Back to Journals » Journal of Experimental Pharmacology » Volume 13
Evaluation of Antidiabetic and Antioxidant Activity of Leaf Extract and Solvent Fractions of Hypoestes forskaolii (Val) (Acanthaceae) in Mice
Authors Wakene W , Asmamaw S, Kahaliw W
Received 4 May 2021
Accepted for publication 5 August 2021
Published 22 August 2021 Volume 2021:13 Pages 859—872
DOI https://doi.org/10.2147/JEP.S318696
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Paola Rogliani
Wakuma Wakene,1 Solomon Asmamaw,2 Wubayehu Kahaliw2
1Department of Pharmacy, College of Health Sciences, Mettu University, Mettu, Ethiopia; 2Department of Pharmacology, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Wakuma Wakene Tel +251 963421238
Email [email protected]
Background: Currently, there is a demand for new antidiabetic drugs from natural therapeutic agents, and diabetes mellitus disease is global epidemic. The leaf of Hypoestes forskaolii has been used in the traditional health system for the management of diabetes mellitus in Ethiopia and Eritrea. The aim of this study was to evaluate in vivo antidiabetic, antihyperlipidemic and in vitro antioxidant activity of leaf extract and solvent fractions of the leaf of H. forskaolii (Vahl) in mice.
Methods: The blood glucose-lowering activities of leaf extract and solvent fractions of the leaf of H. forskaolii were screened in the normoglycemic, glucose loaded, and streptozotocin-induced diabetic mice models. In the treatment of streptozotocin-induced diabetic mice with leaf extract and solvent fractions, weight and lipid profile was measured. Antioxidant activity of the plant leaf extract was determined using DPPH assay.
Results: The leaf extract of H. forskaolii showed significant blood glucose reduction in the normoglycemic model and glucose loaded test at doses of 200 mg/kg (34.1%, p < 0.001 and 55.5%, p < 0.001), respectively, as compared to the normal control. In the streptozotocin-induced diabetic model, extract and solvent fractions significantly (p < 0.001) reduced blood glucose level at all tested doses (100mg/kg, 200mg/kg and 400mg/kg) on the 14th day as compared to diabetic control. In addition, a significant reduction (p < 0.001) of serum TC, VLDL, LDL, TG was observed. In the antioxidant activity test, the IC50 values of extract and a standard drug (ascorbic acid) were 4.87μg/mL and 15.7μg/mL, respectively.
Conclusion: The present study showed that the methanolic leaf extract and solvent fractions of H. forskaolii have antidiabetic, and antioxidant activity that provides a scientific support for the local use of the plant leaves in the treatment of diabetes.
Keywords: diabetic mellitus, H. forskaolii, streptozotocin, hyperlipidemia
Background
Diabetes mellitus is a metabolic disorder featured by chronic hyperglycemia with disturbances of carbohydrate, fat, and protein metabolism resulting from abnormalities in insulin secretion, insulin action, or both.1 According to an IDF report 463 million people with diabetes in the world in 2019, it will rise to 578 million by 2030 and the number will rise to 700 million people with DM in 2045.2 Diabetes mellitus is known to cause hyperlipidemia through various metabolic derangements, which is found in about 40% of diabetic patients and diminishing antioxidant defense mechanism through the process of chronic oxidative stress due to hyperglycemia, which in turn causes defective insulin gene expression and insulin secretion as well as increased apoptosis this is the fact linking diabetes mellitus with oxidative stress.3,4
Even though insulin therapy and oral hypoglycemic agents are the first-line treatment for diabetes mellitus, they have some side effects and fail to significantly alter the course of diabetic complication.5 This limitation of currently available oral anti-diabetic agents either in terms of efficacy/safety coupled with the emergence of the disease into global epidemic have encouraged alternative therapy that can manage diabetes more efficiently and safely.6
H. forskaolii belongs to the family of Acanthaceae.7 In Ethiopia and Eritrea, the leaf of the plant has been used traditionally for the management of diabetes mellitus without scientific evidence of its safety or efficacy.8,9 Of the phytochemical compounds identified, fusicoccane Diterpene compounds were previously isolated from the methanol root extract, and it has verified antioxidative and cytotoxic and genotoxic activities.10 To the best of the authors’ knowledge, there are no previous scientific reports on the antidiabetic activity of H. forskaolii. Therefore, this study aimed evaluation of antidiabetic and antioxidant activity of leaf extract and solvent fractions of hypoestes forskaolii (Val) (Acanthaceae) in mice.
Methods
Materials and Methods
Chemicals and Instruments
The following drugs, chemicals, and instruments were used in the study. Streptozotocin (Fisco Research laboratories, India), glibenclamide (Julphar pharmaceuticals, Ethiopia), citric acid (Lab tech chemicals, India), ascorbic acid (Blulux Labratories, India), n-hexane (Loba chemie, India), chloroform (Aristar, England), sodium hydroxide (BluluxLabratories, India), DPPH (Sigma-Aldrich, Germany), sodium citrate (Lab tech chemicals, India), Methanol (Nice chemicals, India), 5% glucose solution (Reyoung pharmaceuticals, China), What man filter paper No.1, test tube, beakers, funnels, measuring cylinder, glass rod, spatula, pipettes, gavages (oral feeding syringes), Syringes (1 mL, 3 mL, and 5 mL) with needles, desiccators, digital analytical balance (EPH-400 Abron Exports), pH meter (Bante Instruments, UK), Sensor Card glucometer and strip (Alliance international, Taiwan), Lyophilizer (Labfreez, China), Hot air oven (Medit-Medizin Technik, Germany), Automated chemistry analyzer (Beckman coulter, Germany), UV-spectrophotometer (Agilent Technologies, Malaysia), rotary evaporator (Hamato, Japan), Tween-80 (Avishkar Lab Tech chemicals, India). All chemicals used were of analytical grade.
Plant Material
The fresh leaves of H. forskaolii were collected from the Dugda district (located in East Shewa Zone of Oromia Region, 135 km south of Addis Ababa). The plant material was identified and authenticated by Mr. Abiyu Enyew (botanist) and the voucher specimen (001/WWJ/2020) was deposited in the herbarium of the biology department, faculty of natural and computational science, University of Gondar. The leaves of the plant were thoroughly washed with tap water to remove dirt and dried under shade and optimal ventilation. The dried leaves were pulverized to a coarse powder using mortar and pestle.
