Back to Journals » Journal of Experimental Pharmacology » Volume 12
Evaluation of Abortifacient Effect of Rumex nepalensis Spreng Among Pregnant Swiss Albino Rats: Laboratory-Based Study
Authors Dabe NE , Kefale AT , Dadi TL
Received 30 April 2020
Accepted for publication 19 June 2020
Published 31 July 2020 Volume 2020:12 Pages 255—265
DOI https://doi.org/10.2147/JEP.S260719
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bal Lokeshwar
Video abstract presented by Nikodimos Eshetu Dabe.
Views: 325
Nikodimos Eshetu Dabe,1 Adane Teshome Kefale,2 Tegene Legese Dadi3
1Department of Biomedical Science, College of Health Sciences, Mizan–Tepi University, Mizan Teferi, Ethiopia; 2Department of Pharmacy, College of Health Sciences, Debre Berhan University, Debre Berhan, Ethiopia; 3Department of Public Health, College of Health Sciences, Mizan–Tepi University, Mizan Teferi, Ethiopia
Correspondence: Nikodimos Eshetu Dabe Email [email protected]
Background: Rumex nepalensis Spreng (Amharic: Yewsha Tult) belongs to the Polygonaceae (buckwheat) family. In Ethiopia, the plant is traditionally used for the treatment of stomach ache, tonsillitis, ascariasis, uterine bleeding, etc. An ethnobotanical study from Mizan–Tepi University also reported the use of the plant by “Shekicho” people as an abortifacient. As a result, this study aimed at the assessment of the outcome of hydro-ethanolic leaves extract of R. nepalensis on Swiss albino pregnant rats and confirm its abortifacient activity.
Methods: The hydro-alcoholic leaves extract of Rumex nepalensis Spreng was evaluated for its abortifacient activity in Swiss albino rats. The mature female rats were mated overnight to male rats in mating cages. Two different dosage regimens (300 mg/kg, 600 mg/kg) of the extract were administered. Laparotomy was performed on the rats to assess the uterus and ovary, the viable, non-viable, adsorbing sites, and corpora lutea. Differences between the experimental and control groups were compared using one-way analysis of variance (ANOVA), followed by Dunnett’s T-test to determine their level of significance.
Results and Discussion: This study revealed that Rumex nepalensis Spreng had anti-implantation and abortifacient activities at both 300 and 600 mg/kg doses, which was statistically significant as compared with the controls. It was relatively safe up to the dose of 5000 mg/kg, where no mortality and organ toxicity were manifested. Phytochemicals identified were alkaloids, flavonoids, saponins, tannins, steroids, and anthraquinones.
Conclusion: In general, our study showed that R. nepalensis had a significant abortifacient activity that testifies its traditional dibs. Therefore, the use of this plant should be avoided in pregnant women to minimize unintended abortion and further studies are needed to know its mechanism of activity and to identify the phytochemicals corresponding to this activity. Checking its efficacy on other species is also needed.
Keywords: Rumex nepalensis Spreng, traditional medicine, abortifacient activity
Plain Language Summary
Females in the developing world use unknown herbals for discontinuation of pregnancy. Likewise, “Shekicho” women in Ethiopia are using Rumex nepalensis for the same purpose. And some women in “Kaffa Zone”, Ethiopia use the leaves of this plant as cabbage without knowing its claim. So, we conducted this study to:
- Confirm its effectiveness and toxicity to avoid unnecessary complications, unintended abortion, and death of mothers from taking this herb with unknown doses.
- Provide an alternative treatment modality in decreasing unintended pregnancy.
To do so, we collected fresh plant materials of Rumex nepalensis Spreng. The taxonomist identified the specimens and we hired into the extraction and phytochemical screening process. In the meantime, we bred the rats.
Then, we tested the acute toxicity with different doses using female Swiss albino rats aged between 1 and 2.5 months with a weight of 150–250 grams.
There was no death up to the dose of 5000 mg/kg except for some symptoms of toxicity at and above the dose of 2000 mg/kg.
Then after, we checked its preimplantation and abortifacient activity. Eventually, we confirmed a promising significant anti-implantation and abortifacient effect of the plant at doses of 300 and 600 mg/kg.
- So, this result is important to avoid unintended abortion in those pregnant women who are taking the plant as a cabbage.
- It is also important for the country since it will be an alternative means of decreasing importing the contraceptive and abortifacient drugs.
