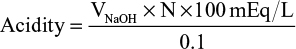Back to Journals » Journal of Experimental Pharmacology » Volume 9
Evaluation of anti-ulcer activity of the leaf extract of Osyris quadripartita Decne. (Santalaceae) in rats
Authors Abebaw M, Mishra B , Gelayee DA
Received 21 October 2016
Accepted for publication 30 November 2016
Published 16 January 2017 Volume 2017:9 Pages 1—11
DOI https://doi.org/10.2147/JEP.S125383
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bal Lokeshwar
Mastewal Abebaw, Bharat Mishra, Dessalegn Asmelashe Gelayee
Department of Pharmacology, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Abstract: Osyris quadripartita (OQ) Salzm. ex Decne. has been used to treat peptic ulcer disease in Ethiopian folk medicine, but its efficacy has not been validated. The present study was therefore carried out to evaluate the anti-ulcer activity of 80% methanol leaf extract of OQ in rats. The effect of OQ extract on gastric ulcer in rats in pylorus ligation-induced and ethanol-induced models was studied using single dosing (100, 200, 400 mg/kg) and repeated dosing (200 mg/kg for 10 and 20 days) approaches. Ranitidine (50 mg/kg) and sucralfate (100 mg/kg) were used as the standard drugs. Depending on the model, outcome measures were volume and pH of gastric fluid, total acidity, ulcer score, percent inhibition of ulcer score, ulcer index as well as percent inhibition of ulcer index. Data were analyzed using one-way analysis of variance followed by Tukey’s post hoc test, and P<0.05 was considered as statistically significant. OQ significantly (P<0.001) reduced gastric ulcer index by 55.82% and 62.11%, respectively, in pylorus ligation-induced and ethanol-induced ulcer models at the 400 mg/kg dose, which is comparable to the standard drugs. Ten and 20 days pre-treatment with OQ200 exhibited significant (P<0.001) ulcer inhibition by 66.48% and 68.36% (pylorus ligation-induced model) as well as 71.48% and 85.35% (ethanol-induced model), respectively. OQ possesses both dose-dependent and time-dependent anti-ulcer effect in the two models. The oral median lethal dose (LD50) is estimated to be higher than 2000 mg/kg for the crude hydroalcoholic extract, and secondary metabolites such as flavonoids, tannins, and saponins were present. The findings of this study confirmed that OQ has anti-ulcer pharmacologic activity due to one or more of the secondary metabolites present in it. Therefore, this study validates its anti-ulcer use in Ethiopian folk medicine. Further investigations on isolation of specific phytochemicals and elucidating mechanisms of action are needed.
Keywords: anti-ulcer activity, in vivo, Osyris quadripartita, rat
Introduction
Peptic ulcer disease and its complications remain the cause of significant morbidity worldwide, representing a major burden for health care resources.1 Although potent anti-ulcer drugs are available, most of them produce several toxicities, thus emphasizing the need to search for new alternatives.2 As high as 80% of the world population depends on plant-derived medicines for the first line of primary health care,3 reinforcing the theory that plant extracts can be good sources of new drugs. Ethiopia is a country characterized by a wide range of climatic and ecological conditions possessing enormous diversity of flora and fauna, including a wide range of potentially useful medicinal plants. The genus Osyris includes more than 34 species and belongs to the family Santalaceae. Osyris quadripartita (OQ) Salzm. ex Decne. (locally called qeret in Amharic and wato in Afaan Oromoo) is an evergreen, dioecious tree or shrub reaching a height of 1–7 m with many branches and the branches sometimes pendant.4 It is hemiparasitic and may opportunistically tap into the root systems of nearby plants and parasitize them, although it can freely grow and survive.5 It grows on rocky slopes and degraded woodland and scrub with an altitude of 1600–2900 m.6. The plant, which is native to Africa, Southwestern Europe, and Asia, is commonly known as wild tea plant. It is widely distributed in Ethiopia4 and used traditionally to treat peptic ulcer disease, cancer, toothache, malaria, skin lesion, abdominal pain, and urine problems among others.7–12 Several studies have been carried out to evaluate pharmacologic effects of OQ. It reduces capillary permeability associated with inflammation13 and has antioxidant,14 antibacterial, and antifungal6 as well as antimalarial10 activities. Phytochemicals such as polyphenols (flavonoids, lignans, coumarins), anthracene derivatives, and sesquiterpene lactones14 are present. OQ not only has several pharmacologic effects, but also it lacks acute toxic effect.10
Methods and materials
Chemicals and drugs
Glacial acetic acid (Sigma -Aldrich Chemie, Steinheim, Germany), benzene (Nice Laboratory Reagent, Kerala, India), chloroform (Super TeK chemicals, Uttar Pradesh, India), ethanol (Indenta Chemicals, Mumbai, India), ferric chloride (Super Tek Chemicals), lead acetate trihydrate (Guangdong Chemical Reagent Engineering, Guangdong, People’s Republic of China), mercuric chloride (Super Tek Chemicals), methanol (Nice Chemicals, Kochi, India), potassium iodide (Super Tek Chemicals), ranitidine (Cadila Pharmaceuticals, Bengaluru, India), sucralfate (Moraceae Pharmaceuticals Pvt. Ltd, Lucknow, India), sulfuric acid (Hi Media Laboratories Pvt. Ltd, Mumbai, India), hydrochloric acid (Nice Laboratory Reagent), sodium hydroxide (Rankem, Mumbai, India), and phenolphthalein (Fine Chemicals, Mumbai, India) were used.
