Back to Journals » Vascular Health and Risk Management » Volume 18
Evaluation of Ameliorative Effect of Quercetin and Candesartan in Doxorubicin-Induced Cardiotoxicity
Authors Majhi S, Singh L, Yasir M
Received 13 July 2022
Accepted for publication 5 December 2022
Published 13 December 2022 Volume 2022:18 Pages 857—866
DOI https://doi.org/10.2147/VHRM.S381485
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Harry Struijker-Boudier
Sagarika Majhi,1 Lubhan Singh,2 Mohd Yasir3
1Department of Pharmacology, I. T. S College of Pharmacy, Ghaziabad, UP, India; 2Department of Pharmacology, Kharvel Subharti College of Pharmacy, Swami Vivekanand Subharti University, Meerut, UP, India; 3Department of Pharmacy, College of Health Sciences, Arsi University, Asella, Ethiopia
Correspondence: Mohd Yasir, Department of Pharmacy, College of Health Sciences, Arsi University, Asella 396, Oromia Region, Ethiopia, Tel +251-904748369, Email [email protected]
Background: Several mechanisms have been explored for the anthracycline myocardial toxicity. These are free-radical generation, myocyte apoptosis, lipid peroxidation, mitochondrial deterioration, and direct repression of muscle-specific gene expression. Adriamycin (Doxorubicin) is a potent anti-cancer agent. Adriamycin in prolonged use is fatal and generates free radicals that lead to dose-dependent cardiac toxicity.
Objective: The intent of the study was to explore the protective activity of candesartan and quercetin in cardiomyopathy induced by doxorubicin in rats.
Methods: To induce cardiac toxicity, rats were intraperitoneally treated with doxorubicin (06 equivalent injections of 2.5 mg/kg, i. p. at 48 hour interval for 02 consecutive weeks to achieve a cumulative dose of 15 mg/kg). Individual and combined oral treatment of candesartan (5 mg/kg/day) and quercetin (10 mg/kg/day) was administered for four weeks.
Results: Following cardiomyopathy, heart/body weight ratio (3.526 × 10− 3), serum creatine kinase (352.4± 16.99 IU/L), lactate dehydrogenase (661.7± 20.45 IU/L) levels were elevated in addition to altered lipid profile (TC – 118.4± 4.25 mg/dL, TG – 263.3± 9.99 mg/dL, VLDL – 52.66± 1.99 mg/dL, LDL – 52.99± 5.80 mg/dL and HDL – 12.78± 0.36 mg/dL). The pre-cotreatment of candesartan and quercetin significantly restored the values to normal. The increased level of lipid peroxides (33.12± 1.63 μmol/mg protein), serum troponin-T (1.82 ± 0.11 pg/mL) and nitric oxide (13.33± 0.73 nmol/mg protein) level along with attenuating antioxidant profile, ie catalase, glutathione and superoxide dismutase (1.43± 0.12 nmol/mg protein, 8.48± 0.42 nmol/mg protein and 2.09± 0.031 U/mg protein) were reversed to normal. Morphometry and histopathologic changes represented a beneficial effect of single and combination pre-cotreatment of drugs which significantly decreases adriamycin cardiac toxicity.
Conclusion: The overall result depicts more beneficial and cardioprotective effect of quercetin and candesartan combination as compared to their individual effects in doxorubicin treated animals. Therefore, this combination might be a suitable option to treat the cardiotoxic effect of doxorubicin.
Keywords: cardiotoxicity, candesartan, quercetin, doxorubicin/adriamycin
Introduction
Adriamycin, also termed as Doxorubicin (DOX), is a member of anthracycline antibiotics. It is isolated from Streptomyces peacetius var. Caesius, effectively and extensively used as a broad-spectrum anti-cancer agent. However, the clinical use of DOX is restricted by its acute and chronic cardiotoxicity.1 Various mechanisms have been recommended for anthracyclines myocardial toxicity. It includes free-radical generation, myocyte apoptosis, lipid peroxidation, mitochondrial deterioration and direct repression of muscle-specific gene expression.2,3 Dexrazoxane is a cytoprotective drug used to prevent and improve cardiomyopathy associated with doxorubicin treatment for metastatic breast cancer. But overall usage of DOX must be monitored in various cases.