Extraction and Fractionation
Extraction
A total of 1.1 kg of powder was macerated in 80% methanol (250 g in 1500 mL) in an erlenmeyer flask for 72 hours with occasional stirring at room temperature. After 72 hours, the mixture was filtered first with a muslin cloth and then by Whatman filter paper No. 1. The filtrate was kept in a refrigerator at 4°C while the marc was re-macerated twice for 72 hours using the same volume of 80% methanol to exhaustively extract the plant material. The combined filtrate obtained from successive maceration was concentrated under reduced pressure using a rotary evaporator (Hamato, Japan).
The extract was further concentrated to dryness by freeze-drying using a lyophilizer (Labfreez, China) to remove water solvent. After drying, the amount of dry extract obtained was harvested and the dried extract was transferred into airtight bottles and stored in a refrigerator at 4°C until used.11
Fractionation
Solvent fractionation of the leaf extract was carried out using water, chloroform, and n-hexane. Briefly, 65 gm extract was suspended in 400 mL of distilled water and transferred to a separating funnel. An equal volume of n-hexane was added to it and it was shaken vigorously. The mixture formed two layers; n-hexane, and aqueous layers, n-hexane layer was removed. The partition with n-hexane was repeated again two times in the same manner. The n-hexane layer was combined and subjected to evaporation using a hot air oven set at 40°C to get the n-hexane fraction. To the separating funnel containing aqueous layer, 400mL of chloroform was added. The mixture formed two layers and then the chloroform layer was removed and the aqueous layer was partitioned as with n-hexane twice again. The chloroform layer was pooled and concentrated using a hot air oven set at 40°C to obtain the chloroform fraction. The remaining aqueous layer was concentrated using a hot air oven (Medit-Medizin Technik, Germany) set at 40°C, frozen in a refrigerator overnight and lyophilized to remove water. After drying, the solvent fractions obtained were put in airtight bottles and stored in a refrigerator at 4°C until used.12
Experimental Animals
For all the models employed in this study, except in the cases of acute oral toxicity male mice were used because male mice are more sensitive to STZ and insulin than female mice.13 Healthy male Swiss albino mice (weighing 20–30 g and age of 6–10 weeks) were used in the study.14 The animals were obtained from the pharmacology department, University of Gondar. The animals were kept in polypropylene cages (6–10 animals per cage) under standard conditions (12 hours light and 12 hours dark cycle) with free access to a pellet diet and water ad libitum. They were acclimatized to the laboratory conditions one week before initiation of the experiment. All experiments were performed according to the National Academy of Sciences, Institute for Laboratory Animal Research, Division on Earth and Life Studies, National Academy of Sciences Guide for the Care and Use of Laboratory Animals, National Academy of Sciences, Institute for Laboratory Animal Research, Division on Earth and Life Studies, Washington, DC, USA, 8th edition, 201115 and approved by the research and ethics committee, Department of pharmacology, University of Gondar.
Preliminary Phytochemical Screening of Leaf Extract and Solvent Fractions
Leaf extract and solvent fractions of H. forskaolii were screened for the presence or absence of secondary metabolites such as alkaloids, steroidal compounds, phenolic compounds, tannin, saponins, terpenes, and flavonoids using standard procedures. Detection of Alkaloids (Mayer’s Test): About 0.2g H. forskaolii leaf extract and solvent fractions were dissolved individually in dilute hydrochloric acid and filtered. Filtrate was treated with Mayer’s reagent (Potassium Mercuric Iodide). Formation of a yellow-colored precipitate indicates the presence of Alkaloids; Detection Saponins (Foam Test): 0.5 g of H. forskaolii leaf extract and solvent fractions were shaken with 2 mL of water. If foam was produced which persists for 10 mins it indicates the presence of Saponins; Detection of Tannins (Braymer’s Test: 1 mL of the H. forskaolii leaf extract and solvent fractions were diluted with distilled water and added with 2 drops of ferric chloride). A transient greenish to black color indicates the presence of Tannins; Detection of Terpenoids (Copper acetate Test): An amount of 0.8 g of H. forskaolii leaf extract and solvent fractions were taken in a test tube, then poured 10 mL of methanol in it, shaken well and filtered to take 5 mL leaf extract and solvent fraction of plant sample. Then, 2 mL of chloroform was mixed in leaf extract and solvent fractions, and 3 mL of sulphuric acid was added in selected sample leaf extract and solvent fractions. Formation of reddish brown color indicates the presence of Terpenoids; Detection of Phenols (Ferric Chloride Test): H. forskaolii leaf extract and solvent fractions were treated with 3 to 4 drops of ferric chloride solution. Formation of bluish-black color indicates the presence of Phenols; Detection of Flavonoids (Alkaline Reagent Test): H. forskaolii leaf extract and solvent fractions were treated with few drops of sodium hydroxide solution. Formation of intense yellow color, which became colorless upon addition of dilute hydrochloric acid, indicates the presence of Flavonoids; Detection of Anthraquinones: H. forskaolii leaf extract and solvent fractions were (equivalent to 100 mg) shaken vigorously with 10 mL of benzene, filtered and 5 mL of 10% ammonia solution was added to the filtrate. The mixture was shaken and observed for the presence of a pink, red or violet color in the ammonia (lower) phase that indicates the presence of free Anthraquinones; Cardiac glycosides (Keller killianis test): About 100mg of H. forskaolii leaf extract and solvent fractions were dissolved in 1mL of glacial acetic acid containing one drop of ferric chloride solution. This was then under layer with 1mL of concentrated sulphuric acid. A brown ring obtained at the interface would indicate the presence of a deoxy sugar characteristic of cardenolides; and Steroids (Salkowski’s test): About 100mg of H. forskaolii leaf extract and solvent fraction were dissolved in 2mL of chloroform. Sulphuric acid was carefully added to form a lower layer. A reddish-brown color at the interface was an indicative of the presence of steroidal ring.16
Acute Oral Toxicity Test
An acute oral toxicity test was performed based on the OECD 425 guideline limit test procedure.17 Nulliparous, non-pregnant and healthy young adult female Swiss albino mice (age of 6–10 weeks and 20 −30 g) were employed. On the first day; one mouse was fasted for 3 hours before and one hour after being provided with 2000 mg/kg of the leaf extract/solvent fractions. Then, the mouse was observed for physical, neurological, autonomic, or behavioral changes at least once during the first 30 minutes, periodically for 24 hours, with special attention during the first 4 hours, and over for 24 hours. Following the results from the first mouse, the other four mice were recruited and fasted for 3–4 hours and administered a single dose of 2000 mg/kg and were observed in the same manner. They were observed for a total of 14 days for any signs of overt toxicity determination.
