Back to Journals » Cancer Management and Research » Volume 11
Evaluating the prognostic significance of tumor-infiltrating lymphocytes in solid tumor: practice of a standardized method from the International Immuno-Oncology Biomarkers Working Group
Authors Liu JY, Yang GF, Chen FF , Peng CW
Received 14 January 2019
Accepted for publication 6 June 2019
Published 23 July 2019 Volume 2019:11 Pages 6815—6827
DOI https://doi.org/10.2147/CMAR.S201538
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Antonella D'Anneo
Jiu-Yang Liu,1,* Gui-Fang Yang,2,* Fang-Fang Chen,2 Chun-Wei Peng1
1Department of Gastrointestinal Surgery, Zhongnan Hospital of Wuhan University, Hubei Key Laboratory of Tumor Biological Behaviors & Hubei Cancer Clinical Study Center, Wuhan 430071, People’s Republic of China; 2Department of Pathology, Zhongnan Hospital of Wuhan University, Wuhan 430071, People’s Republic of China
*These authors contributed equally to this work
Purpose: Tumor-infiltrating lymphocytes (TILs) become increasingly relevant to tumor progression. This study aims to evaluate (a) methods of TILs assessment and (b) their prognostic significance in gastric cancer (GC).
Methods: The percentage of stromal TILs (psTIL) was reported semi-quantitatively by H&E evaluation. Herein, we screened two independent cohorts of breast cancer (n=240) and GC (n=481) for psTIL characterization. Correlations between psTIL and clinic-pathological features, as well as overall survival (OS) were further explored. Additionally, the prediction role of psTIL in GC was evaluated by receiver operating characteristic curve (ROC) analysis.
Results: TILs could be demonstrably distinguished from other stromal areas and surrounding tumor nests according to the assessment method. More importantly, it is reproducible, easily to determine, and quickly performed. In GC, a two-grade scale for psTIL was appropriate to be divided into low and high subgroups by using the median value of 10% as the threshold. High psTIL was correlated with no serosa invasion, earlier TNM stage and better survival state (P<0.05 for all), and identified as a favorable prognostic factor both by univariate (HR: 0.734, P=0.047) and multivariate analyses (HR: 0.722, P=0.030). A beneficial OS of high psTIL was found in a linear manner with increasing TILs infiltrates associated with improved survival by Kaplan–Meier survival curve (P=0.030) and ROC analysis (AUC: 0.432, P=0.012).
Conclusion: TILs provide a reproducible method for assessment that can potentially be used to guide management. The parameter psTIL could be served as an independent, favorable prognostic factor of GC.
Keywords: tumor-infiltrating lymphocytes, gastric cancer, prognosis, biomarker
Introduction
Gastric cancer (GC) is the fifth most common human malignant disease and the fourth leading cause of cancer-related death worldwide.1 The survival of GC patients remains poor even after the surgery-centered multidisciplinary treatments. Large variations in clinical outcomes have been reported in patients with the same stage and similar treatment regimens.2,3 One of the key causes is that GC is a heterogeneous disease in its biologic behaviors, but the present GC staging system provides incomplete prognostic information; thus, additional information is necessary to improve tailored treatment for the individual patient.4,5 Notably, pathologists have long recognized the stromal, immune infiltration, nerves, and vasculature as integral parts of the tumor microenvironment, which often provide important information regarding tumor behavior, prognosis, and response to treatment.6
Tumor-infiltrating lymphocytes (TILs), represented by T cells, B cells, and NK cells, are the major type of infiltrating immune cells. TILs are considered a manifestation of the host immune response against tumor cells, and several studies have already reported the potential of TILs as a prognostic parameter for various human malignancies, such as colorectal cancer,7 non-small-cell lung cancer,8 oropharyngeal cancer,9 and breast cancer (BC).10 Taken together, current data suggest that stromal TILs may be an important biomarker to predict the survival and select patients with the highest likelihood of responding to therapy.11 For TIL evaluation to be incorporated into research (ie, stratification for clinical trials) or routine clinical care, the approach to quantifying TIL within the stromal and the tumor needs to be standardized. According to the International TILs Working Group, a detailed description of how to evaluate stromal TILs instead of total TILs and intratumoral TILs has been recommended as a potential consensus.12,13
Although such methods have also been recommended for other solid cancer,14 only a few studies have investigated the prognostic impact of TILs in GC.15 It is well acknowledged that good biomarkers in cancer must be validated refers to the robustness of the test, in terms of accuracy, reliability, and interobserver reproducibility.16 Herein, the recommended method has been validated in 240 BC tissues, and then percentage of stromal TILs (psTIL) in 481 GC tumors was evaluated according to the recommended method. This study was aimed to evaluate the feasibility and reproducibility of this method, and the possibility for TIL to be considered as a promising factor in terms of GC prognosis for clinical use.