Experimental animals
Healthy adult Wistar albino rats of either sex were selected randomly for the study. The rats were obtained from the animal house of the Department of Pharmacology, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar. Rats of 12–16 weeks, weighing 160–200 g, were used for the experiment. Each rat was housed in a plastic box cage under standard conditions at 19–25°C and was kept under 12/12 h light/dark cycle. The rats were allowed free access to standard pellet feed and water ad libitum. The study was carried out according to the National Research Council Guide for the Care and Use of Laboratory Animals and Organization of Economic Co-operation and Development (OECD) guidelines.15,16 Approval from the Research Review Committee of the Department of Pharmacology was also obtained.
Plant material collection and identification
The fresh leaves of OQ Salzm. ex Decne. (Santalaceae) were collected from Gondar area, Ethiopia, during the month of January 2016. Taxonomic identification was established by Mr Melaku Wondafirash who is an ethnobotanist in the Department of Biology and Biodiversity Management of Addis Ababa University (AAU), and the voucher specimen was deposited in the National Herbarium of AAU with voucher number designated as Mastewal 001.
Preparation of plant extract
The extraction procedure was carried out according to Girma et al10 with a slight modification. Fresh matured leaves of OQ were washed thoroughly, air dried at room temperature under shade, and coarsely powdered using mortar and pestle. The powder was kept in a tightly closed brown bottle until extraction. Then, 600 g of this coarsely powdered plant was macerated in 80% methanol with occasional stirring for 3 days at room temperature to obtain the hydroalcoholic crude extract. After 72 hours, the filtrate was separated from the marc by using a filter paper (Labsman No 1; Jignesh Agency Pvt. Ltd., Mumbai, India). The marc was re-macerated twice. The filtrates were combined and the alcohol was allowed to evaporate in an oven (TF55-1 ALC; France Etuves, Chelles, France) at 40°C. The percentage yield was found to be 35.83% w/w. The dried extract was stored in desiccators until the actual experiment.
Preliminary phytochemical screening
The crude methanol extract was assessed for secondary metabolites such as alkaloids, tannins, glycosides, steroids, terpenoids, flavonoids, saponins, and anthraquinones using standard methods.17,18
Acute toxicity test
Acute toxicity study was carried out using the limit test dose of 2000 mg/kg as described by OECD 425 guideline.16 Three female albino rats were fasted for 24 hours but allowed free access to water. A limit dose of 2000 mg/kg of OQ was administered sequentially and animals were observed individually for behavioral profile (alertness, restlessness, irritability, and fearfulness), autonomic profiles (defecation and urination), neurologic profile (spontaneous activity, reactivity, touch response, pain response, and gait), physical states such as lacrimation, loss of appetite, tremors, hair erection, salivation, diarrhea, and for morbidity or mortality, after dosing continuously for 2 hours, periodically during the first 24 hours (with special attention given during the first 4 hours) and daily thereafter, for a total of 14 days.