Grouping and Dosing of Animals
For both normoglycemic and glucose-loaded mice model, Swiss albino male mice fasted morning for 4–6 hours, but the water was allowed ad libitum and then randomly divided into five different groups (6 mice per group). Group I received distilled water at 10mL/kg, group II, received 5mg/kg glibenclamide, and the remaining three groups (III, IV & V) were treated with 100, 200, and 400 mg/kg dose of the extract, respectively.
In the single-dose STZ-induced model, mice were fasted overnight for 14 hours and were assigned randomly into fifteen groups (each group contained six mice). Then, group I were treated with vehicle (distilled water, for aqueous fraction and leaf extract), group II was treated with vehicle (2% tween-80, for chloroform and n-hexane fractions), group III were treated with glibenclamide 5 mg/kg and the remaining groups were treated with the different dose of H. forskaolii leaf extract (100, 200, and 400mg/kg); aqueous fraction (100, 200, and 400mg/kg); chloroform fraction (100, 200 and 400mg/kg) and n-hexane fraction (100, 200, and 400mg/kg).
In the repeated daily dose-treated STZ-induced model. Mice were fasted overnight for 14 hours and were assigned randomly into fourteen groups. Group- I received vehicle (distilled water, for both aqueous fraction and leaf extract, diabetic control), group-II received vehicle (2% tween-80 for chloroform fractions, diabetic control), group-III received vehicle (2% tween-80 for chloroform fraction, non-diabetic control), group- IV received vehicle (distilled water for aqueous fraction and leaf extract, non-diabetic control), group V, received glibenclamide 5mg/kg and the remaining groups received different dose of the H. forskaolii leaf extract (100, 200, and 400mg/kg); aqueous fraction (100, 200, and 400mg/kg), and chloroform fraction (100, 200 and 400 mg/kg).18
Based on the acute oral toxicity test results, the extract and solvent fraction doses were determined, with volume of distribution is 1 mL/100g of body weight of the mice. The middle dose was one-tenth of the limit test, the higher dose was two wise the middle dose and the lower dose was the half of the middle dose.19 Glibenclamide 5 mg/kg is selected as a standard drug for the study based on earlier studies.20 The study was conducted using the oral route of administration because the plant materials are traditionally used by people via the oral route.21
Measurement of Blood Glucose Level
To measure blood glucose, a blood sample was withdrawn from the tail vein of each mouse by cutting the tip of the tail aseptically in all animal models. Blood glucose levels were measured by using blood glucose meter and it was measured three times, then the average was taken.
Induction of Experimental Diabetes
The male Swiss albino mice fasted for 16 hours, and body weights were recorded before the induction of diabetes. Experimental diabetes was induced by a single intraperitoneal injection of 150mg/ kg of STZ, freshly dissolved in 0.1 M citrate buffer (pH = 4.5) and this smallest single dose of STZ required to induce diabetes in male mice.22,23 Thirty minutes after the administration of STZ, mice were allowed free access to food and water. After 6 hours’ administration of STZ, a 5% glucose solution was provided to the animals for the next 24 hours to prevent death secondary to hypoglycemic shock that is a result of hyperinsulinemia. After the three days of STZ injection, mice were screened for the development of diabetes, and fasting blood glucose level >200 mg/dl were included in the study as diabetes mice and randomly assigned into different groups.20
Evaluation of the Effect of Leaf Extract of Hypoestes forskaolii in Blood Glucose Level of Normoglycemic Mice
Mice were grouped and treated as described in section 3.7. Using aseptic conditions, a blood sample was collected from tips of the tail vein of each mouse to determine BGL. The baseline blood glucose level of each mouse was measured just before treatment (0 hours) then, the blood glucose level of each mouse was measured at 1, 2, 3, and 4 hours after treatment.24
Evaluation of the Effect of Leaf Extract of Hypoestes forskaolii in Oral Glucose Loaded Mice
Mice were grouped and treated as described in section 3.7. Thirty minutes before leaf extract treatment, all of the mice were loaded with 2 g/kg glucose solution orally; then, blood samples were collected before treatment (ie, 0 minutes), 30, 60, and 120 min after administration of extract in order to evaluate their blood glucose level.25
Antihyperglycemic Activity of a Single Dose of the Leaf Extract and Solvent Fractions of Hypoestes forskaolii in Diabetes Mice
Mice were grouped and treated as described in section 3.7. BGL was measured just before treatment (0 hours) baseline, and then at 2, 4, 6, and 8 hours post-treatment.26
Antihyperglycemic Activity of Repeated - Dose of Leaf Extract and Solvent Fractions of Hypoestes forskaolii in Diabetes Mice
Fasting blood glucose level and bodyweight of mice were measured just at day 0 (before treatment), 7th day and 14th day of treatment following overnight fasting for 14 hours. On the 15th day, blood sample was collected in a sterile tube by cardiac puncture under halothane anesthesia from over fasted (14 hours) diabetes mice. The blood sample was left at room temperature for 2 hours and then centrifuged. The supernatant was immediately separated from the pellet to prepare serum samples in order to determine the level of TG, TC, HDL, VLD, and LDL using an automated chemistry analyzer.25,27
Antioxidant Activity of Leaf Extract of Hypoestes forskaolii in Diphenyl-2-Picrylhydrazyl Assay
The free radical scavenging activity of the plant leaf extract was determined according to the method described by Brand William et al.28 The potential antioxidant activity of leaf extract was determined based on the scavenging activity of the stable DPPH free radical. The solution of DPPH was prepared (as 4mg of DPPH and dissolved in absolute methanol of 100mL, each time freshly prepared and stored in a dark and cool place). Ascorbic acid solution (6000 µg/3 mL, stock solution) was prepared by dissolving 6 mg ascorbic acid in 3 mL of absolute methanol. From the stock solution, 62.5, 125, 250, 500, 1000, and 2000 µg/mL dilutions were prepared. Concisely, 100 μL of test samples solution dissolved in methanol at different concentrations (2000, 1000, 500, 250, 125, and 62.5 μg/mL) were prepared from the leaf extract of the plant and subsequently transferred to a distinct test tube containing 3.9 mL of a 0.004% (w/v) DPPH radicals dissolved in methanol in separate test tubes. After keeping the reaction solution in dark for half an hour at room temperature, the absorbance of each solution was read at 517 nm by a UV-spectrophotometer. Vitamin C was used as a reference drug and examined under the same conditions. For the blank solution, DPPH solution (methanol and DPPH) was prepared.