Oxidative stress triggers the release of cytokines to induce cardiac inflammation or cardiomyopathy.4 It is also linked to rise in cardiac angiotensin converting enzyme (ACE) activity. ACE inhibition drastically declines adriamycin toxicity in clinical5,6 and pre-clinical studies.7–9 Ang II enhances cytokine production eg tumor necrosis factor-α (TNF- α) via AT1 receptors present on monocytes, macrophages and vascular smooth muscle cells.10 Ang II play a significant role in the pathology of hypertension and IHD (ischemic heart disease). The cardiac remodelling process is significant with a decrease Ang II activity along with long-term survival in pre-clinical and clinical of cardiac hypertrophy and failure.11,12 AT1 also aggravates cardiac disorders like left ventricular (LV) hypertrophy, myocardial infarction, atherosclerosis, hypertension, and heart failure. Candesartan (CAN) an ARB, potentiates AT2 receptor activity and opposes AT1 stimulation.13–15
Nowadays, edibles from medicinal source are gaining popularity due to their protective effects in cardiovascular disease therapy.16 Quercetin (QRN) is widely and commonly ingested dietary polyphenolic flavonoid.17,18 It possesses various pharmacological actions, including antiviral,19 antidiabetic,20 anti-inflammatory,21 cardioprotective,22 neuroprotective23 and antiproliferative activity.24 Adequate presence of QRN in food such as fruits, vegetables, wine, etc.25 up surged the interest for cardioprotective activity of natural substances.26 Due to oxidative stress, many reactive oxygen species (ROS) are produced, leading to heart diseases like atherosclerosis, IHD, heart failure and hypertension. QRN has high antioxidant properties among all the flavonoids to prevent oxidative stress.27,28
The current protocol examines the consequences of single and combined pre-cotreatment of QRN and CAN on DOX induced cardiac toxicity. QRN (directly captures ROS to show free radical neutralizing activity) and CAN (blocks RAAS by AT1R and improves ROS induced oxidative stress) could produce additional protective effects against chronic DOX cardiotoxicity. Also, candesartan half-life is 9 hrs, and 10,000-fold AT1 affinity as compared to AT2 whereas quercetin has a half-life is ~12.5 hrs as per studies.29–31 This investigation can show the probable preliminary mechanisms underlying these effects.
Materials and Methods
Doxorubicin hydrochloride vial 50mg was purchased from Aaltra Med Health care limited, Hyderabad, India. Quercetin powder 25 g was purchased from Sigma–Aldrich, St. Louis, MO, USA. Other chemicals were purchased from CDH, Hi Media, Reckon diagnostics and ERBA diagnostics. All chemicals used were of analytical grade.
Experimental Design of in-vivo Study
Adult male Wister rats, weighing 200–250 g were used after a one-week acclimatization period. The experimental protocol for animals was performed as per the guidelines of Committee for the purpose of control and supervision of experiments on animals (CPCSEA). The protocol for in-vivo study was approved by Institutional Animal Ethical Committee (IAEC) of Institute of Technology and Science (ITS), College of Pharmacy, Muradnagar, Ghaziabad, UP, India. The study protocol number was ITS/07/IAEC/2013. During the study period, standard environmental conditions viz.12h dark/light cycle and temperature (21 ± 2°C) were maintained. Animals had free access to standard laboratory pellets and water ad libitum. Rats were divided into five groups (08 rats/group) and were administered with drugs viz. DOX, CAN, and QRN as shown in Table 1. Such type of pattern is reported previously32,33 in Table 1.
 |
Table 1 Route, Dose and Description of Drug Administration |
Serum and Tissue Sampling
Rats were sacrificed after 24-hours of last DOX injection by cervical dislocation. Blood was collected and serum was separated by centrifugation at 10,000×g for 10 min. Heart of animals were removed and washed by ice-cold saline. The heart was weighed and homogenized in ice-cold saline to obtain 10% (w/v) homogenate. Serum and heart homogenate were stored at −80°C, until analysis. The heart: body weight ratio was calculated for gravimetric analysis, by weight of the animals before sacrifice and of isolated hearts using a precision balance.