Introduction
Background of the Study
Abortion is the termination of a pregnancy after the implantation of the blastocyst in the endometrium before the fetus has attained “viability”, and it is variously defined in law, usually between 24 and 28 weeks. In our country, Ethiopia, it is before 28 weeks of pregnancy. The two major categories of abortions are induced and spontaneous. Induced abortions are those initiated voluntarily to end the gestation, whether permitted by law or not. All other abortions are called spontaneous.1
In 2010–14 it is estimated that 25.1 million (45.1%) abortions were unsafe, 17.1 million (30.7%) were less safe and 8 million (14.4%) were least safe throughout the world. Half of the abortions in developing countries (49.5%) were unsafe; however, almost all abortions in developed countries (87.5%) are safe. The highest proportion of least safe abortions occurred in middle Africa, followed by western Africa and eastern Africa.2
More proximate causes include poor access to contraceptives and contraceptive failure. Worldwide, an estimated 68,000 women die (367 deaths per 100,000 unsafe abortions) as a result of complications from unsafe induced abortions every year; this means about eight deaths per hour.3
Botanical Background of Experimental Plants
Nearly 70–80% of the world population still uses herbal medicines for their health care,4 which is particularly higher in Africa where access to western medicine is limited; and the public preference for and cultural beliefs on traditional medicines are higher.5,10 Herbal medicines are employed for treatment, prevention, and identification of ailments.8 In addition to these, plant products are also used for the prevention of unwanted pregnancy, abortion, infertility management, treatment of menopausal symptoms, menstrual disorders, management of pregnancy-related disorders, and gynecologic complications.9,11,15 Some studies also showed the abortifacient and antifertility effects of different plants.16,17
The use of traditional medicine in Ethiopia is dated back to ancient times18,19 and it is still under practice.20,23 Thus, it is important to evaluate the efficacy and safety of herbal products commonly used for abortifacient effect by the local community. Hence, in this study, we tried to evaluate the abortifacient activity and safety of the plant Rumex nepalensis.
Rumex nepalensis Spreng (Amharic: Yewsha Tult) belongs to Polygonaceae also known as the buckwheat family. The family comprises over 1200 species in 52 genera.24 The genus Rumex consists of more than 200 species and is widely distributed in the world.24,25
Rumex nepalensis is aa herbaceous, perennial plant producing erect, branched stems 50–180 cm tall from a large rootstock (Figure 1). The plant is sometimes harvested from the wild for local use as a food, medicine, and source of tannin.26,27
 |
Figure 1 Photograph of leaves of R. nepalensis taken from “Sheka” forestry region, Ethiopia during sample collection, November 2018. |
The roots of R. nepalensis are used for the treatment of intestinal parasites.28 Previous studies revealed that roots extract of R. nepalensis has strong purgative activity.25,26,29 The fresh young leaves of the plant are rubbed over the affected areas after an injury from stinging nettles and are used to treat colic and syphilis ulcers.30 The root and leaves extracts of R. nepalensis have also shown significant antimicrobial and antifungal activities.31,35
In Ethiopia, the plant is traditionally used for stomach ache, rheumatism, tonsillitis, ascariasis, uterine bleeding, abdominal bleeding, child diarrhea, toothache, acute febrile illness, amoebiasis, eye infection, liver disease, gastrointestinal and oral ulcers, as an antidote, laxatives, and cabbage.19,22,23,36 An ethnobotanical study from Mizan–Tepi University reports the use of the plant as an abortifacient by “Shakicho” people.
Justification of the Study
Females in developing countries are exposed to unsafe abortion by taking unknown herbals and other products. So, confronting different complications like heavy bleeding, sepsis, disability, and death is inevitable. Even after safe abortion procedures, they might encounter many complications and the pregnancy might continue. As a result, it is very important to study the available alternatives in addition to the prevention of unintended pregnancy. And they might be an alternative treatment modality in the future. In the meantime, confirming whether the plant is abortifacient or not is important to avoid excessive consumption to prevent toxicities and unintended abortion. Currently, this plant is used by the “Shakicho” females in Ethiopia for such purpose. So, this study is justifiable to check its effectiveness, and determine the effective and toxic doses. For this study, we have used Wister albino rats since they are the preferred rodent species for testing of chemicals. Female Wister albino rats were used for acute toxicity tests. This is because literature surveys of conventional LD50 (lethal dose, that resulted in the death of 50% of the tested animals) tests show that, although there is little difference in sensitivity between the sexes, in those cases where differences are observed, females are generally slightly more sensitive than males. That is why 48 healthy, young adult, nulliparous, and non-pregnant female rats weighing between 150 and 250 grams were used in this evaluation. Those rats aged between 1 and 2.5 months initially were brought from the animal laboratory of the Ethiopian Public Health Institute, and then they were bred at the pharmacology laboratory of Mizan–Tepi University.
Due to these and the above-mentioned reasons, we were engaged to do this study.
