and 46.97% chemosuppression, respectively. As a result, it is reasonable to conclude that the plant has antiplasmodial properties, and it also supports a previous study that found Z. scabra to have in vitro antimalarial activity against P. falciparum.11 When a compound suppresses parasitemia by 30% or more, it is called active,39 which supports the results of this research. Furthermore, the crude extract improved the survival time of parasite-infected mice in a dose-dependent manner, with the greatest effect occurring at the maximum dose administered. This is comparable to the activities of Nuxia congesta and Melia azedarach in previous studies.40,41
The crude extract was fractionated using three different solvents (n-butanol, chloroform, and water), each of which had a different polarity index, and the suppressive test on early Plasmodium infection was performed. The chemosuppressive activity of the chloroform, n-butanol, and water fractions of Z.scabra was found to be dose dependent; the higher (74.33%) chemosuppressive activity of the n-butanol fraction may be the product of a single or synergistic effect of secondary metabolites such as flavonoids, alkaloids, and tannins found in fraction2 The result of this study is in line with other invivo antimalarial studies,41–43 where n-butanol fraction of Ajuga integrifolia, Dodonaea angustifolia and Asparagus africanus significantly showed chemosuppression in treated mice compared to negative control. However, as opposed to the chloroform and butanol fractions, the water fraction displayed fewer parasitemia suppression, implying that the various types and concentrations of secondary metabolites in the fractions.40 The crude extract of Z. scabra has significantly increased the survival time of the parasite-infected mice in dose-dependent manner and the maximal effect was achieved at the highest dose. This is comparable with the activities of the previous reports exhibited by Nuxia congesta, melia azedarach and Polyalthial ongifolia.40,41,44 On the other hand, the fractions also extended survival time in a non-dose dependent manner in treated mice; particularly the higher dose of both n-butanol and chloroform fraction and the middle dose of butanol fraction but only the higher dose of the water fraction was capable of significantly increasing survival time compared to negative control. The more active antimalarial secondary metabolites quantitatively and/or qualitatively may reside inside semi-polar and non-polar solvents, which indicates that relative variance in chemosuppressive and prolonging survival time behavior may be due to variation in phytochemical contents. It has been documented that different secondary metabolites found in chloroform and n-butanol have been linked to antimalarial activity.41,45 These negligible variations in parasitemia suppression activity may be due to concentration differences of the right secondary metabolites responsible for chemosuppressive activity.45
This study has revealed that the crude extract and chloroform fraction of Z. scabra did not prevent the drop of weight and temperature. The failure could be attributable to active phytochemical constituent the plant contains such as: saponins and tannins that are known to form complexion reaction with proteins; saponin-protein or protein-tannin complexes; these complexes in turn block protein utilization by inhibiting digestive enzymes; this may have reduced nutrient utilization and reduced food intake. It could also be associated with the amount of hypothermic components present in the crude extracts and chloroform fractions of the test plant46,47. Furthermore it could be attributed to the inability of the extracts and chloroform fractions to completely clear the parasites from the body of the mice other than reducing to different levels.29,37 This is comparably similar with the findings of other plants such as Leonoti socymifolia,47 Calpurnia aurea48 and D. angustifolia extracts,40 where these plants were unable to prevent the drop of weight and temperature, although they showed significant parasitemia suppression. Indeed, it has been reported that the animals those infected with plasmodium showed a decline in food consumption and body weight during the course of the experiment.37 Among the fractions, all doses of n-butanol significantly prevent loss in body weight and rectal temperature, but only highest dose of aqueous fractions showed a significant increment in body weight and prevention of loss in rectal temperature associated with parasitemia in parasite-infected animals. This activity might have been resulted from the overall improvement in PCV and parasite clearance among treated mice.49
In this study it was noted that the crude extract of Z. scabra at dose of 400 and 600 mg/kg showed significant protection in PCV in four-day suppressive test. Among the solvent fractions only butanol fractions at dose of 600 mg/kg significantly prevent reduction in PCV as compared to negative control. This finding is in line with other findings in which the higher doses better protected PCV as compared to the lower dose.50 This protection effect might have been resulted from significant parasite suppression and RBC protective effect induced by active constituent(s) in the administered doses of the extract since the increase in blood parameters corresponded to the decreased parasites load.51
