Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Evaluation of Antidiabetic Effect of Ethanolic Leaves Extract of Becium grandiflorum Lam. (Lamiaceae) in Streptozotocin-Induced Diabetic Mice
Authors Gebremeskel L , Beshir Tuem K , Teklu T
Received 22 January 2020
Accepted for publication 8 April 2020
Published 4 May 2020 Volume 2020:13 Pages 1481—1489
DOI https://doi.org/10.2147/DMSO.S246996
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Antonio Brunetti
Leake Gebremeskel,1 Kald Beshir Tuem,2 Tewolde Teklu1
1Pharmacology and Toxicology Unit, Department of Pharmacy, College of Health Sciences, Aksum University, Aksum, Ethiopia; 2Department of Pharmacology and Toxicology, School of Pharmacy, College of Health Sciences, Mekelle University, Mekelle, Ethiopia
Correspondence: Leake Gebremeskel
Pharmacology and Toxicology Unit, Department of Pharmacy, College of Health Sciences, Aksum University, Postal 298, Aksum, Ethiopia
Tel +251 913726205
Fax +251348750244
Email [email protected]
Background: Becium grandiflorum has been used traditionally for treatment of different ailments including diabetes mellitus although it lacks scientific evidence. Thus, the present study was aimed at evaluating the antidiabetic effect of Becium grandiflorum in streptozotocin (STZ)-induced diabetic mice.
Methods: The antidiabetic activity of hydro-ethanolic (30:70) leaf extract of Becium grandiflorum was evaluated in STZ (45 mg/kg)-induced diabetic and normal mice. Antihyperglycemic, hypoglycemic, oral glucose tolerance and body weight change effects of the extract were assessed after administering three doses of the extract (200, 400 and 600 mg/kg), glibenclamide 5 mg/kg (reference drug) and 2% Tween 80 (vehicle). One-way analysis of variance and Tukey’s post hoc test were used for data analysis.
Results: All doses of the extract (200 mg/kg (p< 0.05), 400 mg/kg (p< 0.05) and 600 mg/kg (p< 0.01)) and glibenclamide 5 mg/kg (p< 0.001) showed statistically significant blood glucose level reduction in normal mice as compared to Tween 80. The hydroalcoholic extract at a dose of 200 mg/kg (p< 0.05), 400 mg/kg (p< 0.01) and 600 mg/kg (p< 0.001) showed better blood glucose tolerance after 60, 120 and 180-minute treatment duration in normal mice as compared to negative control. In diabetic mice, Becium grandiflorum doses and the reference drug caused maximum reduction in blood glucose level at the end of the 15th day of treatment by 17.61%, 22.52%, 24.62% and 34.12%, respectively. The extract’s doses and the standard drug showed significant (p< 0.05) improvement in body weight while the diabetic control continued to lose their body weight.
Conclusion: Thus, Becium grandiflorum exhibits antihyperglycemic activity in STZ-induced diabetic mice, and shows improvement in oral glucose tolerance and body weight, which justifies the claimed use of the plant in ameliorating diabetes mellitus in Ethiopian folk medicine.
Keywords: Becium grandiflorum, antidiabetic, antihyperglycemic, streptozotocin
Background
Diabetes mellitus (DM) is a group metabolic disorder, causing disturbances in the metabolism of lipids, carbohydrates and proteins. It is caused by defects in insulin secretion and/or action, target-tissue resistance and increased hepatic glucose output.1,2 The main clinical presentations of diabetic patients include polyuria, polyphagia, polydipsia and ketosis. DM causes several complications, notably retinopathy, neuropathy, nephropathy and cardiovascular diseases that are known to be the major causes of morbidity and mortality of DM.3 DM is one of the main causes of morbidity and mortality in developed and developing countries, and it is expected to be the 7th leading cause of death in 2030.4 The present scenario of rapid worldwide increase in diabetic patients is associated with ageing, sedentary or unhealthy lifestyle, urbanization, consumption of an energy-rich diet and obesity which caused an increase of cases from 153 million to 368 million in the last three decades.5,6 Globally, in 2017, around 451 million people aged 18–99 years lived with diabetes, and the number was predicted to rise to 693 million by the year 2045.4,7 In developing countries, including Africa, at least 1 in 10 deaths in adults aged 35–64 is attributable to diabetes.8,9 In Ethiopia, although there is no single national-level prevalence study, hospital-based studies indicate that the prevalence increased to 3.4% in 2015 and the number of diabetic cases is expected to rise to 10.6 million by 204010, and other pooled prevalence of DM associated with hypertension was 4.99%.11
Achieving better glycemic control with minimum side effects and ease of access still remains a challenge with the current antidiabetic drugs. The use of medicinal plants nowadays is being strengthened since treatment of DM using phytotherapy is affordable, easily accessible, less costly, very effective and with fewer side effects.12 About 70–90% of the population in developed and developing countries use traditional medicine for their primary health care needs as they are relatively inexpensive and with fewer side effects.13 These facts have encouraged the expansion of the frontiers of scientific evaluation of the hypoglycemic properties of diverse plant species, because herbal preparations are considered a main source of modern medicine.14,15 In spite of the many clinical studies and trials underway, there are still several unmet needs of the community. Therefore, further studies for safer and more effective antidiabetic agents from plant origin are required. Currently, in Ethiopia, a number of plant species like Pentas schimperiana, Justicia Schimperiana16 and Moringa stenopetala17 have been evaluated and their hypoglycemic activity confirmed in animal models.