Therefore, the objective of this study was to evaluate the hydro-ethanolic leaves extract of R. nepalensis on the outcome of rat pregnancy. Testing of the extract’s effectiveness and toxicity was our primary intention.
Methods
Plant Materials and Preparation of Extracts
Fresh plant materials of Rumex nepalensis Spreng were collected based on its ethnobotanical description from forestry regions of Sheka and Bench-Maji zone, Southwest Ethiopia. Specimens of the plant were identified by a taxonomist and samples were deposited at the Herbarium with Voucher specimen number (111/MTU/PHARM) at the College of Health Science, Mizan–Tepi University (MTU). Fresh parts of the plant were cleaned from extraneous materials, dried under shade at room temperature, and ground by a manual crusher into fine particles.37,38 Then, it was extracted with 80% ethanol, end up to crude extract (Figure 2).
 |
Figure 2 Sample photographs showing the extraction process (A and B) and phytochemicals screening (C–F) within the Pharmacology laboratory, Mizan–Tepi University, Ethiopia, January 2019. |
Ethanolic extract of this medicinal plant was qualitatively screened for the presence of secondary metabolites to relate the abortifacient activity of the plant with the presence or absence of these constituents. Phytochemical tests used were as follows.
Test for Tannins
Using the FeCl3 test, 0.5 grams of the extract was dissolved in 50 mL of distilled water and filtered through a muslin filter. Then, a small amount of the filtrate was transferred to a test tube and a few drops of 5% ferric chloride solution were added.
Test for Alkaloids
Using Mayer’s test, the plant extract (0.5 g) was added to 5 mL of dilute H2SO4 (1%) on a steam bath, then filtered. The filtrate was transferred into a test tube and a few drops of Mayer’s were added.
Test for Flavonoids
Using the lead acetate test, 0.5 grams of the extract was dissolved in 50 mL of distilled water and then filtered through a muslin filter. Then after, a small amount of the filtrate was transferred into a test tube and a few drops of 10% lead acetate solution were added.
Test for Anthraquinones
Using Borntrager’s test, a little powder was shaken with an immiscible solvent (chloroform) and then filtered. Then, an equal amount of ammonium hydroxide was added to the filtrate in a test tube and was shaken.
Tests for Saponins
Using the Froth test, 20 mL of water was added to 150 mg of solvent-free extract and shaken vigorously.
Test for Steroids
Using Libermann Burchard’s test, powder of the extract (0.5 g) was dissolved in 2 mL chloroform and filtered. About 2 mL of the filtrate, acetic acid anhydride (2 mL), and concentrated H2SO4 (2 mL) were added.
Test for Terpenoids
Using Salkowski’s test, a small amount of the extract was mixed with 2 mL of chloroform and filtered through a filter paper. Three drops of concentrated H2SO4 were carefully added into the filtrate.
Chemicals Used
Chemicals were 80% ethanol, ferric chloride, suluric acid, lead acetate, ammonium hydroxide, acetic acid anhydride, normal saline, distilled water, diethyl ether, and chloroform. All chemicals were purchased from an Importer called Fine Chemicals, Ethiopia.
Experimental Animals
Swiss albino rats weighing between 150 and 250 grams were used in this evaluation. These rats aged between 1 and 2.5 months were bred at the Pharmacology laboratory of Mizan–Tepi University. They were housed in well-ventilated stainless-steel cages at room temperature (24±2 °C) in the hygienic condition under a natural light and dark schedule. Other than room cleaning, the relative humidity was 30–70%. For feeding, conventional rodent laboratory diets were used with an unlimited supply of drinking water. Then, the animals were acclimatized to laboratory conditions for 1 week before the experiment to alleviate any non-specific stress.39,40
Acute Toxicity Study
An acute toxicity study was carried out for Rumex nepalensis Spreng extracts using female Swiss albino rats. The rats were divided randomly into control and six treatment groups, each group consisting of five rats. Control group received only the vehicle and each treatment group received orally the 80% ethanolic extracts of Rumex nepalensis Spreng with a dose of 300, 500, 1000, 2000, 2500, and 5000 mg/kg. To increase the rate of absorption of the extract, all groups of albino rats fasted overnight before administration. At the end of the fasting period, the body weight of each rat was recorded before dosing and the doses were calculated and administered to the rats in the experimental groups based on their body weight (Figure 3). Animals were kept under close observation for 4 hours after administration of the extract and then they were observed daily for any changes in general behavior and/or other physical activities.41
 |
Figure 3 Sample photographs showing the oral administration process (A–C) within the biomedical science laboratory, Mizan–Tepi University. |
Animal Pairing
The mature female rats were introduced overnight to mature male rats in a plastic mating cage with mesh floor to facilitate detecting the vaginal plug. The day of detecting a vaginal plug or sperm (or both) was considered as day 1 of gestation. Pregnant females were used for assessing the effectiveness of the plant extract on implantation and mid-term abortion.