The effect of the crude extract on existing parasite infection was further evaluated using Rane’s (Curative) test. The parasitemia level started to decrease after the third dose was administered on the fifth day. Despite the lack of cure, all doses of the crude extract showed a substantial (p<0.001) dose-dependent chemosupression on the 6th and 7th days as compared to the negative control. While this may mean that the plant had a fast onset of action,50 the overall lower curative than suppressive effect may be due to the plant’s limited period of action, which would be insufficient to control the parasites’ exponential growth in an existing infection.21 It is desirable that the plants exhibit both suppressive and curative effects.52 Only the maximum dose resulted in a substantial increase in survival time, which is likely due to the higher parasitemia reduction at this dose. This finding is consistent with other findings, which showed a dose-dependent decrease in percent parasitemia from the 5th to 7th day compared to the negative control, as well as a significantly improved survival time at the maximum dose of the crude extract.53
In spite of higher parasite suppression, leaf crude extract of Z. scabra prevent against body weight loss and reduction in temperature in curative test than in 4-day test. This indicates the involvement of other factors for these reductions beyond malaria infection. For example, the weight loss might be due to catabolic activity on stored lipids or anorexogenic effect that may have led to decreased food intake due to the presence of appetite-suppressant metabolites in the crude extract.21 This evidence is further supported by the effect of Myrica salicifolia that the entire doses of the crude extract did not prevent body weight loss in the 4-day test, even though there was a significant suppression of parasitemia.54
The loss of RBCs due to hemolysis of malaria-infected RBCs, degradation of uninfected cells in the spleen, erythropoietic suppression, dyserythropoiesis, and oxidative stress, which increases membrane fragility, causes a drop in PCV. Therefore, plants with antimalarial activity are expected to avoid a drop in PCV by preventing hemolysis and RBC destruction.27,28 In this study, the crude extract of Z. scabra showed significant protection in PCV reduction at higher doses in curative test. This could be as a result of antagonistic effect on oxidative stress and inflammation as well as the destructive anti-plasmodial effect of the extract against the parasitized red blood cell and the parasite in established infection, thereby sustaining the availability of new red blood cells produced and released from bone marrow. This finding is in agreement with other reported findings where the higher doses better protected PCV from decreasing in as compared to lower doses.50
The ability of the extract to prevent parasitemia proliferation was investigated in this study using a prophylactic test model. The results of this test showed that the extract has dose-dependent chemoprophylactic activity at all doses. The crude extract significantly reduced parasitemia at all doses, but the effect was smaller than in the four-day suppressive test and Curative test. This may be due to the fact that the extract is given prior to the onset of infection and thus has been quickly metabolized and/or excreted.55 It can also be due to the invivo model used, which lacks the insect vector, and the manner of inoculation and the doses used that result in rapid infection of the erythrocytes without the parasite going through the liver stages.35 Another possibility was that the extract might have acted through metabolic activation of the immune system and so parasite clearance could not be total.22 Similar results, in which plants had better suppressive and curative effects than prophylactic effects, were reported in other studies48,56–58. The result here was, however, in contrary with other investigations where plants had high residual activity than suppressive and curative activity.59 In this analysis, the crude extract and fractions significantly improved the mean survival time in all tested models as compared to the negative control, with the exception of the lower doses. This adds to the proof that P. berghei is suppressed, resulting in a decreased overall pathologic impact of the parasite in the study mice.21 In all models, the mean survival time of mice treated with the standard drug was substantially longer than the entire doses of the crude extract and solvent fraction treated groups; this may be due to the quick removal process or the extracts’ lower potency.60 In residual infection, the highest dose of Z. scabra and G. ternifolia also showed preventive effect in temperature reduction in line with the largest dose effect by methanolic extract of Syzygium guineense stem barks.61 The crude extract had no significant impact on body weight reduction in prophylactic model than the curative model. This could be attributed by low parasitemia suppression in repository model than in rane’s model and/or other than parasitemia suppression other factors may be involved.
Alkaloids, anthraquinone, flavonoids, saponins, steroids, tannins, and terpenoids were included in the root extracts of Z. scabras, according to preliminary phytochemical screening. These bioactive constituents are also attributed to medicinal plants’ therapeutic effects through a variety of mechanisms, including alkaloids disrupting the parasite’s ability to detoxify heme into nontoxic malaria pigment, anthraquinones intercalation in DNA, phytosteroids and flavonoids’ immunomodulatory effects, tannins’ free radical scavenging effects, and phenols including flavonoids’ antioxidant effect.62–64The extracts may have sparked its action through some of the mechanisms described above, or through some other mechanism that has yet to be discovered. As a result, the antimalarial activity found in this plant may have resulted from the action of these metabolites individually or in combination.