The medicinal plant under study, Becium grandiflorum, is among the claimed antidiabetic species which need scientific justification. It is an endemic plant to Ethiopia and Eritrea, and has vernacular names “Tebeb” (Tigrigna) and “Mentesie” or “Matosh” (Amharic). It is an indigenous perennial aromatic woody shrub, medium sized, grows in highlands and mid-altitude areas (1600–3100 m) above sea level.17,18 Various experimental studies on plants that belong to the same genus and similar species of the plant against different ailments were carried out. For instance, from the root bark of Becium grandiflorum var. obovatum, the two new saponins triterpenoid beciumecine 1 and 2 were investigated for cancer treatment in South Africa.19 Moreover, Ocimum sanctum, the same genus as Becium grandiflorum, stimulates insulin secretion.20 Based on the ethnobotanical reports and interviewing traditional practitioners, Becium grandiflorum Lam. is a potential plant for the treatment of many ailments like bacterial infections, wound healing, malaria, diabetes mellitus, respiratory depression, influenza and inflammatory disorders.21–23 Traditionally, people use fresh leaf of Becium grandiflorum for treatment of different ailments including diabetes although it lacks scientific evidence.18 The aim of this study is to evaluate the antidiabetic effect of 70% ethanolic leaves extract of Becium grandiflorum in streptozotocin-induced diabetic mice.
Methods
Chemicals
Streptozotocin (STZ) (Spectrochem Pvt, Ltd, Mumbai, India), distilled water (Tigray regional laboratory, Mekelle, Ethiopia), ethanol (Carlo Erba Reagents, Italy), Tween 80 (Atlas Chemical Industries Inc., USA), glucose standard strip/kits and glucometer (ACCU-CHEK Active, Germany) and Glibenclamide (Alfa Aesar, Great Britain) were some of the chemicals used during the experiment. All other chemicals used were of analytical grade.
Plant Materials Collection
The plant material was collected from “Adishuhu”, Southern Tigray, which is about 687 km north from Addis Ababa. The medicinal plant specimen was identified by taxonomist Dr Yemane Gberezgabiher, Department of Biology, Mekelle University. Authentication of the plant specimens was done at the National Herbarium, College of Natural and Computational Sciences, Addis Ababa University where a voucher specimen (KBTMU-001) was deposited for future reference.
Extraction of the Plant Material
The leaves were dried at room temperature under shade for 3 weeks. After fully dried and pulverized, 450 grams of the coarse powder of leaves of the Becium grandiflorum was macerated in a sufficient amount of aqueous:ethanol (30:70, v/v) (2.5 liters) for 3 days with timely stirring and shacking, and re-macerated three times to exhaustively extract the leaf. Upon repeated filtration using Whatman no. 1 filter paper, the combined filtrate was evaporated and concentrated using a rotary evaporator and kept in a ventilated oven at 40 ºC until dried. The concentrated and dried extract was weighed and gives a percentage yield of 29.7% (w/w). The dried extract was placed in a sealed container and stored in a refrigerator at 4 °C for further procedure.
Experimental Animals
A total of 106 mice (96 for antidiabetic study (males) and 10 for acute oral toxicity study (females)24,25) were used for this study. They were bred at the animal house facility of School of Pharmacy, Mekelle University. Swiss albino mice weighing 20–30 grams were employed for antidiabetic study. The animals were housed in polypropylene cage (6 mice per cage), kept under laboratory conditions (temperature 23±2 °C, relative humidity 55±10% with a 12 h light:12 h dark cycle), and allowed free access to standard pellet and water ad libitum. Before initiation of the experiment, mice were acclimatized to the laboratory condition for 5 days. Ethical clearance was obtained from the health research and ethics committee of College of Health Sciences, Mekelle University. At the end of each procedure, mice were sacrificed humanely using cervical dislocation and buried into the disposal system of the college.