Grouping and dosing of animals
Animals were randomly assigned to different groups each consisting of six rats. All treatments were given orally 1 hour before the experiment by oral gavage. Doses were determined based on the acute toxicity studies as per OECD guidelines.16
Pylorus ligation model
For the single-dose study: Group 1 (negative control [NC], received distilled water), Group 2 (R50 ie, positive control and received ranitidine 50 mg/kg), Group 3 (OQ100 ie, received OQ 100 mg/kg), Group 4 (OQ200 ie, received OQ 200 mg/kg), and Group 5 (OQ400 ie, received OQ 400 mg/kg).
For the repeated dose in a 10-day study: Group 1 or negative control (distilled water), Group 2 or positive control (ranitidine 50 mg/kg), and Group 3 (OQ 200 mg/kg).
For the repeated dose in a 20-day study: Group 1 or negative control (distilled water), Group 2 or positive control (ranitidine 50 mg/kg), and Group 3 (OQ 200 mg/kg).
Ethanol-induced ulcer model
All treatments were given orally 1 hour before the experiment by oral gavage.
For the single-dose study: Group 1 (negative control [NC], received distilled water), Group 2 (S100 ie, positive control and received sucralfate 100 mg\kg), Group 3 (OQ100 ie, received OQ 100 mg/kg), Group 4 (OQ200 ie, received OQ 200 mg/kg), and Group 5 (OQ400 ie, received OQ 400 mg/kg).
For the repeated dose in a 10-day study: Group 1 or negative control (distilled water), Group 2 or positive control (sucralfate 100 mg/kg), and Group 3 (OQ 200 mg/kg).
For the repeated dose in a 20-day study: Group 1 or negative control (distilled water), Group 2 or positive control (sucralfate 100 mg/kg), and Group 3 (OQ 200 mg/kg).
Anti-ulcer activity evaluation
Pyloric ligation-induced ulcer model
The Shay rat model described by Dashputre and Naikwade was followed with a slight modification.19 Animals were fasted for 48 hours before the study, but had free access to water. After 1 hour of drug treatment, they were anesthetized with ether and the abdomen was opened by a small midline incision below the xiphoid process. Pyloric portion of the stomach was slightly lifted out and ligated. This was performed with caution to avoid traction to the pylorus or damage to its blood supply. The stomach was replaced carefully, and the abdominal wall was closed by interrupted sutures. Rats were sacrificed by an overdose of anesthetic ether after 6 hours of pyloric ligation. The abdomen was opened, cardiac end of the stomach was dissected out, and the contents were drained into a glass tube. The volume of the gastric juice was measured after centrifugation (Eppendorf AG-5703DQ713856) at 2000 rpm for 10 minutes. From the supernatant, aliquots (25 of 1 ml each) were taken for the determination of pH and total acidity. Each stomach was examined for lesions in the forestomach portion and indexed according to severity.
The 10-day and 20-day periods were selected based on a study done by Mohod and Modhankar,20 and the ulcer was induced on the 10th and 20th day of treatment after having the animals fasted for 48 hours. Scoring the ulcers was done as described below.
Macroscopic evaluation of stomachs
The stomachs were opened along the greater curvature and rinsed with water to remove gastric contents and blood clots and examined by a 10× magnifier lens to assess the formation of ulcers. The number of ulcers was counted. Scoring of ulcer was made as follows:19
Normal colored stomach (0),
Red coloration (0.5),
Spot ulcer (1),
Hemorrhagic streak (1.5),
Deep ulcers (2),
Perforation (3).
The total mucosal area and total ulcerated area were measured. The ulcer index was then calculated using the following equation:21
Ulcer index = 10/x
where × is the total mucosal area/ulcerated area.
Percentage inhibition of ulceration was calculated as below:
|
Determination of pH
An aliquot of 1 ml of gastric juice was diluted with 1 ml of distilled water, and pH of the solution was measured using pH meter (Adwa AD8000).19
Determination of total acidity
An aliquot of 1 ml of gastric juice was diluted with 1 ml of distilled water and was taken into a 50 ml conical flask and two drops of phenolphthalein indicator was added and titrated with 0.01N NaOH until a permanent pink color was observed. The volume of 0.01N NaOH consumed was noted. The total acidity was expressed as mEq/L and calculated by the following formula:19
|
where V is volume and N is normality.