Conclusion
In general, the current findings show that Z. scabra root extract has antimalarial activity in Plasmodium berghei-infected mice. In addition, parasitemia, survival time, PCV, weight, and temperature all improved in the plant. As compared to the chloroform and aqueous fractions, the n-butanol fraction was found to be the most involved. This suggests that the n-butanol fraction may contain a possible lead molecule for the production of a new malaria drug. The findings provide statistical evidence for the use of the plant in traditional medicine to treat malaria and its complications. It is suggested that further research be done on the n-butanol fraction.
Abbreviations
ANOVA, Analysis of Variance; OECD, Organization for Economic Cooperation and Development; ACT, Artemisinin-based Combination Therapy; IP, intraperitoneal.
Data Sharing Statement
Almost all of our study’s materials and data are included in the manuscript, and some of it will be made available to other researchers upon fair request.
Ethics Approval
The proposal was reviewed and approved with approval number of (SOP3/10/13) by the ethical review committee of Mizan-Tepi University’s School of Pharmacy in Mizan Teferi, Ethiopia. The experimental animals were handled according to the guidelines for care and use of laboratory animals and OECD-guidelines.17,18
Acknowledgments
The Mizan-Tepi University funded the study, which the authors are grateful for.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kaharudin FA, Zohdi RM, Mukhtar SM, et al. In vitro antiplasmodial and cytotoxicity activities of crude extracts and major compounds from Goniothalamuslanceolatus. J Ethnopharmacol. 2020;23(254):112657. doi:10.1016/j.jep.2020.112657
2. Pérez-Moreno G, Cantizani J, Sánchez-Carrasco P, et al. Discovery of new compounds active against Plasmodium falciparum by high throughput screening of microbial natural products. PLoS One. 2016;11(1):e0145812. doi:10.1371/journal.pone.0145812
3. Muluye AB, Desta AG, Abate SK, Dano GT. Anti-malarial activity of the root extract of Euphorbia abyssinica (Euphorbiaceae) against Plasmodium berghei infection in mice. Malar J. 2019;18(1):1–8. doi:10.1186/s12936-019-2887-7
4. Gedif T, Hahn HJ. The use of medicinal plants in self-care in rural central Ethiopia. J Ethnopharmacol. 2003;87(2–3):155–161. doi:10.1016/S0378-8741(03)00109-0
5. Teklay A, Abera B, Giday M. An ethnobotanical study of medicinal plants used in Kilte Awulaelo District, Tigray Region of Ethiopia. J Ethnobiol Ethnomed. 2013;9(1):1–23. doi:10.1186/1746-4269-9-65
6. Tekle Y. An ethno-veterinary botanical survey of medicinal plants in Kochore district of Gedeo zone, southern nations nationalities and peoples regional state (SNNPRs), Ethiopia. J Sci Innov Res. 2014;3(4):433–445.
7. Kefalew A, Asfaw Z, Kelbessa E. Ethnobotany of medicinal plants in Ada’a District, East Shewa Zone of Oromia regional state, Ethiopia. J Ethnobiol Ethnomed. 2015;11(1):1–28. doi:10.1186/s13002-015-0014-6
8. Anand SP, Jeyachandran R. In vitro multiple shoot regeneration from nodal explants of Zehneriascabra (Lf) Sonder – an important medicinal climber. Plant Tissue Cult. 2004;14(2):101–106.
9. Arulappan MT, Britto JS, Ruckmani K, Kumar MR. Antimicrobial and antifungal activities of Zehneriascabra (LF) sond against human pathogens. Int J Develop Res. 2015;5:3852–3859.
10. Tadesse WT, Hailu AE, Gurmu AE, Mechesso AF. Experimental assessment of antidiarrheal and antisecretory activity of 80% methanolic leaf extract of Zehneriascabra in mice. BMC Complement Altern Med. 2014;14(1):1–8. doi:10.1186/1472-6882-14-460
11. Tesfaye WH, Alamneh EA. In vivo antimalarial activity of the crude extract and solvent fractions of the leaves of Zehenria scabra (Cucurbitaceae) against Plasmodium berghei in mice. J Med Plant Res. 2014;8(42):1230–1236.