Acute Oral Toxicity Study
Acute oral toxicity study of Becium grandiflorum was done based on the Organization for Economic Cooperation and Development (OECD) 425 guideline.26 After acclimating for a week, 5 healthy female mice, weighing 20–30 g, were used. At first, a single mouse was fasted for 4 h and 2000 mg/kg of the leaf extract was administered by oral gavage and observed periodically for 24 h for any acute sign of toxicity. The other four mice were given the same dose and cage side observation was made for gross behavioral changes for 14 days. Then, the dose was raised to 5000 mg/kg and the same procedure was carried out on five female mice.
Induction of Diabetes
Following overnight fasting of male mice, the blood glucose level and body weight were recorded prior to diabetes induction. Experimental diabetic mice were induced by intraperitoneal injection of streptozotocine (STZ) dissolved in 0.1 M sodium citrate buffer pH 4.5 at a single dose of 45 mg/kg body weight,27 and 30 minutes after injection mice were allowed free access to food and water. Three days after STZ injection, the plasma blood glucose level of each animal was determined; and a mouse with fasting blood glucose range above 200 mg/dl was considered diabetic, which was included in the study.3
Experimental Designs and Procedures
For the antihyperglycemic test, six groups (6 mice each) were assigned as group 1: normal mice receiving 2% Tween 80 (normal control); group 2: diabetic mice given 2% Tween 80 (negative control); group 3: diabetic mice given glibenclamide (positive control); and groups 4, 5 and 6: mice treated with three selected (200 mg/kg, 400 mg/kg and 600 mg/kg) doses of Becium grandiflorum extract respectively.28,29 Fasting blood glucose level (BGL) was measured using an auto-analyzer glucometer (ACCU-CHEK Active, Germany) on days 0, 5, 10 and 15 by collecting blood from the tail vein of the experimental mice in which results were expressed in terms of milligrams per deciliter of blood with the same procedure as applied by Geetha et al30 and Joseph et al.31 Moreover, the body weight of diabetic mice was recorded on every 5th day on day 0, 5, 10 and 15 to see the change in body weight among the treated groups.32
In the hypoglycemic activity study on normal mice, five groups (6 mice each) were designed as group 1: mice receiving 2% Tween 80 (normal control); group 2: mice treated with glibenclamide or standard drug (positive control); groups3, 4, and 5: mice given 200 mg/kg, 400 mg/kg and 600 mg/kg doses of Becium grandiflorum extract respectively. Blood samples were collected from the tail of normal mice to determine the blood glucose level at 0, 1, 2, 3 and 4 h post treatment using an auto-analyzer glucometer.16,17 In the oral glucose tolerance test (OGTT), another five groups of normal mice (6 mice each) were employed with the same grouping and dosing as described in the hypoglycemic activity study. In OGTT, normal mice were fasted for 12 h and their blood glucose level was measured immediately prior to treatment. Thirty minutes after treatment, all mice were loaded with glucose solution (2 g/kg body weight) orally in a volume of 1 mL. Blood sugar levels were measured, for each mouse, at 30 min, 1 h and 2 h intervals after treatment. Dose levels were chosen based on acute oral toxicity results described by the OECD;26 and a bit modification was made as the plant was found to be safer at 5000 mg/kg.32,33 In all procedures, grouping and dosing, animals were randomly assigned to their respective groups.
Statistical Analysis
The results were described as means±SEM. The values were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test applied for multiple comparisons of data. The differences were considered significant, by fixing the P value as <0.05. Analysis was performed using SPSS software package Version 21.
Results
Acute Oral Toxicity Test
In the present study, Becium grandiflorum did not produce any change in behaviors (restlessness, motor activity, breathing and diarrhea) in the first 24 h and up to 14 days cage side observation at both 2000 mg/kg and 5000 mg/kg doses; and none of the experimental animals died at 5000 mg/kg, the maximum dose employed. Therefore, the oral median lethal dose (LD50) of the extract causing 50% death of the animals is greater than 5 g/kg.