Ethanol-induced gastric ulcer
The ulcer was induced by administering ethanol following the method by Dashputre and Naikwade with a slight modification.19 All the animals were fasted for 48 hours before administration of ethanol. The gastric ulcers were induced in rats by administrating ethanol (90%) (1 ml/200 g) orally, after 60 minutes of 80% methanolic extract and sucralfate treatment. They were kept in specially constructed cages to prevent coprophagia during the experiment. The animals were anesthetized 1 hour later with anesthetic ether, and the stomach was incised along the greater curvature and ulceration was scored as for the pyloric ligation-induced ulcer model.
The 10-day and 20-day repeated-dose approaches were selected based on a study done by Mohod and Bodhankar.20 The ulcer was induced on the 10th and 20th day of treatment after having the animals fasted for 48 hours. Scoring the ulcers was done as described above.
Statistical analysis
Data were expressed as mean ± standard error of mean and statistically evaluated using one-way analysis of variance, followed by Tukey’s multiple comparison tests using SPSS software version 20. P<0.05 was considered to be significant.
Results
Phytochemical screening
Preliminary phytochemical screening of OQ confirmed the presence of different secondary metabolites, as shown in the Table 1.
  | Table 1 Results for preliminary phytochemical screening of Osyris quadripartita |
Acute toxicity test
With the acute toxicity test at the limit test dose of 2000 mg/kg, neither mortality nor changes related to behavioral, autonomic, neurologic, and physical profiles were observed within the first 24 hours and during the 14-day follow-up.
Anti-ulcer effects of OQ
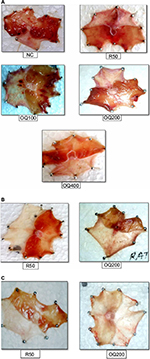
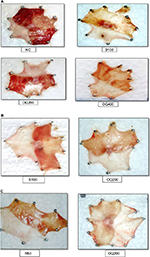
The effect of the hydroalcoholic leaf extract of OQ on gastric ulcers induced by pylorus ligation and ethanol was assessed by macroscopic evaluation (Figures S1 and S2).
Effects of the leaf extract on pylorus ligation-induced ulcer
Single-dose study
As shown in Table 2, treatment with the extract and the standard drug reduced the volume of gastric secretion, but increased pH was noted for OQ200, OQ400, and the standard drug. These changes, however, were not significantly different from those of the negative control. Both R50 and OQ400 produced a statistically significant decrease (P<0.05) in the total acidity when compared to the negative control. Ulcer index was significantly reduced by OQ200 (P<0.05), OQ400 (P<0.001) as well as standard drug R50 (P<0.01). In most of the parameters, the extract exhibited a dose-dependent effect. Interestingly, the extract at the highest dose demonstrated better effects than the standard drug in terms of percent inhibition of ulcer score and ulcer index.
Repeated-dose study
Pretreatment for 10 days
Compared to the negative control, OQ200 exhibited significant reduction in volume of gastric secretion (P<0.001) and total acidity (P<0.001), ulcer score (P<0.001), and ulcer index (P<0.001) similar to the standard drug. The effect of OQ200 was comparable to R50 regarding percent reduction in ulcer scores (75.48% vs 76.87%) and ulcer index (66.48% vs 63.72%) (Table 3).
Pretreatment for 20 days
Compared to the negative control, OQ200 exhibited significant reduction in volume of gastric secretion (P<0.001) and total acidity (P<0.001), ulcer score (P<0.001), and ulcer index (P<0.001) similar to the standard drug. The effect of OQ200 was comparable to R50 regarding percent reduction in ulcer scores (79.83% vs 78.26%) and ulcer index (68.42% vs 66.92%) (Table 4).
Effects of the leaf extract on ethanol-induced ulcer
Single-dose study
Both the ulcer score and ulcer index were significantly reduced by OQ200 (P<0.001), OQ400 (P<0.001), and the standard drug S100 (P<0.001). The effect of OQ400 was comparable to R50 regarding percent reduction in ulcer scores (58.09% vs 59.49%) and ulcer index (62.11% vs 67.4%) (Table 5).
Repeated-dose study
Pretreatment for 10 days
Both OQ200 and the standard drug produced a significant reduction in ulcer score (P<0.001) and ulcer index (P<0.001) as compared to the negative control. The reduction (%) in ulcer score and ulcer index was very high to the extract and sucralfate (Table 6).
Pretreatment for 20 days
Both OQ200 and the standard drug produced a significant reduction in ulcer score (P<0.001) and ulcer index (P<0.001) as compared to the negative control. The percent reduction in ulcer score and ulcer index for the extract was comparable to that of sucralfate (Table 7).