12. Akele B. In vivo anti-inflammatory and antinociceptive activities of aerial part extracts of Zhenriascabra. Int J Pharm Ind Res. 2012;2(4):479–484.
13. Kipkore W, Wanjohi B, Rono H, Kigen G. A study of the medicinal plants used by the Marakwet Community in Kenya. J Ethnobiol Ethnomed. 2014;10(1):1–22. doi:10.1186/1746-4269-10-24
14. Ragunathan M, Abay SM Ethnomedicinal survey of folk drugs used in Bahirdar Zuria district, Northwestern Ethiopia;2009.
15. Giday M, Asfaw Z, Woldu Z. Ethnomedicinal study of plants used by Sheko ethnic group of Ethiopia. J Ethnopharmacol. 2010;132(1):75–85. doi:10.1016/j.jep.2010.07.046
16. Giday M, Teklehaymanot T, Animut A, Mekonnen Y. Medicinal plants of the Shinasha, Agew-awi and Amhara peoples in northwest Ethiopia. J Ethnopharmacol. 2007;110(3):516–525. doi:10.1016/j.jep.2006.10.011
17. Council NR. Guide for the Care and Use of Laboratory Animals. National Academies Press; 2010.
18. Ocde O. Acute oral toxicity: up and down procedure. OECD Guideline for the Testing of Chemicals; 2008: 1–2.
19. Huang BW, Pearman E, Kim CC. Mouse models of uncomplicated and fatal malaria. Bio-Protocol. 2015;5(13). doi:10.21769/BioProtoc.1514
20. Deressa T, Mekonnen Y, Animut A. In Vivo anti-malarial activities of Clerodendrummyricoides, Dodoneaangustifoliaand Aloe debrana against Plasmodium berghei. Ethiop J Health Dev. 2010;24(1). doi:10.4314/ejhd.v24i1.62941
21. Basir R, Rahiman SF, Hasballah K, et al. Plasmodium berghei ANKA infection in ICR mice as a model of cerebral malaria. Iran J Parasitol. 2012;7(4):62.
22. Waako PJ, Gumede B, Smith P, Folb PI. The in vitro and invivo antimalarial activity of Cardiospermum halicacabum L. and Momordica foetida Schumch. Et Thonn. J Ethnopharmacol. 2005;99(1):137–143. doi:10.1016/j.jep.2005.02.017
23. Peters W. The four-day suppressive invivo antimalarial test. Ann Trop Med Parasitol. 1975;69(2):155–171. doi:10.1080/00034983.1975.11686997
24. Ryley JF, Peters W. The antimalarial activity of some quinolone esters. Ann Trop Med Parasitol. 1970;64(2):209–222. doi:10.1080/00034983.1970.11686683
25. Peters W. Drug resistance in Plasmodium berghei. I. Chloroquine resistance. Exp Parasitol. 1965;17(1):80–89. doi:10.1016/0014-4894(65)90012-3
26. Dikasso D, Makonnen E, Debella A, et al. Anti-malarial activity of withaniasomnifera L. Dunal extracts in mice. Ethiop Med J. 2006;44(3):279–285.
27. Bantie L, Assefa S, Teklehaimanot T, Engidawork E. In vivo antimalarial activity of the crude leaf extract and solvent fractions of Croton macrostachyusHocsht. (Euphorbiaceae) against Plasmodium berghei in mice. BMC Complement Altern Med. 2014;14(1):1. doi:10.1186/1472-6882-14-79
28. Adumanya OC, Uwakwe AA, Essien EB Antiplasmodial activity of methanol leaf extract of Salaciasenegalensis Lam (Dc) in albino mice infected with chloroquine-sensitive Plasmodium berghei (NK65); 2014.
29. Mengiste B, Makonnen E, Urga K. In vivo antimalarial activity of DodonaeaAngustifolia seed extracts against Plasmodium berghei in mice model. Momona Ethiop J Sci. 2012;4(1):47–63. doi:10.4314/mejs.v4i1.74056
30. Cathrine L, Ks B. General techniques involved in phytochemical analiysis. Int J Adv Res Chem Sci. 2015;2:4.
31. Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: a review. Int Pharm Sci. 2011;1(1):98–106.
32. Ukpai OM, Amaechi EC. Evaluation of invivo antimalarial activity of the ethanolic leaf extracts of Chromolaena odorata and Cymbopogon citratus in mice. Niger J Biotechnol. 2012;24.