Estimation of Serum Creatine Kinase (CK-MB) and Lactate Dehydrogenase (LDH) Levels
CK-MB found in high concentration in myocardium (14–42%) was determined using diagnostic kit (ERBA diagnostics). Mix and incubate for 3 mins at 37°C. Then, measure the absorbance (340 nm) at time 0 and again exactly after 1, 2 and 3 minutes. The absorbance change (∆A) at 60 second interval was measured using UV spectrometry to calculate CK-MB level as (U/L). LDH, an cytoplasmic enzyme, was determined using diagnostic kit (Reckon diagnostics). Estimation of these enzymes provides a strong base to monitor the progress of onset and status in CHD patients. Mix and read first absorbance of the test exactly at one minute and thereafter at 30, 60 and 90 seconds at 340 nm. Determine the mean change in absorbance per minute and calculate test results as (IU/L).27,28
Estimation of Myocardial Antioxidant Enzyme Activities
Commercial kits were used to estimate myocardial SOD, CAT, GSH activity by colorimetric method. The % inhibition was reported to calculate SOD (U/mg protein), CAT and GSH (nmol/mg protein) concentration.28 Thiobarbituric acid reactive species (TBARS), also termed as malondialdehyde (MDA) is a marker of oxidative stress, can be determined in cardiac homogenates by lipid peroxidation. It is measured by UV spectrometer at 532nm.
Estimation of Lipid Profile
Commercial kits were used to determine serum triglyceride (TG), total cholesterol (TC) and high-density lipoprotein (HDL). Friedewald’s formula was employed to determine low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL).34
Estimation of Myocardial Total Nitrite/Nitrate and Cardiac Troponin T Concentration
Griess reaction was used for the colorimetric assay of nitrate and nitrite (NO indicators in tissue). Total nitrite is then measured as it is proportionately converted into nitrite and nitrate.35 Cardiac troponin T (cTnT) was determined using commercial kit and as per manufacturer’s guidelines.
Morphometric Analysis
Cardiac tissues were taken in thin slices and stained by 1% TTC (triphenyl tetrazolium chloride) in phosphate buffer saline (pH 7.4).36 Viable tissues appear red due to staining whereas no stains were visible on non-viable tissue. The stained heart images were analysed for area of necrosis.
Histopathological Examination of the Cardiac Tissues
Twenty-four hours after last DOX injection, the rats were sacrificed and hearts were isolated. Immediately after isolation, the heart tissue was washed with cold phosphate buffer saline (pH = 7.4) and fixed in 10% buffered formalin. Further, haematoxylin and eosin stain were used and 5μm stained sections were observed under a light microscope. Histopathological changes were identified through the images taken by digital camera.36
Statistical Analysis
Results were expressed as mean ± standard error of the mean (S.E.M). Statistical significance was determined by one-way analysis of variance (ANOVA) followed by Tukey–Kramer post-test to compare all groups. Results were considered to be statistically significant when P < 0.001, 0.01 and 0.05.
Results
General Data (Heart/Body Weight Ratio)
The results indicated a significant rise (P < 0.05) in ratio in DOX-treated group (3.526 × 10−3) as compared to control (2.145 × 10−3) in Table 2. QRN administration (2.767 × 10−3) significantly reduced the heart/body weight ratio (P < 0.05), while combination of CAN and QRN (2.276 × 10−3) resulted in a more pronounced decline in heart/body weight ratio at P < 0.05.
 |
Table 2 Effect of CAN, QRN and CAND+ QRN on Heart/Body Weight Ratio |
Effect of the Single and Combined Pre-Cotreatment of CAN and QRN on the Serum CK-MB and LDH Levels
DOX caused a significant rise (P < 0.01) in serum CK-MB and LDH levels as 352.4±16.99 IU/L and 661.7±20.45 IU/L, respectively, as shown in Table 3. CAN or QRN individually suggested a significant decrease in serum CK-MB and LDH levels as compared to DOX-treated rats. Further, pre-cotreatment showed enhanced effect 164.7±7.605 IU/L and 251.3±20.49 IU/L rather than the individuals.