Implantation Experiments
In this experiment, timed-pregnant rats on day 3 of gestation were randomly divided into three groups of five rats each. In the first group, each pregnant rat received a daily 300 mg/kg dose of hydroalcoholic leaves extract orally for 3 consecutive days starting from day 3 to 5 of gestation.17 The second group received a 600 mg/kg dose. The third group (control) received 1.0 mL of distilled water similarly. On day 13, as in a similar study on other plants,1,7 rats were laparotomized under ether anesthesia to minimize the painful effect of sacrificing steps. The uterus and ovary units were removed from the animal, the implantation, adsorbing sites, and corpora lutea were counted.
Abortion Experiments
In this experiment, timed-pregnant rats on day 10 of gestation were randomly divided into three groups of five rats each. In the first group, each pregnant rat received a daily dose of 300 mg/kg hydro-alcoholic leaves extract orally for 3 consecutive days starting from days 10 to 12 of gestation,17 the second group received a doubled dose of 600 mg/kg, and the third group (control) similarly received 1.0 mL normal saline. On day 13, the rats underwent laparotomies using anesthesia to minimize the painful effect of sacrificing steps. The uterus and ovary units were removed from the animal, the viable, non-viable, adsorbing sites and corpora lutea were counted.
The body weight of all experimental animals was measured using a digital electronic balance. Animals were observed individually at least once during the first 30 minutes after dosing and periodically during the first 24 hours (with special attention given during the first 4 hours). All gross pathological changes were recorded for individual animals in each group.
Statistical Analysis
The mean and the standard deviation for the results of each group of rats were calculated. Differences between the experimental and control groups were compared using one-way analysis of variance (ANOVA), followed by Dunnett’s T-test to determine their level of significance.
Differences at p<0.05 were considered statistically significant.
Ethical Consideration
The study was conducted after having an approval letter from the research ethics review committee of the College of Health Sciences with number MTU/chs/2005, Mizan–Tepi University. Additionally, as to the OECD 423 guideline, animals used in this study were protected from any unnecessary painful and terrifying situations.40 They were handled as per recommended standard and anesthetized for any painful procedure.38,40,42
Results
Rumex nepalensis: Extraction Yield
A maceration technique was used for crude extraction. Initially, 345 grams of the crushed plant material was weighed using a digital electrical balance and transferred into a maceration chamber. Then, 80% ethanol was added and the chamber was covered with a lid. After this, the chamber was starred occasionally for 3 days, and then it was first filtered using muslin cloth followed by Grade 1 Whatman filter paper. The filtrate was transferred into an evaporating dish and kept over 80 °C sated thermostat water bath until the solvent completely evaporated.
- Weight of plant material= 345 grams
- Weight of evaporating dish= 74.9 grams
- Weight of dish + extract= 180.9 grams
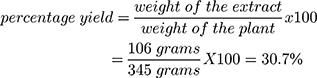
Standard phytochemical screening tests were used to assess the presence of secondary metabolites. The findings are depicted in Table 1.
 |
Table 1 Phytochemicals Identified from R. nepalensis Extract |
Effects of Acute Administration of Extracts on Behavior, Body Weight, and Gross Morphology of the Vital Organs
Hydro-alcoholic extract of Rumex nepalensis Spreng did not show any mortality with a single oral dose of up to 5000 mg/kg body weight. Behavioral changes like loss of appetite, hypo-activity, piloerection, lethargy, and dizziness were observed at and above the dose of 2000 mg/kg with increased severity as the dose increased. The symptoms, however, disappeared after a washout period of the first week of observation (Table 2).
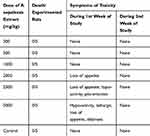 |
Table 2 Acute Toxic Effects of Rumex nepalensis Spreng Leaves Extract in Rats as Compared to the Controls |
Concerning body weight change, both the treated and control groups of rats gained proportional body weight during the 2-week observation period, which is not significant (P>0.05) (Table 3).
 |
Table 3 Mean Body Weight of Rats Treated with R. nepalensis Extract as Compared to the Controls During the 2 Weeks Observation Period (Expressed as Mean±SDE (p value), n=5) |
Observation on the gross appearance of internal organs including the liver and kidney of experimental rats did not show any abnormal changes in texture, shape, size, and color as they are compared to that of the control. Also, no lesion was noted in these organs in all groups of experimental rats.