IC50 is the amount of sample needed to inhibit 50% DPPH free radicals. Radical scavenging property of the test samples was described using percent inhibition and computed by the equation:
Where Abs control is the absorbance of the blank control in the absence of the test sample and Abs test sample is the absorbance of the solutions that contain test samples.
Statistical Analysis
The data were expressed as mean ± standard error of mean. Means of all parameters among groups were analyzed using ANOVA followed by the Tukey post hoc test. Statistical package for social sciences version 23 Software was used for statistical analysis. The result was considered significant when p < 0.05.
Results
Preliminary Phytochemical Screening of Leaf Extract and Solvent Fractions
Preliminary phytochemical analysis of the leaf extract and solvent fractions of H. forskaolii showed the presence of alkaloids, flavonoids, Terpenoids, tannins, phenolic compounds, saponins, and anthraquinones as shown in Table 1.
 |
Table 1 Preliminary Phytochemical Screening of Leaf Extract and Solvent Fractions |
The Effect of Leaf Extract of Hypoestes forskaolii on Blood Glucose Level of Normoglycemic Mice
The effect of H. forskaolii leaf extract on the blood glucose level in normal fasted mice is shown in Table 2. Blood glucose level of normal mice was significantly (p < 0.001) reduced at all tested doses at all-time points except 0 hour as compared to negative control and baseline data. On the other hand, at a time point of 3 and 4 hours, 200 and 400 mg/kg doses showed statistically insignificant blood lowering effect when compared to each other. The maximum BGL reduction was attained at the 4th hours, for CE 100, 200, 400mg/kg and glibenclamide 5mg/kg with percentage reduction of (22.95%, 34.2%, 31.1%, and 41.8%, respectively).
 |
Table 2 The Effect of Leaf Extract of Hypoestes forskaolii on Blood Glucose Level of Normoglycemic Mice |
The Effect of Leaf Extract of Hypoestes forskaolii on Oral Glucose Loaded Mice
The effects of leaf extract of H. forskaolii on oral glucose loaded mice are summarized in Table 3. The administration of glucose (2g/kg) to the mice showed peak BGL following one hour after glucose loaded, confirming the induction of hyperglycemia. The extract and GLC5mg showed the significant (p < 0.001) reduction in BGL from 60 minutes onwards as compared to 30 minutes and negative control.
 |
Table 3 The Effects of the Leaf Extract of Hypoestes forskaolii on Oral Glucose Loaded Mice |
Besides, DW 10ml also showed significant (p < 0.05 and p < 0.01) BGL reduction starting from 60 minutes to 120 minutes, respectively, compared to 30 minutes.
Antihyperglycemic Activity of a Single Dose of the Leaf Extract and Solvent Fractions of Hypoestes forskaolii in Diabetic Mice
The antihyperglycemic activity of a single dose of the leaf extract and solvent fractions were studied in diabetic mice and summarized in Table 4. The leaf extract, aqueous fraction, and chloroform fraction showed significant (p < 0.001) BGL reduction at a dose of 200mg/kg and 400mg/kg after 4 hours of treatment as compared to diabetic control while after 2 hours of treatment compared to baseline data. In addition, at a dose of 100mg/kg, the extract and aqueous fraction revealed significant (p < 0.01) BGL reduction after 6 hours of treatment compared to baseline and diabetic control data. Moreover, GLC5mg/kg showed significant (p < 0.001) BGL reduction beginning from 2 hours compared to baseline as well as diabetic control. In contrast, n-hexane fraction at a dose of 100 and 200mg/kg had no significant effect on BGL reduction compared to both the baseline and diabetic control. Moreover, no significant BGL reduction was observed with 2% tween-80 and DW treated group compared to baseline time.
 |
Table 4 Single Dose Antihyperglycemic Effect of Leaf Extract and Solvent Fractions of Hypoestes forskaolii in Diabetic Mice |
The maximal percentage reduction of BGL was observed with 48.5% in AF400mg, 46.4% in CE400mg, and 49.9% in GLC5mg treated group at the 8th hour compared to the respective.
Baseline line and the low BGL reduction were observed with HF100mg/kg, HF400mg/kg and HF200mg/kg with 22.5%, 22.12%, and 26.23% blood glucose level reduction.
The Effect of Repeated Doses of Leaf Extract and Solvent Fractions of Hypoestes forskaolii in Diabetic Mice
The effect of repeated doses of leaf extract and solvent fractions of H. forskaolii in diabetes mice has been shown in Table 5. Streptozotocin-induced mice showed a significant (p < 0.001) difference in BGL as compared to normal control at day zero. The leaf extract and chloroform fraction showed significant ((p < 0.01 and p < 0.001) BGL reduction at a dose of 200mg and 400mg/kg on the 7th and 14th days, respectively, as compared to diabetic control. Besides, the leaf extract and chloroform fraction, at a dose of 100mg/kg showed significant (p < 0.05 and p < 0.01) BGL reduction at the 7th and 14th days, respectively, compared to diabetic control. Besides, treatment with all tested doses of aqueous fraction and GLC5mg/kg showed a significant (p < 0.001) reduction of BGL at the 7th and 14th days as compared to diabetic control. Moreover, at all the tested dose of leaf extract, GLC 5mg/kg, and solvent fractions showed significant (p < 0.001) BGL reduction compared to baseline at 14th days. In contrast, diabetic control and the normal control group showed statistically insignificant BGL reduction on the 7th and 14th days compared to the respective baseline level.
 |
Table 5 The Effect of Repeated-Dose of Leaf Extract and Solvent Fractions of Hypoestes forskaolii in Diabetic Mice |
The highest BGL reduction was achieved by the three highest doses of leaf extract and each fraction at 14 days with the percentage reduction of BGL (45.3%), 45.31%), (47.92%) for leaf extract, chloroform fraction, and aqueous fraction, respectively.