 |
Table 3 Effect of CAN, QRN and CAND+ QRN on Plasma Markers of Cardiac Damage |
Effect of Individual and Combined Pre-Cotreatment of CAN and QRN on Myocardial Antioxidant Levels, Lipid Peroxidation and Total Nitrite Levels
DOX treated animals showed a marked decrease in myocardial antioxidant activity ie CAT, GSH and SOD (1.43±0.12 nmol/mg protein, 8.48±0.42 nmol/mg protein and 2.09±0.031 U/mg protein) at p<0.001 as compared to control (4.08±0.12 nmol/mg protein, 28.29±0.43 nmol/mg protein and 5.51±0.088 U/mg protein) in Table 4. CAN or QRN single administration improved CAT, GSH and SOD levels. This effect was markedly enhanced viz. 3.54±0.32 nmol/mg protein, 23.12±0.64 nmol/mg protein and 3.23±0.015 U/mg protein on collateral administration (p<0.001).
 |
Table 4 Effect of CAN, QRN and CAND+ QRN on Antioxidant Level |
DOX induced a significant rise (P < 0.01) in myocardial MDA, 33.12±1.63 µmol/mg protein and NO, 13.33±0.73 nmol/mg protein level as compared to control 19.17±1.20 µmol/mg protein and 5.62±0.61 nmol/mg protein, respectively, Table 5. The combined pre-cotreatment of CAN and QRN resulted in a more significant (P < 0.01) decrease in MDA and NO levels ie 18.59±0.65 µmol/mg protein and 5.80±0.39 nmol/mg protein.
 |
Table 5 Effect of CAN, QRN and CAN+ QRN on Cardiac LPO Levels and NO Level |
Effects of Individual and Combined Pre-Cotreatment of CAN and QRN on Lipoprotein Fractions and Serum Troponin-T Levels
DOX induction resulted in a significant rise (p<0.001) in the levels of TC, TG, VLDL and LDL (118.4±4.25 mg/dL, 263.3±9.99 mg/dL, 52.66±1.99 mg/dL and 52.99±5.80 mg/dL) along with significant decrease in HDL cholesterol levels (12.78±0.36 mg/dL), Tables 6 and 7. Pre-treatment with CAN and QRN individually significantly (p<0.001) lowered TC, TG, LDL, VLDL and increased HDL level in serum. Pre-cotreatment of combination significantly (p<0.001) lowered TC, TG, VLDL, LDL and increased HDL level in serum, ie 81.83±3.59 mg/dL, 109.2±8.97 mg/dL, 21.84±1.79 mg/dL, 21.29±3.96 mg/dL and 19.08±2.20 mg/dL, respectively.
 |
Table 6 Effect of CAN, QRN and CAN+QRN on Plasma Lipid Profile |
 |
Table 7 Effect of CAN, QRN and CAN+QRN on Plasma Lipid Profile and Serum Troponin-T Levels |
Rats treated with DOX showed increased serum cTnT level, 1.82 ± 0.11 pg/mL suggesting myocardial lesions (p<0.001), Table 7. Groups treated with CAN (0.39 ± 0.03 pg/mL) or QRN (0.42 ± 0.01 pg/mL) or the combination (0.34 ± 0.02 pg/mL) showed significant reduction (p<0.01) in serum cTnT level compared to the DOX-treated group.
Morphometry and Histopathological Analysis
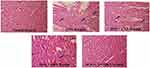
TTC staining of heart of control rats showed brick red coloration indicative of a greater number of viable cells. Rats treated with doxorubicin exhibited pale coloration indicating areas of necrosis as shown in Figure 1. However, Doxorubicin rats pre-treated with candesartan, quercetin and the combination showed a protective effect with a minimal pale coloration. Histological analysis of control group showed normal cardiac tissue without any myonecrosis. DOX-treated group developed cardiac necrosis with oedema, leukocyte infiltration and intramuscular haemorrhage. The single and pre-cotreatment of CAN and QRN ameliorated the histopathological changes of cardiomyocytes in Figure 2.
Discussion
Doxorubicin (DOX) is one of the main anticancer drugs that can induce oxidative damage to cardiac tissues. It mainly targets the cardiac antioxidant defence mechanism. In recent times, many endogenous myocardial antioxidants have become promising therapeutic targets of treatment associated with increased oxidative stress.37 Doxorubicin causes rats to lose weight over a period following injection, as confirmed by other reports.38 DOX-induced cardiac toxicity is identified by decreased body weight and increased heart weight.39 This study confirms the earlier findings.