Preimplantation and Abortifacient Effect of Hydroalcoholic Extract of Rumex nepalensis
Female virgin mated rats received hydro-alcoholic extracts for 3 consecutive days, starting from day 3 of gestation. The treatments with 300 mg/kg and 600 mg/kg body weight significantly inhibited implantation in four out of five (80%) rats in both doses. On the other hand, the mean numbers of corpora lutea were 4.33±3.92 (P<0.007) and 1.83±4.99 (P<0.002) of respective doses as compared to control (12±2.1) (Table 4).
 |
Table 4 Preimplantation Effects of Hydro-Alcoholic Extracts of R. nepalensis on Female Pregnant Rats |
Again, timed-pregnant rats received a hydro-alcoholic extract of 300 mg/kg and 600 mg/kg body weight for 3 consecutive days, from day 10 to 12 of gestation. This treatment significantly induced strong abortive action in pregnant rats in four out of five (80%) for 300 mg/kg and in five out of five (100%) rats for 600 mg/kg. Meanwhile, the mean numbers of viable fetuses were 1.5±4.17 (P<0.003) and 1.25±2.23 (P<0.004) for rats who received a hydro-alcoholic extract of 300 mg/kg and 600 mg/kg body weight respectively as compared to control rats, which was 11±2.34 (Table 5). But, the hydro-alcoholic extract did not affect the mean number of corpora lutea.
 |
Table 5 Abortifacient Effects of Hydro-Alcoholic Extracts of R. nepalensis on Female Pregnant Rats |
Discussion
Phytochemicals of R. nepalensis extracts were tested using standard methods. Accordingly, this plant is positive for alkaloids, hydrolyzable tannins, flavonoids, steroids, saponins, and anthraquinones. Previous studies also reported an abundance of anthraquinones,25,30,32,35,43 saponins, tannins,44 and flavonoids from the extraction of R. nepalensis.25,35 A study also showed that extract was negative for alkaloids.35 Whereas, contrary to our findings, terpenoids were reported from the hydro-alcoholic extract of R. nepalensis in the aforementioned study.
In the present acute toxicity test, the hydro-alcoholic extract of R. nepalensis did not show any mortality with a single oral dose up to 5000 mg/kg body weight. A similar finding was reported by Habtamu, where R. nepalensis was safe up to the dose of 5000 mg/kg in mice.35 Dosing of the animals above 5000 mg/kg was not done with the recognition of the need to protect animal welfare.40
Behavioral changes such as loss of appetite, hypo-activity, piloerection, lethargy, and dizziness started to appear at a dose of 2000 mg/kg. The symptoms, however, disappeared after the 1st week of observation. The present result, therefore, suggests that the oral LD50 of the extract is greater than 5000 mg/kg. Hence, the plants’ extract may be considered to be safe when administered orally. In line with this, the tender leaves of R. nepalensis are cooked and used as vegetables, which impart an acidic-lemon flavor in dishes, safely used as nutraceuticals.27,45
This study was designed to evaluate the preimplantation and abortifacient effect of hydro-alcoholic leaves extracts of R. nepalensis following the confirmation of its acute toxicity profile. In this investigation, the hydro-alcoholic extract of R. nepalensis inhibited implantation in pregnant rats. That means it has a dose-dependent anti-preimplantation effect. The plant’s leaves extract administrated orally on days 3–5 to pregnant rats results in diminished fetal implantation and caused a significant reduction of corpora lutea. A study on Artemisia monosperma extract by Hijazi and Salhab also showed similar effects on female pregnant rats.17 Hydro-alcoholic leaves extract of Alstonia scholaris Linn is also in line with our findings. The researchers reported that the oral administration of Alstonia scholaris Linn extract inhibited implantation activity in rats.46
The hydro-alcoholic extracts of R. nepalensis also exhibited a pronounced abortifacient effect. It has shown a dose-dependent loss of implants and an increased number of resorption in female rats at both doses. The results of this study are in line with the traditional reputation of this plant and antifertility activities of different species of the genus Rumex and the family Polygonaceae25,47,49 were previously reported as an abortifacient plant.25,50,52 Also, several plants are used traditionally for antifertility claims,17,20,50,55 and experimentally evaluated for their claim.12,17,25,46,49,56,60
The progesterone and estrogen hormones facilitate the process of implantation and in the maintenance of implanted embryo.46,50,56,57 The anti-implantation and abortifacient activity of metabolites of R. nepalensis may be due to their effects on hormonal balance, blood progesterone level, and estrogenic activities, and this makes it have an antagonist effect and interferes with the fertility process. But the exact mechanism(s) of this observed anti-fertility and abortifacient activity of the plant extract remains unclear. Likewise, the study of athe bortifacient effect of leaf extracts of Inula viscosa was also found to be unclear.57 The present study elucidates the fact that the R. nepalensis extract has no toxic effect on major vital organs such as the liver, spleen, and kidney, but is active against implantation and causes abortion.