Discussion
This study was carried out to evaluate the anti-ulcer effect of 80% methanol leaf extract of OQ on pylorus ligation-induced and ethanol-induced gastric ulcer models. The 35.83% plant extraction yield is similar with the 40.11%6 and 32.75%10 reported in previous studies done in Ethiopia, thus supporting that hydroalcoholic solvent possesses a good extracting potential. Methanol releases more diverse phytochemicals such as anthocyanins, tannins, saponins, terpenoids, xanthoxylines, totarol, quassinoids, lectones, flavones, phenols, and polyphenols than other extraction solvents.22,23 The acute toxicity study revealed that the plant extract was safe in rats at a limit dose of 2000 mg/kg and that the median lethal dose (LD50) of the extract is above 2000 mg/kg. The finding supports the work done on mice in another study.10 In the preliminary phytochemical screening, the 80% methanol leaf extract of OQ was positive for flavonoids, saponins, tannins, phenols, terpenoids, and alkaloids. These secondary metabolites are effective as antioxidant, antineoplastic, anti-ulcer, anti-inflammatory, and immune stimulating agents.20,24–28 Flavonoids are thought to increase mucosal prostaglandin content, decrease histamine secretion from mast cells by inhibition of histidine decarboxylase, inhibit Helicobacter pylori growth, act as free radical scavengers, and inhibit H+/K+-ATPase.25,27,29 Saponins may activate mucous membrane protective factors, and tannins render the outermost layer of the mucosa less permeable, for instance, to chemical irritation.27 In addition, terpenoids and alkaloid compounds are also reported to have potent activity against gastric ulcers.30,31
The etiology of peptic ulcer is unknown in most of the cases, yet it is generally accepted that it results from an imbalance between aggressive factors and the maintenance of mucosal integrity through the endogenous defense mechanisms. To regain the balance, different therapeutic agents are used to inhibit the gastric acid secretion or to boost the mucosal defense mechanisms by increasing mucosal production, stabilizing the surface epithelial cells, or interfering with the prostaglandin synthesis.20,29,32–34
Pyloric ligation-induced ulcer model is an important method for the measurement of mean ulcer index in ulcerogenesis. Gastric ulceration in this method may be the stress-induced secretion of HCl in excess amounts from the parietal cells and autodigestion of mucosa by the gastric juice.20,29,32 Free radicals may also be associated since studies have shown changes in the antioxidant status following pylorus ligation-induced ulceration in rats.29 In the present study, different doses of the extract (100, 200, and 400 mg/kg) were evaluated for their effect on volume of gastric secretion, pH, total acidity, ulcer score, and ulcer index along with the standard drug ranitidine (50 mg/kg). In the single-dose study of the pylori ligation model, OQ100 did not show any better activity when compared to the negative control. This indicates that the low dose of the extract is not an adequate dose to produce ulcer healing. This model showed that the highest dose of the plant extract (OQ400) has got better antisecretory activity as evidenced by reduction in the mean volume of gastric secretion, rise in pH, and reduction in total acidity (P<0.01) compared to the negative control. Significant reduction in ulcer index (measure of ulcerated area) was noted for OQ200 (P<0.05) and OQ400 (P<0.001) as compared to the negative control. While R50 has more antisecretory effect than the extract, OQ400 amazingly exhibited a better reduction of ulcer index than the standard drug (2.35±0.25 vs 2.72±0.66), which might be due to more combined cytoprotective and antisecretory activity effect of the extract than of the standard drug. Flavonoids are suggested to possess both these effects.25,27 The better reduction in ulcer index noted for OQ400 compared with the standard drug R50 is in line with a report by Melese et al21 where the aqueous extract of Plantago lanceolata L. at a dose of 400 mg/kg showed a better ulcer inhibition than the standard drug ranitidine. Such findings strengthen the search for novel agents by tapping the rich herbal drugs used in folk medicine.