One of the important markers of early and late-stage cardiac injury is CK-MB (specific for cardiac muscle)40. In the study, a reasonable increase in serum cardiac isoenzymes (LDH and CK-MB) was observed due to DOX-induced cardiac necrosis. CAN inhibits AT1R-mediated ROS generation, thereby preventing CK-MB and LDH leakage.41 QRN inhibit angiotensin converting enzyme activity, thereby decreasing the conversion of Ang I to Ang II.42 The results indicate that QRN pre-treatment can potentiate the cardioprotective effect of CAN due to its free radical scavenging and antioxidant properties. QRN and its derivatives may represent promising cardioprotective activity for prevention and treatment of wide range of cardiac disease.43
Rise in lipid peroxidation products is considered as biochemical marker of oxidative stress in DOX-treated rats. Due to lipid peroxidation, inflammatory cells accumulate in cardiac myocytes.44 Thus, inactivity of GSH, SOD and CAT in DOX-treated group is due to superoxide anion generation at the site of damage in myocardium. Current study showed that combined effect of CAN and QRN treatment could decrease the lipid peroxide levels33 by neutralizing ROS.
CAN treatment could decline DOX-induced elevation of myocardial NO levels. This could be due to decreased TNF-α level and blockade of Ang II-mediated elevation of ROS. Our data showed that CAN produced more pronounced effect in lowering NO levels than QRN. The probable reason for this effect can be that AT1R blockade increases circulating levels of Ang II that could in theory act on the unopposed AT2R effects.45
Lipids consist of cholesterol (HDL and LDL cholesterol), triglycerides, phospholipids and free fatty acids. Observations concluded a significant rise of serum total cholesterol, triglyceride LDL and VLDL in our studies. This showed that DOX reduced the rate of lipolysis, which is deleterious for heart function46,47 Any change in lipid metabolism directly alters the concentration of lipoproteins. The present combination showed a potent antioxidant activity. It stabilizes lipid metabolizing enzymes by its hypolipidemic effect thereby proving its potential as a cardio-protector.
Metabolically viable cells and tissues can be easily identified using TTC staining (redox indicator). It provides the infarct size to assess myonecrosis of cardiac tissue. The myocardial respiration generates enzymes that reduces TTC to a brick red precipitate ie TPF (1,3,5-triphenylformazan). Thus, viable tissues appear red as compared to the pale non-viable tissues.48 The single or combined pre-cotreatment of CAN and QRN portrayed minimal pale-coloration demonstrating normal cardiac tissues. Histoarchitectural observations verifies DOX-induced necrosis in cardiac fibers. These detrimental changes are remarkably countered by pre-treatment with CAN, QRN or both providing maximal safeguard. Myocardial lesions were diminished to marked extent in groups treated with CAN, QRN or both providing microscopic evidences of cardioprotective.31,43
Conclusion
In conclusion, our study provides an explanation for the cardioprotective effect of quercetin individually as well as in combination with candesartan in doxorubicin-treated animals. Quercetin’s antioxidant properties might have shown the additional effect to candesartan. The results indicate that QRN pre-treatment can potentiate the cardioprotective effect of CAN due to its free radical scavenging and antioxidant properties. Therefore, this combination might be a suitable option to treat the cardiotoxic effect of doxorubicin. Nevertheless, in future additional studies are required to demonstrate and approve the efficacy and safety of quercetin/candesartan combination regimens in clinical practice.
Abbreviations
CAN, candesartan; QRN, quercetin; DOX, doxorubicin; CK-MB, serum creatine kinase; LDH, lactate dehydrogenase; ACE, angiotensin-converting enzyme; AT, angiotensin II receptor; GSH, reduced glutathione; MDA, malondialdehyde; SOD, superoxide dismutase; CAT, catalase; ROS, reactive oxygen species; cTnT, serum troponin-T; NO, nitric oxide.