Materials and methods
Patients and follow-up
There were 240 BC and 481 GC cases included in this study, all of which have received radical operation at the Department of Oncology, Zhongnan Hospital of Wuhan University (Wuhan, China) from December 2002 to February 2011. Major clinic-pathological characteristics including age, gender, tumor location, pathological types, lymph node metastasis, invasion depth, and distant metastasis were collected. In addition, the information of follow-up was available. TNM stages were determined according to the UICC/AJCC 7th TNM staging system. The primary end point for this study was overall survival (OS), which was defined as the interval from the date of surgery to cancer-related death. Additionally, the 3-year disease-free survival (DFS) and 5-year DFS were also assessed in the BC analysis.
Ethics statement
All patients provided written informed consent for their information to be stored in the hospital database, and we obtained separate consent for use of research. Study approval was obtained from independent ethics committees from Zhongnan Hospital of Wuhan University. The study was undertaken in accordance with the ethical standards of the World Medical Association Declaration of Helsinki.
H&E staining and image acquisition
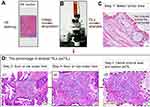
Serial tissue sections (4-μm thickness) of BC and GC were prepared for H&E staining before proceeding to the following imaging studies (Figure 1A). Routine H&E staining was performed as previously published.17 The digital images of H&E staining were captured under Olympus BX51 fluorescence microscope equipped with Olympus DP72 camera (Olympus Optical Co., Ltd., Tokyo, Japan), at 100× or 200× magnification (Figure 1B). Identical settings were used to avoid selection bias.
Morphological evaluation and quantification of stromal TILs
A standardized method for stromal TILs morphological evaluation was conducted according to the International Immuno-Oncology Biomarkers Working Group. The exclusion criteria were as follows: TILs outside of the borders of tumor invasive front, TILs in tumor zones with crush artifacts, necrosis, and regressive hyalinization (Figure 1C). In the selected areas, stromal TILs were considered no co-evolution with cancer if not containing any tumor cells. Therefore, tumor cells should be present at all borders of the image field. To minimize selection bias, one section with at least 4 standard-compliant vision fields per patient was randomly selected and considered as adequate, with no focus on hotspots.
Then, the percentage of TILs (psTIL) were obtained from selected images. The clinical information was unknown by two independent pathologists in advance. The parameter psTIL was reported for the stromal compartment and calculated by the area occupied by stromal TILs over the total intratumoral stromal area, which was round up to the nearest 5–10% in this practice for as much detailed information. A magnification of ×100 or ×200 was optional if psTIL value was 0–10%, and 50–100%, while a higher magnification of ×200 was optimized if psTIL value was located in the intermediate group (Figure 1D). Then, a full assessment of average psTIL was determined. In this study, levels of psTIL were categorized according to the median value, which was determined as a threshold for survival analysis.
Statistical analysis
Statistical analyses were performed with IBM SPSS 19.0 software (SPSS Institute, Chicago, IL, USA). Correlations between psTIL and clinic-pathological parameters were calculated with the chi-square test. Survivals were analyzed with the Kaplan–Meier method, by the use of the log-rank test for univariate analyses, and by use of Cox regression model for multivariate analyses. Receiver operating characteristic curve (ROC) analysis was used to determine the predictive value of the parameters. Two-sided P-value of <0.05 was considered to be statistically significant.