A repeated-dose study for two different time durations (10 and 20 days) was also carried out in the pylorus ligation-induced ulcer model and the effect of the extract on improvement of ulcer lesions was observed. The lowest effective dose (OQ200) in the single-dose study was chosen since repeated administration of the higher dose may not be safe. Compared to the negative control, pretreatment with R50 and OQ200 for 10 and 20 days produced a significant reduction in volume of gastric secretion (P<0.001) and total acidity (P<0.001), strengthening the antisecretory activity of the plant extract. Ulcer score (P<0.001) and ulcer index (P<0.001) were also decreased significantly. The percentage of ulcer inhibition slightly increased in a time-dependent manner from 10-day pretreatment of OQ200 (66.48%) to 20-day pretreatment (68.36%). When compared to 55.82% ulcer inhibition by the most effective dose (OQ400) in the single-dose study, the 10 and 20 days of pretreatment of OQ200 showed a better ulcer inhibition (66.48% and 68.42%, respectively). This suggests repeated dosing produces cumulative effect, which is better than single dosing.
The ability of the gastric mucosa to resist injury by endogenous secretions (acid, pepsin, and bile) and ingested irritants (eg, alcohol, nonsteroidal anti-inflammatory drugs [NSAIDs]) can be attributed to a number of factors that have been collectively referred to as “mucosal defense”.35 The concept of gastric cytoprotection against various necrotizing agents has been routinely used to assess the anti-ulcer potential of different compounds. The ethanol-induced acute gastric mucosal injury model is considered to be one of the widely used experimental models of ulcer disease.36 Ethanol easily penetrates the gastric mucosa and causes gastric ulcer 1 hour after administration. Ulceration is due to a decrease in gastric mucus, prostaglandin levels, glutathione, mucosal blood flow, and bicarbonate secretion as well as an increase in lipid peroxidation, oxidative stress, leukotriene production, and generation of free radicals leading to cell and membrane damage. It has been reported that leukotriene antagonist and 5-lipoxygenase inhibitors are capable of inhibiting alcohol and NSAID-induced gastric ulceration in rats.20,37,38
In the present study, the ethanol-induced model was employed to confirm the gastric cytoprotective effect of the plant extract. This test excluded the 100 mg/kg dose as it was noted to be least effective in the pylorus ligation model. Extract- and standard drug-treated groups showed a significant reduction (at least P<0.01) in both the ulcer score and ulcer index. The extract effect increases with the dose and is comparable with that of the standard drug. The repeated-dose study for ethanol-induced ulcer model showed a significant reduction in ulcer score (P<0.001) and ulcer index (P<0.001) in the 10-day and 20-day pretreatment with OQ200 and S100. This finding signifies that the extract possesses a gastroprotective effect, which is as good as the standard drug. The repeated-dose study with OQ200 showed a better reduction in percentage of ulcer inhibition, (71.48% for 10 days and 85.35% for 20 days) as compared to the single-dose study for OQ200 (54.3%) and OQ400 (62.11%). This suggests that the cumulative ulcer healing effect of the extract is better than that of the single dose in this model. The results of the present study showed that the OQ leaf extract was capable of inhibiting gastric lesions formed by ethanol. Absolute alcohol would extensively damage the gastric mucosa leading to increased infiltration of neutrophils, which are a major source of inflammatory mediators. Therefore, suppression of neutrophil infiltration during inflammation was found to enhance gastric ulcer healing.33,34,39 OQ leaf extract has been shown to contain anti-inflammatory activity,13 and it is speculated that the gastroprotective effect exerted by this plant could be credited to its anti-inflammatory property. Compounds that act as antioxidants or activate the redox system are important for restoring gastric tissue.30,40 Therefore, strong ulcer healing effect of the extract in the ethanol-induced model might also be related to the antioxidant activity of the plant, which is well demonstrated in previous studies.14 Flattening of the mucosal folds observed in the present study also suggests the gastroprotective effect of the extract. Different reports showed that the changes in the gastric motility may play a role in the development and prevention of experimental gastric lesions. Relaxation of circular muscles will increase the mucosal area to being exposed to necrotizing agents and reduce the volume of the gastric irritants on rugal crest, leading to protection of the gastric mucosa against damage.33,34,39
Conclusion
The findings in this study confirm, the absence of oral acute toxicity at the doses employed, and presence of anti-ulcer pharmacologic activity of OQ. Its efficacy is comparable to the standard drugs, with repeated administration being more effective than single dosing. Anti-ulcer effects may be related to antisecretory as well as cytoprotective activities of one or more of the identified phytochemicals. Thus, the present work validates the use of OQ for gastric ulcer in the Ethiopian folk medicine, and further studies shall focus on isolation of specific phytochemicals and elucidating mechanisms of action.