Human and Animal Rights
No humans were involved in the study. All animal-related experiments were conducted as per the guidelines by CPCSEA (Committee for the purpose of control and supervision of experiments on animals). The study protocol (ITS/07/IAEC/2013) was approved by members of the Institutional Animal Ethical Committee (IAEC).
Ethics Approval and Consent to Participate
The experimental protocol for animals was performed as per the guidelines of Committee for the purpose of control and supervision of experiments on animals (CPCSEA). The protocol for in-vivo study was approved by Institutional Animal Ethical Committee (IAEC) of Institute of Technology and Science (ITS), College of Pharmacy, Muradnagar, Ghaziabad, UP, India. The study protocol number was ITS/07/IAEC/2013.
Acknowledgment
The authors are grateful to the management of I.T.S College of Pharmacy for providing the necessary support for the successful completion of the work.
Funding
The project was non-funded and self-financed.
Disclosure
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
1. Abu-Gazia M, El-Magd MA. Ameliorative effect of cardamom aqueous extract on doxorubicin-induced cardiotoxicity in rats. Cells Tissues Organs. 2018;206:62–72. doi:10.1159/000496109
2. Sawyer DB, Fukazawa R, Arstall MA, Kelly RA. Daunorubicin induced apoptosis in rat cardiac myocytes is inhibited by dexrazoxane. Circ Res. 1999;84:257–265. doi:10.1161/01.RES.84.3.257
3. Hashish FE, Abdel-Wahed MM, El-Odemi MH, El-Naidany SS, ElBatsh MM. Possible protective effects of quercetin on doxorubicin-induced cardiotoxicity in rats. Menoufia Med J. 2021;34:333–339. doi:10.4103/mmj.mmj_5_20
4. Bien S, Riad A, Ritter CA, et al. The endothelin receptor blocker bosentan inhibits doxorubicin-induced cardiomyopathy. Cancer Res. 2007;67:10428–10435. doi:10.1158/0008-5472.CAN-07-1344
5. Jensen BV, Nielsen SL, Skovsgaard T. Treatment with angiotensin converting- enzyme inhibitor for epirubicin-induced dilated cardiomyopathy. Lancet. 1996;347(8997):297–299. doi:10.1016/S0140-6736(96)90469-9
6. Jensen BV, Nielsen SL, Jensen TS. Angiotensin-converting enzyme inhibitor in the treatment of epirubicin-induced dilated cardiomyopathy. Ugeskr Laeger. 1997;159(13):1945–1949.
7. Sacco G, Bigioni M, Evangelista S, Goso C, Manzini S, Maggi CA. Cardioprotective effects of zofenopril, a new angiotensin-converting enzyme inhibitor, on doxorubicin-induced cardiotoxicity in the rat. Eur J Pharmacol. 2001;414(1):71–78. doi:10.1016/S0014-2999(01)00782-8
8. Hauser M, Wilson N. Anthracycline induced cardiomyopathy: successful treatment with angiotensin converting enzyme inhibitors. Eur J Pediatr. 2000;159(5):389. doi:10.1007/s004310051294
9. Hatake K, Miura Y. Angiotensin-converting enzyme inhibitor for epirubicin-induced dilated cardiomyopathy. Lancet. 1996;347(9013):1485. doi:10.1016/S0140-6736(96)91721-3
10. Hahn AW, Jonas U, Buhler FR, Resink TJ. Activation of human peripheral monocytes by angiotensin II. FEBS Lett. 1994;347:178–180. doi:10.1016/0014-5793(94)00531-1
11. Kawabata H, Ryomoto T, Ishikawa K. Cardioprotection with angiotensin converting enzyme inhibitor and angiotensin II type 1 receptor antagonist is not abolished by nitric oxide synthase inhibitor in ischemia-reperfused rabbit hearts. Hypertens Res. 2001;24:403–409. doi:10.1291/hypres.24.403