Despite it is difficult to exactly attribute which metabolites are anti-implantation and abortifacient effect, it was postulated that polyphenols were isolated in many plants with antifertility activities.46,49,50,52
Conclusion
In conclusion, the present study showed that the administration of R. nepalensis demonstrates anti-implantation and abortifacient activity. This also reduces the number of neonates that are consistent with its use in folk medicine as an anti-conceptional agent. This plant was non-toxic up to a dose of 5000 mg/kg. Further studies are recommended for understanding the exact mechanism(s) of this plant and the probable changes in hormonal levels, fractionation, and elucidation of constituent potentially responsible for their effects. Besides, extraction using a non-polar solvent and its activities should be assessed to fully understand the activities of the plant. Moreover, sexually active reproductive age groups and pregnant women should avoid consumption of this plant to prevent unintended abortion.
Acknowledgments
We greatly thank Mr. Andualem Henok (ass. prof.) dean of the College of Health Science for his genuine effort in opening animal laboratories and helping us in breeding the animals there.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Landy U, Ratnam SS. Prevention and Treatment of Contraceptive Failure. plenum press; 1985:11. Available from: https://link.springer.com/chapter/10.1007/978-1-4684-5248-8_13.
2. Ganatra B, Gerdts C, Rossier C, et al. Global, regional, and subregional classification of abortions by safety estimates from a bayesian hierarchical model. Lancet. 2017;36(17):1–10.
3. Grimes DA, Benson J, Singh S, et al. Sexual and reproductive health for unsafe abortion, the preventable pandemic. Lancet. 2006;368(9550):1908–1919.
4.. Bahekar S, Kale R. Herbal plants used for the treatment of malaria: a literature review. Pharmacogn Phytochem. 2013;1(6):141–146.
5. World Health Organization (WHO). Traditional Medicine Strategy 2002-2005. Geneva: World Heal Organ. 2002; 1–74. Available from: https://apps.who.int/medicinedocs/en/d/Js2297.
6. Peltzer K. Utilization and practice of traditional/complementary/alternative medicine (TM/CAM) in South Africa. African J Tradit Complement Altern Med. 2009;6(2):175–185.
7. Abdullahi AA. Trends and challenges of traditional medicine in Africa. African J Tradit Complement Altern Med. 2011;8(5suppl.5):115–123.
8. Steenkamp V. Traditional herbal remedies used by South African women for gynaecological complaints. J Ethnopharmacol. 2003;86(1):97–108. doi:10.1016/S0378-8741(03)00053-9
9. Shewamene Z, Dune T, Smith C. The use of traditional medicine in maternity care among African women in Africa and the diaspora: a systematic review. BMC Complement Altern Med. 2017;17(1):382. doi:10.1186/s12906-017-1886-x
10. Mahomoodally MF. Evidence-based complementary and alternative medicine traditional medicines in Africa: an Appraisal of ten potent african medicinal plants. Evidence-Based Complement Altern Med. 2013;2013:14. doi:10.1155/2013/617459
11. Rajeswari J, Rani S. Medicinal plants used as abortifacients: a review. Int J Pharm Sci Rev Res. 2014;24(1):129–133.
12. Akah PA, Abortifacient activity of some Nigerian medicinal plants. Phyther Res. 1994;8(2):106–108. doi:10.1002/ptr.2650080212
13. The Royal Women’s Hospital. Herbal and traditional medicines in pregnancy. 2013 June;1–3. Available from: https://www.thewomens.org.au/images/uploads/fact-sheets/Medicines-in-pregnancy-140.
14. Ramasubramania RR, Vital role of herbal medicines in womens health: A perspective review. African J Plant Sci. 2015;9(8):320–326. doi:10.5897/AJPS2015.1315
15. Dewet HD, Ngubane SC. Traditional herbal remedies used by women in a rural community in northern maputaland (South Africa) for the treatment of gynaecology and obstetric complaints. South African J Bot. 2014;94:129–139. doi:10.1016/j.sajb.2014.06.009
16. Domaracky M, Rehak P, Juhas S, Koppel J. Effects of selected plant essential oils on the growth and development of mouse preimplantation embryos in vivo. Physiol Res. 2007;56:97–104.
17. Hijazi AM, Salhab AS. Effects of Artemisia monosperma ethanolic leaves extract on implantation, mid-term abortion and parturition of pregnant rats. J Ethnopharmacol. 2010;128(2):446–451. doi:10.1016/j.jep.2010.01.030
18. Pankhurst R, The history and traditional treatment of rabies in Ethiopia. Med Hist. 1970;14(4):378–389. doi:10.1017/S0025727300015829
19. Getahun A. Some of Common Medicinal and Poisonous Plants Used in Ethiopian. Addis Ababa University; 1976:126. Available from: https://www.semanticscholar.org/paper/.