The Effect of Leaf Extract and the Solvent Fraction of Hypoestes forskaolii on Body Weight of Diabetic Mice
The effects of the leaf extract and solvent fractions on body weight in diabetic mice are shown in Table 6. All groups before leaf extract and solvent fractions administration (day 0) showed no apparent difference in the bodyweight compared to normal control. All the tested doses of the leaf extract, solvent fractions of chloroform, and aqueous showed significant (p < 0.001) body weight improvement after the day 7th of treatment compared to the diabetic control. Moreover, glibenclamide significantly (P<0.01 and p < 0.001) improved the body weight after the day 7th of treatment as compared to diabetic control. In contrast, the bodyweight of the diabetic control was decreased significantly (p<0.001) after the day 7th of treatment compared to the normal control group.
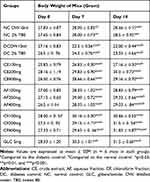 |
Table 6 The Effect of Leaf Extract and Solvent Fractions of Hypoestes forskaolii on Body Weight of Diabetic Mice |
The Effect of Leaf Extract and Solvent Fractions ofHypoestes forskaolii on Lipid Profile of Diabetic Mice
There was a significant (P<0.001) elevation of TC, LDL, VLDL, and TG level with significant (p < 0.001) reduction in HDL cholesterol in diabetic controls of each group of a fraction compared normal control, confirming the induction of diabetic dyslipidemia as shown in Table 7. The aqueous fraction at a dose of 100 mg/kg showed a significant reduction of STC and LDL (p < 0.01 and p < 0.001), respectively, compared to the diabetic control group. Besides, at a dose of 200mg/kg showed a significant (p < 0.001) reduction in serum level of TG and a significant (p < 0.05) increment in serum level of HDL-c compared to diabetic control. At all the tested doses of leaf extract (CE100, CE200, and CE400), chloroform fraction (CF100, 200, 400), and GLC5 to diabetic mice for 14 days showed a significant (p < 0.001) in a dose-dependent reduction in the serum level of TC, TG, LDL, and VLDL at the same time increasing HDL level compared to the diabetic control.
 |
Table 7 The Effect of Leaf Extract and Solvent Fractions of Hypoestes forskaolii on the Lipid Profile of Diabetic Mice |
Antioxidant Activity of Leaf Extract of Hypoestes forskaolii in Diphenyl-2-Picrylhydrazyl Assay
The antioxidant activities for leaf extract of H. forskaolii as well as the standard drug which is ascorbic acid, the least %RSA was obtained at the lowest test concentration (62.5μg/mL) while the highest %RSA was obtained at the highest test concentration (2000 μg/mL) antioxidant activity of the leaf extract was increased in a concentration-dependent manner like ascorbic acid (Figure 1). The IC50 for extracts was 15.7 µg/mL and for that of ascorbic acid was 4.87µg/mL (Figure 2). All tests were independently performed in triplicate and the definition of IC50 values of the tested compounds in the concentration required scavenging 50% of DPPH free radicals.
 |
Figure 1 Free radical scavenging activity of the leaf extract of Hypoestes forskaolii. Abbreviations: SAA, scavenging percent of ascorbic acid; shef, scavenging percent for H. forskaolii. |
Discussion
The present study aimed to investigate the antidiabetic and antioxidant activity of the 80% methanolic leaf extract and solvent fractions of H. forskaolii on normal and diabetic mice. Streptozotocin was used to induce diabetes in the current study. Streptozotocin is a potent methylating agent for DNA and acts as a nitric oxide donor in the pancreatic cells. Pancreatic cells are particularly sensitive to damage by nitric oxide (via inhibition of aconitase activity) and free radicals because of their low levels of free radical scavenging enzymes.29
The acute oral toxicity profile of the methanolic leaf extract of H. forskaolii was determined based on OECD guidelines 2008:425.30 At a dose of 2 g/kg; mortality and any toxicity were not observed. This suggests that the LD50 value of the methanolic leaf extract of H. forskaolii was found to be >2g/kg and the extract is better tolerated and safe on oral administration.31
The preliminary phytochemical screening of the methanolic leaf extract and solvent fractions of H. forskaolii showed the presence of saponins, tannins, Terpenoids, phenols, flavonoids, glycosides, and anthraquinones. These phytochemicals may have a protective or therapeutic potential for the treatment of diabetes via regenerating the damaged beta cells and stopping oxidative stress on beta cells in experimental diabetic rats.32
In the current study, the methanolic leaf extract of H. forskaolii and glibenclamide showed significant (p < 0.001) hypoglycemic effect in normal mice with maximum percentage reduction (34.1%) of BGL at a dose of 200mg/kg. It was observed that the leaf extract at 200mg/kg exerted its action in a non-dose dependent manner; particularly the higher dose (400mg/kg) produced less activity. This observation suggests that activity might decrease with dose. BGL reduction, probably, is the net effect of the interplay between various constituents of the extract. It is likely that higher doses may activate non-specifically both BGL lowering and raising mechanisms. Indeed, it has been reported that the presence of interfering substances in plant extracts may diminish hypoglycemic effect.33 This finding is in line with Mamdouh et al of the previously observed hypoglycemic effect of the related family R. tuberosa in rabbit at 100mg/kg showed significant (p < 0.05) with a percentage reduction of BGL (34.31%).34 In this study, phytochemical analysis of the leaf extract indicated the presence of flavonoid, phenol, saponins, and alkaloid and these phytochemicals have been reported to have insulin secretion effect from pancreatic β- cells.35 Thus, the hypoglycemic effect of the 80% methanolic leaf extract of H. forskaolii could possibly be related to its composition.