Acknowledgment
The authors are very thankful to the University of Gondar for allowing them to use the laboratory facilities.
Disclosure
The authors report no conflicts of interest in this work.
References
Tanih NF, Ndip LM, Clarke AM, Ndip RN. An overview of pathogenesis and epidemiology of Helicobacter pylori infection. Afr J Microbiol Res. 2010;4(6):426–436. | ||
Lavnya A, Kumar MP, Anbu J, Anjana A, Ayyasay S. Antiulcer activity of Canavalia virosa (ROXB) W&A leaves in animal model. Int J Life Sci Pharma Res. 2012;2(4):39–43. | ||
Panda V, Sonkamble M. Anti-ulcer activity of Ipomoea batatas tubers (sweet potato). Funct Foods Health Dis. 2012;2(3):48–61. | ||
Hedberg I, Edwards S. Pittosporaceae to Araliaceae. In: Hedberg I, Edwards S, editors. Flora of Ethiopia and Eritrea. Vol 3. Ethiopia: Addis Ababa: National Herbarium, Biology Department, Science Faculty, Addis Ababa University and Sweden: The Department of Systematic Botany, Uppsala University; 1989. | ||
Herrera CM. The annual cycle of Osyris quadripartita, a hemiparasitic dioecious shrub of Mediterranean scrublands. J Ecol. 1984;72(3):1065–1078. | ||
Taddese S, Asres K, Gbre-Mariam T. In vivo antimicrobial activities of some selected topically applied medical plants. Ethiop Pharma J. 2003;21:39–41. | ||
Suleman S, Alemu T. Survey on utilization of ethnomedicinal plants in Nekemte town, East Wellega (Oromia), Ethiopia. J Herbs Spices Med Plants. 2012;18(1):34–57. | ||
Enyew A, Asfaw Z, Kelbessa E, Nagappan R. Ethnobotanical study of traditional medicinal plants in and around Fiche district, Central Ethiopia. Curr Res J Biol Sci. 2014;6(4):154–167. | ||
Yineger H, Kelbessa E, Bekele T, Lulekal E. Plants used in traditional management of human ailments at Bale Mountains National Park, Southeastern Ethiopia. J Med Plants Res. 2008;2(6):132–153. | ||
Girma S, Giday M, Erko B, Mamo H. Effect of crude leaf extract of Osyris quadripartita on Plasmodium berghei in Swiss albino mice. BMC Complement Altern Med. 2015;15:184. | ||
Gebeyehu G, Asfaw Z, Enyew A, Raja N. Ethnobotanical study of traditional medicinal plants and their conservation status in Mecha Woreda, West Gojjam zone of Ethiopia. Int J Pharm Health Care Res. 2014;2(3):137–154. | ||
Getaneh S, Girma Z. An ethnobotanical study of medicinal plants in Debre Libanos Wereda, Central Ethiopia. Afr J Plant Sci. 2014;8(7):366–379. | ||
Gómez ME, Ayuso MJ, Toro MV. Activity of Osyris quadripartita salzm. methanol extract on capillary permeability in rats. Phytother Res. 1995;9(7):528–530. | ||
Rached W, Benamar H, Bennaceur M, Marouf A. Screening of the antioxidant potential of some Algerian indigenous plants. J Biol Sci. 2010;10(4):316–324. | ||
National Research Council. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, DC: The National Academies Press; 1996. | ||
Organization for Economic Cooperation and Development. OECD Guidelines for the Testing of Chemicals. Acute Oral Toxicity -Up-And-Down-Procedure. UDP; 425. France: OECD Publishing; 2008. | ||
Njoku VO. and Obi C. Phytochemical constituents of some selected medicinal plants. Afr J Pure Appl Chem. 2009;3(11):228–233. | ||
Wadood A, Ghufran M, Jamal SB, Naeem M, Khan A, Ghaffar R. Phytochemical analysis of medicinal plants occurring in local area of Mardan. Biochem Anal Biochem. 2013;2(4):1–4. | ||
Dashputre NL, Naikwade NS. Evaluation of anti-ulcer activity of methanolic extract of Abutilon indicum Linn leaves in experimental rats. Int J Pharm Sci Drug Res. 2011;3(2):97–100. | ||
Mohod SM, Bodhankar SL. Evaluation of antiulcer activity of methanolic extract of leaves of Madhuca indica J.F Gmel in rats. Pharmacologyonline. 2011;3:203–213. | ||