33. Trigg PI. The current global malaria situation. Malaria. 1998;11–22.
34. Zhang QW, Lin LG, Ye WC. Techniques for extraction and isolation of natural products: a comprehensive review. Chin Med. 2018;13(1):1–26. doi:10.1186/s13020-018-0177-x
35. Adzu B, Haruna AK, Salawu OA, Katsayal UD, Njan A. In vivo antiplasmodial activity of ZS-2A: a fraction from chloroform extract of Zizyphus spina-christi root bark against Plasmodium berghei berghei in mice. Int J Biol Chem Sci. 2007;1(3):281–286.
36. Mesfin A, Giday M, Animut A, Teklehaymanot T. Ethnobotanical study of antimalarial plants in Shinile District, Somali Region, Ethiopia, and invivo evaluation of selected ones against Plasmodium berghei. J Ethnopharmacol. 2012;139(1):221–227. doi:10.1016/j.jep.2011.11.006
37. Nureye D, Assefa S, Nedi T, Engidawork E. In vivo antimalarial activity of the 80 Methanolic root bark extract and solvent fractions of Gardenia ternifolia Schumach. & Thonn. (Rubiaceae) against Plasmodium berghei. Evid Based Complement Alternat Med. 2018;Jan:2018.
38. Dibessa TT, Engidawork E, Nedi T, Teklehaymanot T. Antimalarial activity of the aqueous extract of the latex of Aloe pirottae Berger. (Aloaceae) against Plasmodium berghei in mice. J Ethnopharmacol. 2020;255:112763. doi:10.1016/j.jep.2020.112763
39. Adugna M. In vivo antimalarial activity of crude extract of aerial part of Artemisia abyssinica against Plasmodium berghei in mice. Glob J Pharmacol. 2014;8(3):460–468.
40. Fenta M, Kahaliw W. Evaluation of antimalarial activity of hydromethanolic crude extract and solvent fractions of the leaves of Nuxiacongesta R. Br. Ex Fresen (Buddlejaceae) in Plasmodium berghei infected mice. J Exp Pharmacol. 2019;11:121. doi:10.2147/JEP.S230636
41. Asnake S, Teklehaymanot T, Hymete A, Erko B, Giday M. Evaluation of the antiplasmodial properties of selected plants in Southern Ethiopia. BMC Complement Altern Med. 2015;15(1):448. doi:10.1186/s12906-015-0976-x
42. Amelo W, Nagpal P, Makonnen E. Antiplasmodial activity of solvent fractions of methanolic root extract of Dodonaeaangustifolia in Plasmodium berghei infected mice. BMC Complement Altern Med. 2014;14(1):462–469. doi:10.1186/1472-6882-14-462
43. Yared D, Mekonnen Y, Debella A. In vivo antimalarial activities of fractionated extracts of Asparagus africanus in mice infected with Plasmodium berghei. Pharmacologyonline. 2012;3:88–94.
44. Bankole AE, Adekunle AA, Sowemimo AA, Umebese CE, Abiodun O, Gbotosho GO. Phytochemical screening and in vivo antimalarial activity of extracts from three medicinal plants used in malaria treatment in Nigeria. Parasitol Res. 2016;115(1):299–305. doi:10.1007/s00436-015-4747-x
45. Anza M, Worku F, Libsu S, Mamo F, Endale M. Phytochemical screening and antibacterial activity of leaves extract of Bersama abyssinica. J Adv Bot Zool. 2015;3(2):1–5.
46. Baumann E, Stoya G, Völkner A, Richter W, Lemke C, Linss W. Hemolysis of human erythrocytes with saponin affects the membrane structure. Acta Histochem. 2000;102(1):21–35. doi:10.1078/0065-1281-00534
47. Teklu T, Engidawork E, Nedi T, Teklehaymanot T, Gebremeskel L. Evaluation of the antimalarial activity of the hydroalcoholic extract of leaf of leonotis ocymifolia (Burm. f.) Iwarsson (Lamiaceae) against plasmodium berghei in mice. Evid Based Complement Alternat Med. 2020;2020:1–8. doi:10.1155/2020/5384804
48. Eyasu M, Shibeshi W, Giday M. In vivo antimalarial activity of hydromethanolic leaf extract of Calpurnia aurea (Fabaceae) in mice infected with chloroquine sensitive Plasmodium berghei. Int J Pharmacol. 2013;2(9):131–142.