Oral glucose tolerance test measures the body’s ability to use glucose, the body’s main source of energy, and is used to diagnose prediabetes and diabetes.36 In the current study, mice treated with all test doses of extract and positive control showed significant (p < 0.001) blood glucose level reduction within 60 minutes. These results suggest that the methanolic leaf extract of H. forskaolii has antihyperglycemic activity by improving the glucose tolerance, by improving glucose utilization capacity within 60 minutes after extract administration. It has been demonstrated that flavonoids act against diabetes mellitus either through their capacity to avoid glucose absorption (inhibition of α-glycosidase activity in the intestine) or to improve glucose tolerance,37 and this could be the possible mechanisms of H. forskaolii extract to improve glucose tolerance.
In the current study, the results of in vitro DPPH scavenging assay suggested that the dose dependent radical scavenging activity of H. forskaolii could be the possible mechanism of antihyperglycemic activity of extract since this active constituent exists in H. forskaolii. This finding is in agreement with the previous study reported by Mothana et al.38 Plants having potent phytochemicals including phenolic and flavonoid compounds have strong antioxidant properties.39 Antioxidant activities of H. forskaolii extract might be due to the presence of phenolic and flavonoid compounds that have the capacity to donate hydrogen atoms or electrons and capture the free radical.40
In the single-dose diabetic model, 100mg/kg of chloroform and 100mg/kg and 200mg/kg of n-hexane fraction showed statistically insignificant BGL reduction as compared with baseline blood glucose level. This might be due to the insufficient concentration of active metabolite (s) in the lower dose of 100 mg/kg in contrast to the highest 400 mg/kg dose. Furthermore, low BGL reduction activity was observed with n-hexane fraction when compared with leaf extract and the other fraction. The possible reason could be due to the absence of secondary metabolites like alkaloids, phenols, and glycoside, which are present in other fraction and leaf extract of H. forskaolii. Moreover, in the single-dose diabetic model, the highest dose of n-hexane fraction produced the least BGL reduction. This observation suggests that activity might decrease with dose probably due to the net effect of the interplay between various constituents of the fraction and higher doses may likely activate non-specifically both BGL lowering and raising mechanisms.41,42 Due to its less BGL reduction of n-hexane fraction, it was not further investigated for their in vivo antihyperglycemic activity of repeated daily doses on diabetic mice.
In the present work, it was observed that all tested doses of leaf extract and solvent fractions in STZ-induced diabetic mice significantly reduced fasting BGL upon 14 days of repeated dose administration. The maximum percentage reduction of BGL was observed with the highest dose of aqueous fraction and this might be because of the polar character of secondary metabolites such as flavonoids,43 triterpenoids,44 and phenolic.45
Excessive free radicals generated from hyperglycemia-induced glucose autoxidation and protein glycosylation play an imperative role in DM pathogenesis.46 Preceding studies revealed that different plant extracts have shown pancreas β cell-protective activity due to their antioxidant activities47 and this could be the possible mechanism to lower blood glucose levels for H. forskaolii since it has antioxidant activity.
Previously, studies have been done on the same family of the plant. For instance, the antidiabetic activity of p. orientale was well known in normal, glucose loaded and STZ induced diabetic mice and the results have revealed a significant (P < 0.001) decrease in blood sugar level by the enhancement of pancreatic secretion of insulin from β- cells of islets in the streptozotocin-induced rat and again significantly reduces the serum cholesterol level (P < 0.01) in STZ diabetic mice.48 The present study is in line with this study as H. forskaolii has those effects and belongs to the same family. This finding was not in line with another study which reported that the n-hexane fraction had moderate glucose-lowering activity.34
In the current study, STZ-induced diabetic mice developed significant hyperglycemia, bodyweight loss, and diabetic dyslipidemia. In the STZ-induced diabetic mice, bodyweight loss is due to increased protein catabolism secondary to insulin deficiency.49 Previous studies demonstrated that various plant extracts improved body weight loss in STZ-induced diabetic animals.50 In this study, all the tested dose of extract and fractions showed significant (p < 0.001) improvement in body weight suggesting that the plant extract possibly could have protective effects against protein catabolism and muscle wasting possibly due to the enhancement of insulin secretion and/or action, and then improvement of glycemic control.
Lipoprotein lipase which can degrade TG and VLDL is less active in diabetic patients due to insulin deficiency that leads to the development of diabetic dyslipidemia.51 In diabetes; hyperlipidemia is featured with enhanced TG, TC, LDL, and VLDL and decreased HDL cholesterol levels. These changes increased the risk of coronary heart disease in patients with DM.52
In the current study, repeated dose administration of all tested doses of extract and solvent fractions for 14 days significantly (p < 0.001) decreased STC, STG, VLDL-C, and LDL-C levels and elevation of HDL-C level in a dose-dependent compared to diabetic control. The possibilities could be due to improved glucose utilization in the diabetic mice and the plant extract may have a direct effect on lipid absorption and metabolism that can lead to the improvement of diabetic dyslipidemia.53
Conclusion
This study revealed that the 80% methanolic leaf extract, aqueous fraction, and chloroform solvent fractions of H. forskaolii have shown significant lowering of blood glucose level on diabetic, normoglycemic, and oral glucose loaded mice and bodyweight improved in diabetic mice. The results also revealed that the n-hexane fraction has low blood glucose reduction in streptozotocin-induced mice.
The results also verified that free radical scavenging activity by the extract may contribute to the antihyperglycemic and antihyperlipidemic activity.
The results provide scientific support for the use of the plant in folk medicine for the management of diabetes and its associated complications. H. forskaolii would be promising for further clinical studies in the management of DM.
Abbreviations
ANOVA, Analysis of Variance; BGL, Blood Glucose Level; DPP-4, Dipeptidyl Peptidase-4; DPPH, Diphenyl-1-Picrylhydrazyl; DM, Diabetes Mellitus; FPG, Fasting Plasma Glucose; GABA, Gamma Amino butyric Acid; GDM, Gestational Diabetes Mellitus; HbA1c, Hemoglobin A1C; HNF, Hepatocyte Nuclear Factor; IDF, International Diabetes Federation.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethical Approval
The experimental protocol was approved by the ethical review committee of School of Pharmacy, University of Gondar.