12. Wang P, Li HW, Wang YP, Chen H, Zhang P. Effects of recombinant human relaxin upon proliferation of cardiac fibroblast and synthesis of collagen under high glucose condition. J Endocrinol Invest. 2009;32:242–247. doi:10.1007/BF03346460
13. Burnier M. Angiotensin II type 1 receptor blockers. Circ J. 2001;103:904–912. doi:10.1161/01.CIR.103.6.904
14. Hoogwerf BJ. Renin–angiotensin system blockade and cardiovascular and renal protection. Am J Cardiol. 2010;105:30A–35A. doi:10.1016/j.amjcard.2009.10.009
15. Venkatesh RC, Ravindra B, Siva RC, Benito J, Maheswari C. Pharmacokinetic interaction study between quercetin and valsartan in rats and in vitro models. Drug Dev Ind Pharm. 2013;39(6):865–872. doi:10.3109/03639045.2012.693502
16. Syahputra RA, Harahap U, Dalimunthe A, Nasution MP, Satria D. The role of flavonoids as a cardioprotective strategy against doxorubicin-induced cardiotoxicity: a review. Molecules. 2022;27(4):1320. doi:10.3390/molecules27041320
17. Tene K, Kalyan Kumar M, Basveshwar G, et al. Polyphenolic rich compounds from Dillenia pentagyna (Roxb) attenuates the doxorubicin-induced cardiotoxicity: a high-frequency ultrasonography assisted approach. Front Pharmacol. 2021;12:624706. doi:10.3389/fphar.2021.624706
18. Boots AW, Haenen GRMM, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585:325–337. doi:10.1016/j.ejphar.2008.03.008
19. Ohnishi E, Bannai H. Quercetin potentiates TNF-induced antiviral activity. Antiviral Res. 1993;22:327–331. doi:10.1016/0166-3542(93)90041-G
20. Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in stz-induced diabetic rats. Comp. Biochem. Physiol. C Toxicol Pharmacol. 2003;135:357–364.
21. Rotelli AE, Guardia T, Juarez AO, DelaRocha NE, Pelzer LE. Comparative study of flavonoids in experimental models of inflammation. Pharmacol Res. 2003;48:601–606. doi:10.1016/S1043-6618(03)00225-1
22. Alasmari AF. Cardioprotective and nephroprotective effects of Quercetin against different toxic agents. Eur Rev Med Pharmacol Sci. 2021;25:7425–7439. doi:10.26355/eurrev_202112_27440
23. Dok-Go H, Lee KH, Kim HJ, et al. Neuroprotective effects of antioxidative flavonoids, quercetin, (+)-dihydroquercetinandquercetin3-methylether, isolated from Opuntia ficus-indica var. saboten. Brain Res. 2003;965:130–136. doi:10.1016/S0006-8993(02)04150-1
24. Garcia MV, Crespo I, Collado PS, et al. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappa B pathway in Chang liver cells. Eur J Pharmacol. 2007;557:221–229. doi:10.1016/j.ejphar.2006.11.014
25. Sharma A, Parikh M, Shah H, Gandhi T. Modulation of Nrf2 by quercetin in doxorubicin-treated rats. Heliyon. 2020;6(4):e03803. doi:10.1016/j.heliyon.2020.e03803
26. Hertog MG, Bueno-de-mesquita HB, Fehily AM, Sweetnam PM, Elwood PC, Kromhout D. Fruit and vegetable consumption and cancer mortality in the Caerphilly Study. Cancer Epidemiol Biomarkers Prev. 1996;5:673–677.
27. Mariee AD, Abd-Allah GM, El-Beshbishy HA. Protective effect of dietary flavonoid quercetin against lipemic–oxidative hepatic injury in hypercholesterolemic rats. Pharm Biol. 2012;50:1019–1025. doi:10.3109/13880209.2012.655424
28. Larson A, Witman MAH, Guo Y, et al. Acute, quercetin-induced reductions in blood pressure in hypertensive individuals are not secondary to lower plasma angiotensin- converting enzyme activity or endothelin-1: nitric oxide. Nutr Res. 2012;32:557–564. doi:10.1016/j.nutres.2012.06.018
29. Munger MA. Use of angiotensin receptor blockers in cardiovascular protection. Current evidence and future directions. Cardiovascul TherPrevent. 2011;10(7):93–104.