49. Mohammed MI, Desmulliez MP. Autonomous capillary microfluidic system with embedded optics for improved troponin I cardiac biomarker detection. Biosens Bioelectron. 2014;15(61):478–484. doi:10.1016/j.bios.2014.05.042
50. Fentahun S, Makonnen E, Awas T, Giday M. In vivo antimalarial activity of crude extracts and solvent fractions of leaves of Strychnosmitis in Plasmodium berghei infected mice. BMC Complement Altern Med. 2017;17(1):1–2. doi:10.1186/s12906-016-1529-7
51. Adinew GM. Antimalarial activity of methanolic extract of Phytolacca dodecandra leaves against Plasmodium berghei infected Swiss albino mice. Int J Pharmacol Clin Sci. 2014;3(3).
52. Oliveira AB, Dolabela MF, Braga FC, Jácome RL, Varotti FP, Póvoa MM. Plant-derived antimalarial agents: new leads and efficient phythomedicines. Part I. Alkaloids. An Acad Bras Cienc. 2009;81(4):715–740. doi:10.1590/S0001-37652009000400011
53. Shibeshi MA, Enyew EF, Adinew GM, Aragaw TJ. Antimalarial activity of methanolic extracts and solvent fractions of combretummolle leaves in Plasmodium berghei infected mice. J Exp Pharmacol. 2021;13:69. doi:10.2147/JEP.S285117
54. Kifle ZD, Adinew GM, Mengistie MG, et al. Evaluations of antimalarial activity of methanolic root extract of Myrica salicifolia A Rich (Myricaceae) against Plasmodium berghei–infected mice. J Evid Based Integr Med. 2020;25:2515690X20920539. doi:10.1177/2515690X20920539
55. Lim H-S, Im J-S, Cho J-Y, et al. “Pharmacokinetics of hydroxychloroquine and its clinical implications in chemoprophylaxis against malaria caused by Plasmodium vivax”. Antimicrob Agents Chemother. 2009;53(4):1468–1475. doi:10.1128/AAC.00339-08
56. Salawu OA, Tijani AY, Babayi H, Nwaeze AC, Anagbogu RA, Agbakwuru VA. Antimalarial activity of ethanolic stem bark extract of Faidherbia Albida (Del) a. Chev (Mimosoidae) in mice. Arch Appl Sci Res. 2010;2(5):261–268.
57. Al-Adhroey AH, Nor ZM, Al-Mekhlafi HM, Mahmud R. Median lethal dose, antimalarial activity, phytochemical screening and radical scavenging of methanolic Languas galanga rhizome extract. Molecules. 2010;15(11):8366–8376. doi:10.3390/molecules15118366
58. Onwusonye JC, Uwakwe AA. The antiplasmodial activity of methanol root bark extract of alstonia boonei against Plasmodium Berghei infection in mice. Int J Sci Res. 2014;3:2199.
59. Uwakwe A, Monago C. Antiplasmodial activity of methanolic stem bark extract of Anthocleista grandiflora in mice. Int J Appl. 2012;2(4).
60. Alehegn AA, Yesuf JS, Birru EM. Antimalarial activity of crude extract and solvent fractions of the leaves of bersama abyssinica fresen. (Melianthaceae) against Plasmodium berghei infection in Swiss Albino Mice. Evid Based Complement Alternat Med. 2020;2020:2020. doi:10.1155/2020/9467359
61. Zeleke G Antimalarial activity of 80% methanol extract of the stem bark of syzygium guineense (willd.) dc. (myrtaceae) in mice infected with plasmodium berghei (Doctoral dissertation, Addis Ababa University).
62. Azwanida NN. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med Aromat Plants. 2015;4(196):2167–2412.
63. Roth M, Araya JJ, Timmermann BN, Hagenbuch B. Isolation of modulators of the liver-specific organic anion-transporting polypeptides (OATPs) 1B1 and 1B3 from Rollinia emarginata Schlecht (Annonaceae). J Pharmacol Exp Ther. 2011;339(2):624–632. doi:10.1124/jpet.111.184564
64. Chikezie PC, Ibegbulem CO, Mbagwu FN. Medicinal potentials and toxicity concerns of bioactive principles. Med Aromat Plants. 2015;4(3):1–5.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.