Hypoglycemic Activity of Leaf Extract of Becium grandiflorum on Normal Mice
After administering the extract and standard drug to the experimental animal, the blood glucose level was measured every 30 minutes for the first hour and every hour for the next 4 h. Based on the results described in Table 1, statistical difference in blood glucose level was not observed in the first hour post administration. Significant blood glucose reduction was noted at 2 h after administering 400 mg/kg (p<0.05) and 600 mg/kg (p<0.01) of the extract, and glibenclamide (p<0.001), as compared to mice receiving 2% Tween 80 (negative control). However, 200 mg/kg of hydroalcoholic extract did not show significant difference in blood glucose level up to 2 h as compared to negative control. At 3 h, all doses of the extract (200 mg/kg (p<0.05), 400 mg/kg (p<0.05) and 600 mg/kg (p<0.01)) and glibenclamide 5 mg/kg (p<0.001) showed statistically significant blood glucose reduction as compared to the negative control group. Moreover, at 4 h post administration, very significant (p<0.001) blood glucose was reduced by all treatment groups when compared to groups receiving Tween 80.
 |
Table 1 Hypoglycemic Effect of Becium grandiflorum Leaf Extract on Fasting BGL (Blood Glucose Level) in Normoglycemic Mice |
Effect of Becium grandiflorum Leaf Extract on Postprandial Glycemia
After the oral glucose challenge test (2 g/kg) for all groups, the blood glucose elevated to maximum level at 30 minutes. In this test only glibenclamide-treated mice showed statistically significant (p<0.05) tolerance as compared to Tween 80 administered groups. The hydroalcoholic extract at a dose of 200 mg/kg (p<0.05), 400 mg/kg (p<0.01) and 600 mg/kg (p<0.001), and the standard drug (p<0.001) significantly reduced the blood glucose level after 60, 120 and 180 minutes post administration as compared with negative controls (Table 2)
 |
Table 2 Effect of Becium grandiflorum Leaf Extracts on Postprandial NonDiabetic Mice |
Effect of Becium grandiflorum Leaf Extract on BGL in Normal and Diabetic Mice
The antihyperglycemic effect of hydroalcoholic extract of Becium grandiflorum on fasting blood glucose level in diabetic mice was measured once every 5th day during a 15-day treatment period as described in Table 3. All diabetic-induced mice show significant blood glucose level variation (p<0.05) as compared to non-diabetic mice across all time intervals. Glibenclamide 5 mg/kg and 600 mg/kg dose of the extract indicated statistically significant variation (P<0.001) in blood glucose level at the 5th day of treatment as compared to diabetic control. Significant difference in blood glucose level (p<0.001) was observed in all treatment groups at both 10 and 15 days of duration as compared to diabetic control. On the 10th and 15th days, the reference drug and 600 mg/kg of the extract showed significant reduction in blood glucose level (p<0.001) when compared with the 200 mg/kg dose. As shown in Figure 1, daily administration of extracts of Becium grandiflorum at doses of 200 mg/kg, 400 mg/kg and 600 mg/kg caused maximum reduction in blood glucose level at the end of the 15th day by 17.61%, 22.52% and 24.62% respectively. The reference drug, glibenclamide 5 mg/kg, resulted in decreased blood glucose level by 34.12% at the end of the experiment period. Percent reduction in BGL was calculated using the formula:
 |
Table 3 The Effect of Hydroalcoholic Extract of Becium grandiflorum on Fasting Blood Glucose Level in Diabetic Animals |
 |
Figure 2 Effect of Becium grandiflorum on body weight of mice. |
 |
Figure 1 Percent reduction of blood glucose level (BGL). |
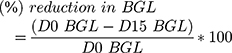
where D0 BGL=blood glucose level at day 0; D15 BGL=blood glucose level at 15th day of treatment.
Effect of Becium grandiflorum Leaf Extract on Body Weight of Normal and Diabetic Mice
Figure 2 represents the changes in body weight in diabetic mice. There was no significant variation in body weight of diabetic and normal mice up to the 5th day of treatment. At the 10th (p<0.05) and 15th (p<0.01) days, the diabetic control showed significant body weight loss as compared to normal control. At the end of the experiment period, the 15th day, significant weight change was observed in mice treated with glibenclamide 5 mg/kg (p<0.05), and 200 mg/kg (p<0.05), 400 mg/kg (p<0.01) and 600 mg/kg (p<0.001) of the extract as compared to the diabetic control group.