20. Seifu T. Ethnobotanical and Ethnopharmaceutical Studies on Medicinal Plants of Chifra District, Afar Region, North Eastern Ethiopia. Addis Ababa University; 2004. Available from: https://www.ajol.info/index.php/epj/article/view/35097.
21. Davigdor E, Wohlmuth H, Asfaw Z, Awas T, The current status of knowledge of herbal medicine and medicinal plants in Fiche, Ethiopia. J Ethnobiol Ethnomed. 2014;10(1):38. doi:10.1186/1746-4269-10-38
22. Wubetu M, Abula T, Dejenu G. Ethnopharmacologic survey of medicinal plants used to treat human diseases by traditional medical practitioners in Dega Damot district, Amhara, Northwestern. BMC Res Notes. 2017;10(157):1–13.
23. Ragunathan M, Solomon M. The study of spiritual remedies in orthodox rural churches and traditional medicinal practice in Gondar Zuria district, Northwestern Ethiopia. Pharmacogn J. 2009;1(3):178–183.
24. Singh G. Plant Systematics: An Integrated Approach.
25. Vasas A, Orbangyapai O, Hohmann J. The genus rumex: a review of traditonal uses, phytochemistry and pharmacology. J Ethnopharmacol. 2015;175:198–228. doi:10.1016/j.jep.2015.09.001
26. Informa G, Hazards K, Refer B. Rumex nepalensis. Asian Journa of Tropical Medicen. 2017;4–6. www.apjtm.org/article.asp.
27. Rao KN, Ch S, Banji D, Sandhya S, Mahesh V. A study on the nutraceuticals from the genus Rumex. Hygeia J Drugs Med. 2011;3(1):76–87.
28. Mei R, Liang H, Wang J, Zeng L, Lu Q, Cheng Y. New seco-anthraquinone glucosides from rumex nepalensis. Planta Med. 2009;75(10):1162–1164. doi:10.1055/s-0029-1185467
29. Prasad B, Subedi L. Medicinal plant diversity and their pharmacological aspects of Nepal Himalayas. Pharmacognnosy J. 2011;3(25):6–17. doi:10.5530/pj.2011.25.2
30. Gautam R, Karkhile KV, Bhutani KK, Jachak SM. Anti-inflammatory, Cyclooxygenase (COX)-2, COX-1 Inhibitory, and Free radical scavenging Effects of Rumex nepalensis. Planta Med. 2010;76(14):1564–1569. doi:10.1055/s-0030-1249779
31. Hussain F, Ahmad B, Hameed I, Dastagir G, Sanaullah P, Azam S. Antibacterial, antifungal and insecticidal activities of some selected medicinal plants of polygonaceae. African J Biotechnol. 2010;9(31):5032–5036.
32. Ghosh L, Rahaman GJ, Sinha S, Pal S, Pal M, Saha BP. Antibacterial efficacy of Rumex nepalensis spreng. Phyther Res. 2003;17(5):558–559.
33. Waqas M, Afridi S, Ahmad N, et al. Antimicrobial activity of Rumex Nepalensis and Urtica Diocia. Int J Sci Res. 2016;5(3):1563–1566.
34. Yadav S, Kumar S, Jain P, et al. Antimicrobial activity of different extracts of roots of Rumex nepalensis spreng. Indian J Nat Prod Resour. 2011;2(1):65–69.
35. Habtamu A. Evaluation of the Antiplasmodial and Antimicrobial Properties of the Medicinal Plants Rumex Nepalensis Spreng and Centella Asiatica. Addis Ababa University; 2017. Available from: http://etd.aau.edu.et/handle/123456789/8510.
36. Mirutse G, Zemede A, Zerihun W. Medicinal plants of the Meinit ethnic group of Ethiopia: an ethnobotanical study. J Ethnobiol Ethnomed. 2009;124(3):513–521.
37. Eshetu N, Afework M, Makonnen E, Debella A, Ergete W, Tolesssa T. Evaluation of the acute and sub-chronic toxic effects of aqueous leaf extracts of artemisia afra on liver, kidney and some blood parameters in Wistar Rats. Adv Biosci Bioeng. 2016;4(1):1–9.
38. World Health Organization. Research guidelines for evaluating the safety and efficacy of herbal medicines. 1993;35. Available from: https://apps.who.int/medicinedocs/en/d/Jh2946e/.