Melese E, Asres K, Asad M, Engidawork E. Evaluation of the antipeptic ulcer activity of the leaf extract of Plantago lanceolata L. in rodents. Phytother Res. 2011;25(8):1174–1180. | ||
Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: a review. Int Pharm Sci. 2011;1(1):98–106. | ||
Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12(4):564–582. | ||
Paguigan ND, Castillo DH, Chichioco-Hernandez CL. Anti-ulcer activity of leguminosae plants. Arq Gastroenterol. 2014;51(1):64–67. | ||
Repetto MG, Llesuy SF. Antioxidant properties of natural compounds used in popular medicine for gastric ulcers. Braz J Med Biol Res. 2002;35(5):523–534. | ||
de Sousa Falcão H, Leite JA, Barbosa-Filho JM, et al. Gastric and duodenal antiulcer activity of alkaloids: a review. Molecules. 2008;13(12):3198–3223. | ||
Borrelli F, Izzo AA. The plant kingdom as a source of anti-ulcer remedies. Phytother Res. 2000;14(8):581–591. | ||
Reddy VP, Sudheshna G, Afsar SK, et al. Evaluation of anti-ulcer activity of Citrullus colocynthis fruit against pylorus ligation induced ulcers in male Wistar rats. Int J Pharm Pharm Sci. 2012;4(2):446–451. | ||
Sharath SS, Preethy J, Kumar GS, et al. Screening for anti-ulcer activity of Convolvulus pluricaulis using pyloric ligation method in Wister rats. Int J Pharm Sci Res. 2015;6(1):89–99. | ||
Klein-Júnior LC, Santin JR, Niero R, de Andrade SF, Cechinel-Filho V. The therapeutic lead potential of metabolites obtained from natural sources for the treatment of peptic ulcer. Phytochem Rev. 2012:11(4):567–616. | ||
Mitra P, Ghosh T, Mitra PK. Anti gastric ulcer activity of Amaranthus spinosus Linn. leaves in aspirin induced gastric ulcer in rats and the underlying mechanism. SMU Med J. 2014;1(2):313–328. | ||
Raju D, Ilango K, Chitra V, Ashish K. Evaluation of anti-ulcer activity of methanolic extract of Terminalia chebula fruits in experimental rats. J Pharm Sci Res. 2009;1(3):101–107. | ||
Al-Radahe S, Ahmed KA, Salama S, et al. Anti-ulcer activity of Swietenia mahagoni leaf extract in ethanol-induced gastric mucosal damage in rats. J Med Plants Res. 2013;7(16):988–997. | ||
Abdulla MA, AL-Bayaty FH, Younis LT, Abu Hassa MI. Anti-ulcer activity of Centella asiatica leaf extract against ethanol-induced gastric mucosal injury in rats. J Med Plants Res. 2010;4(13):1253–1259. | ||
Wallace JL. Nonsteroidal anti-inflammatory drugs and the gastrointestinal tract. Mechanisms of protection and healing: current knowledge and future research. Am J Med. 2001;110(1A):19S–23S. | ||
Shawon L, Gautam P. An overview of the current methodologies used for evaluation of gastric and duodenal anti-ulcer agents. Pharmacologia. 2012;3(8):249–257. | ||
Nordin N, Salama SM, Golbabapour S, et al. Anti-ulcerogenic effect of methanolic extracts from Enicosanthellum pulchrum (King) Heusden against ethanol-induced acute gastric lesion in animal models. PLoS One. 2014;9(11):e111925. | ||
Roy SP, Prajapati K, Gupta R, et al. Evaluation of anti-ulcer effects of ethanolic extract of Delonix regia flower. Indian J Res Pharm Biotechnol. 2013;1(3):440–445. | ||
Al Batran R, Al-Bayaty F, Jamil Al-Obaidi MM, et al. In vivo antioxidant and antiulcer activity of Parkia speciosa ethanolic leaf extract against ethanol-induced gastric ulcer in rats. PLoS One. 2013;8(5):e64751. | ||
Hussain L, Akash MS, Naseem S, Rehman K, Ahmed KZ. Anti-ulcerogenic effects of Salmalia malabarica in gastric ulceration--pilot study. Adv Clin Exp Med. 2015;24(4):595–605. |
Supplementary materials
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.