Acknowledgments
The authors would like to express their thanks to the Department of Pharmacology, School of Pharmacy of the University of Gondar, for providing all the necessary lab facilities. We also like to appreciate Mr. Abiyu Enyew (botanist), for the identification and authentication of the plant material. The Principal investigator (wakuma wakene) wishes to express his heartfelt gratitude to the Department of Pharmacy of Mettu-University for covering his living expenses throughout the study period.
Author Contributions
Wakuma Wakene conceived the idea, drafted the proposal, collected the plant materials, carried out all experiments, and prepared the final manuscript for publication. Solomon Asmamaw and Wubayehu Kahaliw were involved in the design of the study and in revising the manuscript critically for important intellectual content. All authors equally contributed to conception, design of the study, acquisition of data supervision of data collection, analysis and interpretation, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation. 2006.
2. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas. Diabetes Res Clin Pract. 2019;157:107843. doi:10.1016/j.diabres.2019.107843
3. Bandeira DM, Da Fonseca LJS, Guedes DS, Rabelo LA, Goulart MO, Vasconcelos SML. Oxidative stress as an underlying contributor in the development of chronic complications in diabetes mellitus. Int J Mol Sci. 2013;14(2):3265–3284. doi:10.3390/ijms14023265
4. Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress—a concise review. Saudi Pharm J. 2016;24(5):547–553. doi:10.1016/j.jsps.2015.03.013
5. Patel D, Prasad SK, Kumar R, Hemalatha S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac J Trop Biomed. 2012;2(4):320–330. doi:10.1016/S2221-1691(12)60032-X
6. Erasto P, Adebola P, Grierson D, Afolayan A. An ethnobotanical study of plants used for the treatment of diabetes in the Eastern Cape Province, South Africa. Afr J Biotech. 2005;4(12).
7. Al Musayeib NM, Mothana RA, Mohamed GA, Ibrahim SR, Maes L. Hypoestenonols A and B, new fusicoccane diterpenes from Hypoestes forskalei. Phytochem Lett. 2014;10:23–27. doi:10.1016/j.phytol.2014.06.020
8. Beyi MW. Ethnobotanical investigation of traditional medicinal plants in Dugda District, Oromia Regio. J Med Plants Stud. 2018;2(1):1007.
9. Yemane B, Andebrhan M, Surender Reddy K. Traditional medicinal plants used by Tigrigna Ethnic Group in Central Region of Eritrea. IOSR J Pharm Biol Sci. 2017;12(3):40–46.
10. D’Ambola M, Fiengo L, Chini MG, et al. Fusicoccane diterpenes from hypoestes forsskaolii as heat shock protein 90 (Hsp90) modulators. J Nat Prod. 2019;82(3):539–549. doi:10.1021/acs.jnatprod.8b00924
11. Geleta B, Makonnen E, Debella A, Tadele A. In vivo antihypertensive and antihyperlipidemic effects of the crude extracts and fractions of Moringa stenopetala (Baker f.) Cufod. leaves in rats. Front Pharmacol. 2016;7:97. doi:10.3389/fphar.2016.00097
12. Molla M, Gemeda N, Abay SM. Investigating potential modes of actions of mimusops kummel fruit extract and solvent fractions for their antidiarrheal activities in mice. Evid Based Complement Alternat Med. 2017;2017:1–11. doi:10.1155/2017/4103410
13. Vital P, Larrieta E, Hiriart M. Sexual dimorphism in insulin sensitivity and susceptibility to develop diabetes in rats. J Endocrinol. 2006;190(2):425–432. doi:10.1677/joe.1.06596
14. Etuk EU. Animal model for studying diabetes mellitus. Agric Biol J N Am.2010;1(2):130–134.
15. National Academy of Sciences, Institute for Laboratory Animal Research, Division on Earth and Life Studies. National Academy of Sciences Guide for the Care and Use of Laboratory Animals.
16. Pandey A, Tripathi S. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. Int J Pharmacogn Phytochem. 2014;2(5):115–119.
17. Guideline OO. 425: Acute oral toxicity—up-and-down procedure. OECD Guide Test Chem. 2001;2:12–16.
18. Tesfaye A, Makonnen E, Gedamu S. Hypoglycemic and antihyperglycemic activity of aqueous extract of Justicia Schimperiana leaves in normal and streptozotocin-induced diabetic mice. Int J Pharm Sci Res. 2016;7(2):110–113.
19. Toxicity–Up AO. OECD Guideline for the Testing of Chemicals. Vol. 425. 2008:1–2
20. Tamiru W, Engidawork E, Asres K. Evaluation of the effects of 80% methanolic leaf extract of Caylusea abyssinica (fresen.) fisch. & Mey. on glucose handling in normal, glucose loaded and diabetic rodents. BMC Complement Altern Med. 2012;12(1):151. doi:10.1186/1472-6882-12-151
21. Beyi M. Ethnobotanical investigation of traditional medicinal plants in Dugda District, Oromia Region. J Med Plants Stud. 2018;2(1):1007.
22. Deeds M, Anderson J, Armstrong A, et al. Single dose streptozotocin-induced diabetes: considerations for study design in islet transplantation models. Lab Anim. 2011;45(3):131–140. doi:10.1258/la.2010.010090
23. Etuk E. Animals models for studying diabetes mellitus. Agric Biol JN Am. 2010;1(2):130–134.
24. Gebreyohannis T, Shibeshi W, Asres K. Effects of solvent fractions of caylusea abyssinica (Fresen.) Fisch. and Mey. on blood glucose levels of normoglycemic, glucose loaded and streptozotocin-induced diabetic rodents. J Nat Remedies. 2013;14(1):67–75.