Discussion
Antidiabetic activity of Becium grandiflorum was performed on STZ-induced diabetic mice, which resembles human diabetes.34,35 Streptozotocin is mostly used to study in vivo antidiabetic activities in mice as it has a longer half-life (15 minutes), sustained hyperglycemia with fewer incidence of ketosis and less mortality.36 The vital foundation of diabetes mellitus occurs after STZ enters pancreatic β-cells via glucose transporter type 2 (GLUT2) and then it splits into glucose and methyl nitrosourea which has deoxyribonucleic acid (DNA) alkylating properties to damage β-cells of Langerhans and then leads the progression to type-2 diabetes.37,38
In normal fasted mice, 400 mg/kg and 600 mg/kg leaf extract doses started to show significant hypoglycemic effect after 2 h of treatment. At 3 and 4 h of administration, all extract doses (including 200 mg/kg) showed dose-dependent significant hypoglycemic activity which was comparable with glibenclamide with a bit higher significance level observed in the reference drug. Previous reports show that glibenclamide results in hypoglycemia in normal experimental animals by stimulation of insulin release from pancreatic β-cells.39 It has also been reported that 70% ethanol leaf extract of Becium grandiflorum possesses flavonoids, terpenoids, tannins, saponins, phenols and steroids,18 and these secondary metabolites isolated from other medicinal plants have been found to stimulate insulin secretion from pancreatic β-cells.40 Hence, the hypoglycemic activity of 70% of ethanolic leaf extract of Becium grandiflorum may be attributed to the presence of any of these bioactive phytochemicals that act individually or synergistically to either stimulate insulin secretion or mimic insulin action which share a similar mechanism with glibenclamide.
To assess altered carbohydrate metabolism during post glucose administration, the oral glucose tolerance test (OGTT) model was used.16,17,41 It is understood that a high amount of glucose in blood induces insulin secretion and then stimulates peripheral glucose consumption, and in 2–3 h insulin brings down the elevated BGL back to the normal level.17,39 After loading glucose (2 g/kg) for all groups, the blood glucose raised to maximum level at 30 minutes. At 200, 400 and 600 mg/kg doses of the extract, significant improvement in glucose tolerance was observed after 60,120 and 180 minutes of administration. These results suggest that the improvement in glucose tolerance by the extract could be due to the effect of Becium grandiflorum in the β-cells of the pancreas or insulin sensitizing through PPARγ (peroxisome proliferator activated receptor gamma) activation or extra pancreatic activity involving promoting peripheral glucose utilization.7,41 Previous studies described the mechanism to reduce postprandial hyperglycemia via inhibition of carbohydrate hydrolyzing enzymes α-amylase, and α-glycosidase in the digestive system, which prevents postprandial hyperglycemia in type-2 DM.42,43 The other possible antihyperglycemic effect of Becium grandiflorum on the OGTT could be attributed to the presence of secondary metabolites like flavonoids18 because flavonoids have shown their effect to inhibit α-glycosidase, ability to regenerate pancreatic β-cells, increasing the peripheral utilization of glucose and inhibiting the glucose transporter activity from the intestine.44
Significant reduction in fasting BGL of all diabetic mice administered with 70% ethanolic extract of Becium grandiflorum and reference drug has been noted as compared to diabetic control. Maximum percentage reduction in BGL was observed at doses 200 mg/kg (17.61%), 400 mg/kg (22.52%) and 600 mg/kg (24.62%) of the extract, and glibenclamide 5 mg/kg (34.12%) at the 15th day of treatment (Figure 1). This study was almost concordant with a previous report in which maximum reduction in BGL was achieved by glibenclamide (38%) and by other plant leaf extract (27%).45 As aforementioned, many secondary metabolites (flavonoids, saponins, phenols, etc.) of different plant species have been shown to have potent hypoglycemic, antihyperglycemic and glucose suppressive effects.
The effect of 70% ethanolic leaf extract of Becium grandiflorum on body weight change was assessed in STZ-induced diabetic mice (Figure 2). There was no significant change in body weight of diabetic-induced and normal mice up to the 5th day of treatment, but at the 10th and 15th days the diabetic control showed significant body weight loss as compared to normal control. The noticed weight loss in diabetic mice may be due to protein wasting caused by the unavailability of carbohydrate for utilization as an energy source.46 Diabetic mice administered different doses of the extract and glibenclamide showed improvement in body weight while diabetic control mice continued to lose their body weight.35,47 The capability of Becium grandiflorum to protect the body from weight loss could be due to reversal of proteolysis, gluconeogenesis and glycogenolysis and its ability to reduce the bioactive compounds of the plant which might help in suppressing the free radicals generated via hyperglycemia.35,47,48
Conclusion
The results of this study demonstrated that the 70% ethanolic leaf extract of Becium grandiflorum exhibited antihyperglycemic activity in STZ-induced diabetic mice; and the plant extract has shown improvement in oral glucose tolerance, hypoglycemic and body weight. Therefore, this study corroborates the claim of the traditional system for Becium grandiflorum in the treatment of diabetes mellitus. It is worth undertaking further research to establish a solid foundation for this finding and understand the mechanism(s) of action of the plant constituent(s).