39. Organisation For Economic Cooperation and Development. guideline for testing of chemicals. 2008;1–26. Available from: www.vivotecnia.com.
40. Organisation For Economic Cooperation and Development. Guidelines for the Testing of Chemicals, OECD 423. Acute oral toxicity. 2001. Available from: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecdtg425.pdf.
41. Hayelom K, Mekbeb A, Eyasu M, Wondwossen E, Kelbesa U. Methanolic effect of Clerodendrum myricoides root extract on blood, liver and kidney tissues of mice. Afr Health Sci. 2012;4:489–497.
42. World Health organization. General guidelines for methodologies on research and evaluation of traditional medicine. 2000. Available from: https://www.who.int/iris/handle/10665/66783.
43. Liang HX, Dai HQ, Fu HA, et al. Bioactive compounds from Rumex plants. Phytochem Lett. 2010;3(4):181–184.
44. Ghosh L, Gayen JR, Sinha S, Saha BP, Pal M. Pharmacognostical profile of roots of Rumex nepalensis spreng. Anc Sci Life. 2001;20(4):93–96.
45. Anusuya N, Gomathi R, Manian S, Sivaram S, Vnkataramegowda A. Evaluation of Basella rubra. Rumex nepalensis spreng, and Commelina benghalensis L. for antioxidant activity. Int J Pharm Pharmaceuitical Sci. 2012;4:3.
46. Choudhary M, Rani S, Sharma P, Choudhary N, Budhwaar V. Anti-fertility and abortifacient potential of hydroalcoholic leaves extract of Alstonia scholaris in female rats: an ethnomedicine used by Papua women in New Guinea. Bull Fac Pharmacy. 2017;55(1):123–127. doi:10.1016/j.bfopcu.2017.01.005
47. Solomon T, Largesse Z, Mekbeb A, Eyasu M, Asfaw D. Effect of Rumex steudelii methanolic root extract on ovarian folliculogenesis and uterine histology in female albino rats. Afr Health Sci. 2010;10(4):353–361.
48. Gebrie E, Makonnen E, Zerihun L, Debella A. The possible mechanisms for the antifertility action of methanolic root extract of Rumex steudelii. Afr Health Sci. 2005;5(2):119–125.
49. Gashaw T, Mekonnin E, Debella A. In vivo Evaluation of antifertility activity of aqueous and butanol fractions of methanolic root extract of rumex steudelii in female mice and Rats. East African J Heal Biomed Sci. 2016;1(1):1–11.
50. Daniyal M, Akram M. Antifertility activity of medicinal plants. J Chinese Med Assoc. 2015;78(7):382–388. doi:10.1016/j.jcma.2015.03.008
51. Maurya R, Srivastava S, Kulshreshta DK, Gupta CM, Traditional remedies for fertility regulation. Curr Med Chem. 2004;11(11):1431–1450. doi:10.2174/0929867043365215
52. Kumar D, Kumar A, Prakash O. Potential antifertility agents from plants: A comprehensive review. J Ethnopharmacol. 2012;140(1):1–32. doi:10.1016/j.jep.2011.12.039
53. Gradwohl A. Herbal abortifacients and their classical heritage in Tudor England. Penn History Review. 2013;20(1):45–71.
54. Gul S, Rubab B, Ahmad N, Iqbal U. Herbal drugs for abortion may prove as better option in terms of safety. Journal of Scientific and Innovative Research. 2015;4(2):105–108.
55. Ciganda C, Laborde A. Herbal infusions used for induced abortion. J Clin Toxicol. 2003;41(3):235–239.
56. Shaik A, Kanhere RS, Cuddapah R, Nelson KS, Vara PR, Sibyala S. Antifertility activity of Artemisia vulgaris leaves on female Wistar rats. Chin J Nat Med. 2014;12(3):180–185. doi:10.1016/S1875-5364(14)60030-3
57. Al-Dissi NM, Salhab AS, Al-Hajj HA. Effects of Inula viscosa leaf extracts on abortion and implantation in rats. J Ethnopharmacoll. 2001;77(1):117–121. doi:10.1016/S0378-8741(01)00261-6
58. Dabhadkar D, Patil U, Varsha Z, Wikhe M, Dawada S. Antifertility efficay of Cannabis Sativa leaves on female albino rats. Int J Sci Invent Today. 2013;2(2):107–117.
59. Uchendu CN, Isek T. Antifertility activity of aqueous ethanolic leaf extract of Spondias mombin (Anacardiaceae) in rats. Afr Health Sci. 2008;8(3):163–167.
60. Dabhadkar D, Varsha Z. Abortificient efficacy of Indigofera trifoliata leaves extract on female albino rats. Asian J Pharm Clin Res. 2013;6(3):75–79.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