30. Moon YJ, Wanga L, DiCenzob R, Morris ME. Quercetin pharmacokinetics in humans. Biopharm Drug Dispos. 2008;4:205–217. doi:10.1002/bdd.605
31. Sobczuk P, Czerwinska M, Kleibert M, Agnieszka C. Anthracycline-induced cardiotoxicity and renin-angiotensin-aldosterone system—from molecular mechanisms to therapeutic applications. Heart Fail Rev. 2022;27:295–319. doi:10.1007/s10741-020-09977-1
32. Haleagrahara N, Radhakrishnan A, Lee N, Kumar P. Flavonoid quercetin protects against swimming stress-induced changes in oxidative biomarkers in the hypothalamus of rats. Eur J Pharmacol. 2009;621:46–52. doi:10.1016/j.ejphar.2009.08.030
33. Soga M, Kamal F, Watanabe K, et al. Effects of angiotensin II receptor blocker (candesartan) in daunorubicin-induced cardiomyopathic rats. Int J Cardiol. 2006;110(3):378–385. doi:10.1016/j.ijcard.2005.08.061
34. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low- density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. doi:10.1093/clinchem/18.6.499
35. Tsikas D. Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: appraisal of the Griess reaction in the l-arginine/nitric oxide area of research. J Chromatogr B. 2007;851:51–70. doi:10.1016/j.jchromb.2006.07.054
36. Ytrehus K, Liu Y, Tsuchida A, et al. Rat and rabbit heart infarction: effects of anaesthesia, perfusate, risk zone, and method of infarct sizing. Am J of Physiol. 1994;267:H2383–H2390.
37. Elblehi SS, El-Sayed YS, Soliman MM, Shukry M. Date palm pollen extract avert doxorubicin induced cardiomyopathy fibrosis and associated oxidative/nitrosative stress, inflammatory cascade, and apoptosis-targeting bax/bcl-2 and caspase-3 signaling pathways. Animals. 2021;11:88–92. doi:10.3390/ani11030886
38. Rephaeli A, Waks-Yona S, Nudelman A, et al. Anticancer prodrugs of butyric acid and formaldehyde protect against doxorubicin-induced cardiotoxicity. Br J Cancer. 2007;96:1667–1674. doi:10.1038/sj.bjc.6603781
39. Antonio A, Jose M, Jose S, et al. Endurance training attenuates doxorubicin-induced cardiac oxidative damage in mice. Int J Cardiol. 2005;100:451–460. doi:10.1016/j.ijcard.2004.11.004
40. Fadillioglu E, Erdogan H. Effects of erdosteine treatment against doxorubicin-induced toxicity through erythrocyte and plasma oxidant/antioxidant status in rats. Pharmacol Res. 2003;47:317–322. doi:10.1016/S1043-6618(03)00010-0
41. Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi:10.1161/01.RES.74.6.1141
42. Loizzo MR, Said A, Tundis R, et al. Inhibition of angiotensin converting enzyme (ACE) by flavonoids isolated from Ailanthus excelsa (Roxb) (Simaroubaceae). Phytother Res. 2007;21:32–36. doi:10.1002/ptr.2008
43. Ferenczyova K, Kalocayova B, Bartekova M. Potential Implications of Quercetin and its Derivatives in Cardioprotection. Int J Mol Sci. 2020;21(5):1585. doi:10.3390/ijms21051585
44. Saad SY, Najjar TA, Al-Rikabi AC. The preventive role of deferoxamine against acute doxorubicin-induced cardiac, renal and hepatic toxicity in rats. Pharmacol Res. 2001;43:211–218. doi:10.1006/phrs.2000.0769
45. Widdop RE, Jones ES, Hannan RE, Gaspari TA. Angiotensin AT2 receptors: cardiovascular hope or hype? Br J Pharmacol. 2003;140:809–824. doi:10.1038/sj.bjp.0705448
46. Iliskovic SPK. Lipid lowering: an important factor in preventing doxorubicin-induced heart failure. Am J Pathol. 1997;150:727–734.
47. Koutinos G, Stathopoulos GP, Dontas I, et al. The effect of doxorubicin and its analogue mitoxantrone on cardiac muscle and on serum lipids: an experimental study. Anticancer Res. 2002;22:815–820.
48. Altman FP. Tetrazolium salts and formazans. Prog Histochem Cytochem. 1976;9:1–56. doi:10.1016/S0079-6336(76)80015-0
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.