Abbreviations
ADA, American Diabetic Association; ANOVA, Analysis of Variance; BGL, Blood Glucose Level; DM, Diabetes Mellitus; IDF, International Diabetes Federation; OECD, Organization for Economic Cooperation and Development; OGTT, Oral Glucose Tolerance Test; SPSS, Statistical Software Package for Social Science; STZ, Streptozotocin; WHO, World Health Organization.
Data Sharing Statement
The raw data used in this study are available from the corresponding author upon reasonable request.
Ethics and Consent Statement
Ethical approval was taken from the health research and ethics committee of College of Health Sciences, Mekelle University, Ethiopia.
Consent for Publication
All authors reach a consensus to publish this study in a reputable journal, and are accountable for the work.
Acknowledgments
Authors would like to thank the Axum University and Mekelle University for laboratory access.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Ross SA, Gulve EA, Wang M. Chemistry and biochemistry of type 2 diabetes. Chem Rev. 2004;104(3):1255–1282. doi:10.1021/cr0204653
2. Edwin E, Sheeja E, Dhanabal S, Suresh B. Antihyperglycemic activity of Passiflora mollissima Bailey. Indian J Pharm Sci. 2007;69(4):570. doi:10.4103/0250-474X.36947
3. Oyedemi S, Yakubu M, Afolayan A. Antidiabetic activities of aqueous leaves extract of Leonotis leonurus in streptozotocin induced diabetic rats. J Med Plant Res. 2011;5(1):119–125.
4. Cho N, Shaw J, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi:10.1016/j.diabres.2018.02.023
5. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–149. doi:10.1016/j.diabres.2013.11.002
6. Shanker K, Naradala J, Mohan GK, Kumar G, Pravallika P. A sub-acute oral toxicity analysis and comparative in vivo anti-diabetic activity of zinc oxide, cerium oxide, silver nanoparticles, and Momordica charantia in streptozotocin-induced diabetic Wistar rats. RSC Adv. 2017;7(59):37158–37167. doi:10.1039/C7RA05693A
7. Chávez-Silva F, Cerón-Romero L, Arias-Durán L, et al. Antidiabetic effect of Achillea millefollium through multitarget interactions: α-glucosidases inhibition, insulin sensitization and insulin secretagogue activities. J Ethnopharmacol. 2018;212:1–7. doi:10.1016/j.jep.2017.10.005
8. Chimere U, Arome O, Affiong E, Ogechukwu A, Ike C. Anti-diabetic effect of the methanolic leaf extract of Axonopus compressus (P. Beauv) in Alloxan Induced Diabetic Rats. Int J Biochem Res Rev. 2016;12(1):1–5. doi:10.9734/IJBCRR/2016/25425
9. Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94(3):311–321. doi:10.1016/j.diabres.2011.10.029
10. Atlas D. International Diabetes Federation. IDF Diabetes Atlas.
11. Yazew KG, Walle TA, Azagew AW. Prevalence of anti-diabetic medication adherence and determinant factors in Ethiopia: a systemic review and meta-analysis, 2019. Int J Afr Nurs Sci. 2019;11:100167. doi:10.1016/j.ijans.2019.100167
12. Tafesse TB, Hymete A, Mekonnen Y, Tadesse M. Antidiabetic activity and phytochemical screening of extracts of the leaves of Ajuga remota Benth on alloxan-induced diabetic mice. BMC Complement Altern Med. 2017;17(1):243. doi:10.1186/s12906-017-1757-5
13. Robinson MM, Zhang X. Traditional medicines: global situation, issues and challenges. World Med Situation. 2011;1–14.
14. Mihailova S, Tsvetkova A, Todorova A. Pharmacological trends in the treatment of diabetes type 2-New classes of antidiabetic drugs. Int Arch Integr Med. 2015;2(4):223–228.