25. Toma A, Makonnen E, Mekonnen Y, Debella A, Adisakwattana S. Antidiabetic activities of aqueous ethanol and n-butanol fraction of Moringa stenopetala leaves in streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2015;15(1):242. doi:10.1186/s12906-015-0779-0
26. Birru EM, Abdelwuhab M, Shewamene Z. Effect of hydroalcoholic leaves extract of Indigofera spicata Forssk. on blood glucose level of normal, glucose loaded and diabetic rodents. BMC Complement Altern Med. 2015;15(1):321. doi:10.1186/s12906-015-0852-8
27. Kifle ZD, Yesuf JS, Atnafie SA. Evaluation of in vitro and in vivo anti-diabetic, anti-hyperlipidemic and anti-oxidant activity of flower crude extract and solvent fractions of hagenia abyssinica (rosaceae). J Exp Pharmacol. 2020;12:151–167. doi:10.2147/JEP.S249964
28. Brand-Williams W, Cuvelier M-E, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28(1):25–30. doi:10.1016/S0023-6438(95)80008-5
29. Spinas GA. The dual role of nitric oxide in islet β-cells. Physiology. 1999;14(2):49–54. doi:10.1152/physiologyonline.1999.14.2.49
30. Sosa S, Pelin M, Cavion F, Hervé F, Hess P, Tubaro A. Acute oral toxicity of pinnatoxin G in mice. Toxins. 2020;12(2):87. doi:10.3390/toxins12020087
31. Oliver-Bever B. Medicinal Plants in Tropical West Africa. Cambridge university press; 1986.
32. Pourmorad F, Hosseinimehr S, Shahabimajd N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr J Biotech. 2006;5:11.
33. Tamiru W, Engidawork E, Asres K. Evaluation of the effects of 80% methanolic leaf extract of Caylusea abyssinica (fresen.) fisch. & Mey. on glucose handling in normal, glucose loaded and diabetic rodents. BMC Complement Altern Med. 2012;12(1):1–7.
34. Shahwar D, Ullah S, Ahmad M, Ullah S, Ahmad N, Akmal KM. Hypoglycemic Activity of Ruellia Tuberosa Linn (Acanthaceae) in Normal and Alloxan-Induced Diabetic Rabbits. Iran J Pharm Sci. 2011;7(2):107–115.
35. Tanko Y, Jimoh AG, Mohammed A, Musa K. Hypoglycaemic effects of the methanolic extract of aerial part of Chrysanthellum indicum in rats. J Nat Prod Plant Resour. 2011;1:1–7.
36. Fatokun FK, Danckwerts MP, Crowther N. Oral glucose tolerance of traditional medicines in a diabetes induced rat model. Int J Pharmacogn Phytochem Res. 2012;4:41–48.
37. Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol C Toxicol Pharmacol. 2003;135(3):357–364.
38. Al Haidari RA. A review of traditional uses, phytochemicals and bioactivities of the genus hypoestes. Afr J Tradit Complement Altern Med. 2018;15(3):1–17.
39. Meshram A, Srivastava N. Phytochemical screening and in vitro antioxidant potential of methanolic extract of epipremnum aureum (Linden and Andre) GS bunting. Int J Pharm Res Allied Sci. 2016;5(2):1–6.
40. Rahman M, Hossain S, Rahaman A, et al. Antioxidant activity of centella asiatica (Linn.) urban: impact of extraction solvent polarity. Int J Pharmacogn Phytochem. 2013;1(6):27–32.
41. Kesari AN, Gupta RK, Singh SK, Diwakar S, Watal G. Hypoglycemic and antihyperglycemic activity of Aegle marmelos seed extract in normal and diabetic rats. J Ethnopharmacol. 2006;107(3):374–379. doi:10.1016/j.jep.2006.03.042
42. Alarcon-Aguilar F, Jimenez-Estrada M, Reyes-Chilpa R, Roman-Ramos R. Hypoglycemic effect of extracts and fractions from Psacalium decompositum in healthy and alloxan-diabetic mice. J Ethnopharmacol. 2000;72(1–2):21–27. doi:10.1016/S0378-8741(00)00202-6
43. Sharma B, Salunke R, Balomajumder C, Daniel S, Roy P. Anti-diabetic potential of alkaloid rich fraction from Capparis decidua on diabetic mice. J Ethnopharmacol. 2010;127(2):457–462. doi:10.1016/j.jep.2009.10.013
44. Ivorra M, Paya M, Villar A. A review of natural products and plants as potential antidiabetic drugs. J Ethnopharmacol. 1989;27(3):243–275. doi:10.1016/0378-8741(89)90001-9
45. Arul B, Kothai R, Christina AJM. Hypoglycemic Activity of Casearia Esculenta Roxb in Normal and Diabetic Albino Rats. Iran J Pharm Sci. 2006;5(1):47–51.
46. Hameed I, Masoodi SR, Mir SA, Nabi M, Ghazanfar K, Ganai BA. Type 2 diabetes mellitus: from a metabolic disorder to an inflammatory condition. World J Diabetes. 2015;6(4):598. doi:10.4239/wjd.v6.i4.598
47. Masjedi F, Gol A, Dabiri S. Preventive effect of garlic (Allium sativum L.) on serum biochemical factors and histopathology of pancreas and liver in streptozotocin-induced diabetic rats. Iran J Pharm Res. 2013;12(3):325.
48. Nigam V. Antihyperglycaemic activity on flower of Polygonum orientale Linn. using steptozotocin induced diabetic mice model. Int J Pharm Teach Pract. 2013;4(3):1–7.
49. Eleazu C, Iroaganachi M, Okafor P, Ijeh I, Eleazu K. Ameliorative potentials of ginger (Z. officinale Roscoe) on relative organ weights in streptozotocin induced diabetic rats. Int J Biomed Sci. 2013;9(2):82.
50. Sulyman A, Akolade J, Sabiu S, Aladodo R, Muritala H. Antidiabetic potentials of ethanolic extract of Aristolochia ringens (Vahl.) roots. J Ethnopharmacol. 2016;182:122–128. doi:10.1016/j.jep.2016.02.002
51. Poungvarin N, Lee J, Yechoor V, et al. Carbohydrate response element-binding protein (ChREBP) plays a pivotal role in beta cell glucotoxicity. Diabetologia. 2012;55(6):1783–1796. doi:10.1007/s00125-012-2506-4
52. Liu Y, Sun J, Rao S, et al. Antidiabetic activity of mycelia selenium-polysaccharide from Catathelasma ventricosum in STZ-induced diabetic mice. Food Chem Toxicol. 2013;62:285–291. doi:10.1016/j.fct.2013.08.082
53. Rouhi-Boroujeni H, Rouhi-Boroujeni H, Heidarian E, Mohammadizadeh F, Rafieian-Kopaei M. Herbs with anti-lipid effects and their interactions with statins as a chemical anti-hyperlipidemia group drugs: a systematic review. ARYA Atheroscler. 2015;11(4):244.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