15. Gebremeskel L, Bhoumik D, Sibhat GG, Tuem KB. In vivo wound healing and anti-inflammatory activities of leaf latex of aloe megalacantha baker (Xanthorrhoeaceae). Evid Based Complement Alternat Med. 2018;2018:1–7. doi:10.1155/2018/5037912
16. Tesfaye A, Makonnen E, Gedamu S. Hypoglycemic and antihyperglycemic activity of aqueous extract of Justicia Schimperiana leaves in normal and streptozotocin-induced diabetic mice. Int J Pharma Sci Res. 2016;7(2):110–113.
17. Toma A, Makonnen E, Mekonnen Y, Debella A, Adisakwattana S. Antidiabetic activities of aqueous ethanol and n-butanol fraction of Moringa stenopetala leaves in streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2015;15(1):242. doi:10.1186/s12906-015-0779-0
18. Beshir K, Shibeshi W, Ejigu A, Engidawork E. In-vivo wound healing activity of 70% ethanol leaf extract of Beciumgrandiflorum Lam. (Lamiaceae) in mice. Ethiop Pharm J. 2016;32:117–130. doi:10.4314/epj.v32i2.3
19. Burger I, Burger BV, Albrecht CF, Spies HS, Sándor P. Triterpenoid saponins from Becium grandiflorum var. obovatum. Phytochemistry. 1998;49(7):2087–2095. doi:10.1016/S0031-9422(98)00413-0
20. Somasundaram G, Manimekalai K, Salwe KJ, Pandiamunian J. Evaluation of the antidiabetic effect of Ocimum sanctum in type 2 diabetic patients. Int J Life Sci Pharm Res. 2012;5:75–81.
21. Teklehaymanot T, Giday M, Medhin G, Mekonnen Y. Knowledge and use of medicinal plants by people around Debre Libanos monastery in Ethiopia. J Ethnopharmacol. 2007;111(2):271–283. doi:10.1016/j.jep.2006.11.019
22. Teklay A, Abera B, Giday M. An ethnobotanical study of medicinal plants used in Kilte Awulaelo District, Tigray Region of Ethiopia. J Ethnobiol Ethnomed. 2013;9(1):65. doi:10.1186/1746-4269-9-65
23. Equar G, Gebremedhin G, Gebrekidan A, Asmelash T, Yirga G. Determination of antimicrobial activity and phytochemical screening of selected medicinal plants in Tigray region of Northern Ethiopia. Momona Ethiop j Sci. 2018;10(2):255–270. doi:10.4314/mejs.v10i2.6
24. Kolb H. Mouse models of insulin dependent diabetes: low‐dose streptozocin‐induced diabetes and nonobese diabetic (NOD) mice. Diabetes Metab Rev. 1987;3(3):751–778. doi:10.1002/dmr.5610030308
25. Furman BL. Streptozotocin‐induced diabetic models in mice and rats. Curr Protoc Pharmacol. 2015;70(1):
26. OECD. Organization for Economic Growth Development(OECD)guidelines for the Testing of Chemicals: Acute Oral Toxicity Up and DownProcedure (UDP); 2008.
27. Zhang M, Lv X-Y, Li J, Xu Z-G, Chen L. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp Diabetes Res. 2009;2008.
28. Yohannes K, Gomathi P, Aman K In vivo antidiabetic and in vitro antioxidant activity of Myrica salicifolia Hochst. ex A. Rich. (Myricaceae) root extract in streptozotocin-induced diabetic mice.
29. Vareda PMP, Saldanha LL, NAdP C, Violato NM, Dokkedal AL, Bosqueiro JR. Myrcia bella Leaf Extract Presents Hypoglycemic Activity via PI3k/Akt Insulin Signaling Pathway. Evid Based Complement Alternat Med. 2014;2014:1–11. doi:10.1155/2014/543606
30. Geetha G, Kalavalarasariel PG, Sankar V. Anti diabetic effect of Achyranthes rubrofusca leaf extracts on alloxan induced diabetic rats. Pak J Pharm Sci. 2011;24(2):193–199.
31. Joseph S, Kumar L, Bai VN. Evaluation of anti-diabetic activity of Strobilanthes cuspidata in alloxan induced diabetic rats and the effect of bioactive compounds on inhibition of [alpha]-amylase enzyme. J Pharmacogn Phytochem. 2016;5(3):169.
32. Vidhya R, Gandhi GR, Jothi G, Radhika J, Brindha P. Evaluation of antidiabetic potential of Achyranthes aspera Linn. on alloxan induced diabetic animals. Int J Pharm Pharm Sci. 2012;4(5):577–580.
33. Kabbaoui M, Chda A, Mejrhit N, et al. Antidiabetic effect of Thymus satureioides aqueous extract in streptozotocin-induced diabetic rats. Int J Pharm Pharm Sci. 2016;8(9):140–145. doi:10.22159/ijpps.2016v8i9.12647
34. Adisa RA, Choudhary MI, Olorunsogo OO. Hypoglycemic activity of Buchholzia coriacea (Capparaceae) seeds in streptozotocin-induced diabetic rats and mice. Exp Toxicol Pathol. 2011;63(7–8):619–625. doi:10.1016/j.etp.2010.05.002
35. Hammeso WW, Emiru YK, Ayalew Getahun K, Kahaliw W. Antidiabetic and antihyperlipidemic activities of the leaf latex extract of aloe megalacantha baker (Aloaceae) in Streptozotocin-induced diabetic model. Evid Based Complement Alternat Med. 2019;2019:1–9. doi:10.1155/2019/8263786
36. Aboonabi A, Rahmat A, Othman F. Antioxidant effect of pomegranate against streptozotocin-nicotinamide generated oxidative stress induced diabetic rats. Toxicol Rep. 2014;1:915–922. doi:10.1016/j.toxrep.2014.10.022
37. Lukic ML, Stošic-Grujicic S, Shahin A. Effector mechanisms in low-dose streptozotocin-induced diabetes. J Immunol Res. 1998;6(1–2):119–128.
38. Parmar GR, Pundarikakshudu K, Balaraman R. Antidiabetic and antihyperlipidemic activity of Euphorbia thymifolia L. extracts on streptozotocin-nicotinamide induced type 2 diabetic rats. J Appl Pharm Sci. 2017;7(08):078–084.
39. Gebreyohannis T, Shibeshi W, Asres K. Effects of Solvent Fractions of Caylusea abyssinica (Fresen.) Fisch. and Mey. on Blood Glucose Levels of Normoglycemic, Glucose Loaded and Streptozotocin-Induced Diabetic Rodents. J Nat Remedies. 2013;14(1):67–75.
40. Tanko Y, Jimoh AG, Mohammed A, Musa K. Hypoglycaemic effects of the methanolic extract of aerial part of Chrysanthellum indicum in rats. J Nat Prod Plant Res. 2011;1:1–7.
41. Nagmoti DM, Kothavade PS, Bulani VD, Gawali NB, Juvekar AR. Antidiabetic and antihyperlipidemic activity of Pithecellobium dulce (Roxb.) Benth seeds extract in streptozotocin-induced diabetic rats. Eur J Integr Med. 2015;7(3):263–273. doi:10.1016/j.eujim.2015.01.001
42. Tekulu GH, Araya EM, Mengesha HG. In vitro α-amylase inhibitory effect of TLC isolates of Aloe megalacantha baker and Aloe monticola Reynolds. BMC Complement Altern Med. 2019;19(1):206. doi:10.1186/s12906-019-2622-5
43. Aloulou A, Hamden K, Elloumi D, et al. Hypoglycemic and antilipidemic properties of kombucha tea in alloxan-induced diabetic rats. BMC Complement Altern Med. 2012;12(1):63. doi:10.1186/1472-6882-12-63
44. Jadhav R, Puchchakayala G. Hypoglycemic and antidiabetic activity of flavonoids: boswellic acid, ellagic acid, quercetin, rutin on streptozotocin-nicotinamide induced type 2 diabetic rats. Group. 2012;1:100g.
45. Dinku T, Tadesse S, Asres K. Antidiabetic activity of the leaf extracts of Pentas schimperiana subsp. schimperiana (A. Rich) Vatke on alloxan-induced diabetic mice. Ethiop Pharm J. 2010;28:22–26.
46. Ramkumar KM, Vanitha P, Uma C, Suganya N, Bhakkiyalakshmi E, Sujatha J. Antidiabetic activity of alcoholic stem extract of Gymnema montanum in streptozotocin-induced diabetic rats. Food Chem Toxicol. 2011;49(12):3390–3394. doi:10.1016/j.fct.2011.09.027
47. Prakash O, Kumar R, Srivastava R, Tripathi P, Ajeet MS. Plants explored with Anti-diabetic properties: a review. Am J Pharmacol Sci. 2015;3(3):55–66.
48. Dessalegn E, Bultosa G, Haki GD, Rupasinghe HV. Antioxidant and-amylase inhibition activities in vitro of various solvent extracts of Thymus schimperi Ronniger. J Med Plant Res. 2015;9(15):515–524. doi:10.5897/JMPR2014.5431
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