Results
Prognostic values of psTIL in BC
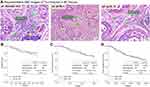
A total of 240 BC patients were included in the present study. Baseline characteristics were summarized in Table 1. The median age was 48.0 years, ranging from 29 to 78 years. According to the recommended methods, TILs have been evaluated in all patients. TILs were distributed in both intratumoral and stromal areas within tumor borders, whereas were more frequent and abundant in the stromal area through direct visualization of H&E staining results, as shown in Figure 2A1. The median psTIL value was 5%, ranging from 0% to 100%. There were 142 (59.2%) and 98 (40.8%) cases divided into psTIL-Low (L) and psTIL-High (H) subgroups, respectively. Representative views of psTIL-L and psTIL-H are shown in Figure 2A2 and A3. Consequently, no statistically significant correlations could be found between psTIL and age, menopausal status, tumor size, clinical nodal status, TNM stage, histological grade, and OS status (P>0.05 for all). Kaplan–Meier survival analysis was conducted according to OS (Figure 2B), 3-year (DFS (Figure 2C), and 5-year DFS (Figure 2D), respectively. Among 98 patients in psTIL-H subgroup, no significant difference was observed (P>0.05 for all).
 |
Table 1 Patients’ demographics and correlations between psTIL and major clinic-pathological features in breast cancer |
Stromal TILs distribution features in GC
TILs could be evaluated in all GC patients (n=481). As shown in Figure 3, TILs were frequently distributed in stromal compartments within tumor borders. Through direct visualization of H&E staining results, TILs were infrequently scattered in stromal areas in some GC cases (Figure 3A, psTIL=1%), whereas abundantly accumulated in others (Figure 3B, psTIL=70%). All psTIL values were determined and included for next-step analyses. The potential relationship of psTIL and histological grade seemed to be found by qualitative analysis. Compared with poorly differentiated adenocarcinoma (Figure 3A) and moderately differentiated adenocarcinoma (Figure 3C, psTIL=30%), highly differentiated cases (Figure 3D, psTIL=100%) had a tendency of higher psTIL values, which should be further confirmed by statistical analysis.
Correlations between psTIL and clinic-pathological characteristics in GC
Among the 481 GC cases included in this study were 338 (70.3%) males and 143 (29.7%) females, ranging in age from 25 to 87 (mean ± SD: 59.0±11.9) years. According to the 7th UICC/AJCC TNM staging system for GC, there were 67 (13.9%), 108 (22.5%), 286 (59.5%), and 20 (4.2%) patients with stage I, stage II, stage III, and stage IV, respectively. The main demographic and clinic-pathological characteristics are presented in Table 2.
 |
Table 2 Patients’ demographics and correlations between psTIL and major clinic-pathological features in gastric cancer |
The median value of psTIL in GC was 10%, ranging from 0% to 100%. The threshold was determined according to the median value. Thus, psTIL-L (≤10.0%) and psTIL-H (>10.0%) subgroups were observed in 269 (55.9%) and 212 (44.1%) patients, respectively. Correlations between psTIL and major clinic-pathological features in GC were summarized in Table 2. psTIL-L was correlated with serosa invasion (P<0.001), advanced TNM stage (P=0.028), and poorer survival state (P=0.004). No statistically significant correlations could be observed with age, gender, tumor location, histological grade, and pN status (P>0.05 for all).
Survival analysis of psTIL in GC
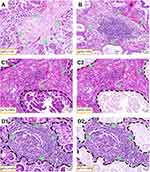
For 481 GC cases, the median OS was 42.6 (95%CI: 32.7–52.5) months, and the 1-, 3-, and 5-year survival rate was 83.84%, 56.26%, and 43.35%, respectively. As expected, the traditional factors were associated with GC patients’ OS, such as histological grade (Figure 4A), serosa invasion (pT) status, lymph node (pN) status (Figure 4B), distant metastasis, and TNM stage (Figure 4C) (P<0.05 for all).
There were 123 (45.7%) and 69 (32.5%) patients dead in psTIL-L and psTIL-H subgroups, respectively. The estimated 3-year survival rates (%) in the above 2 subgroups were 53.89% and 59.77%, respectively. The estimated 5-year survival rate (%) was 36.81% and 52.10%, respectively. The difference between psTIL-L and psTIL-H groups was statistically significant in terms of OS (Figure 4D, P=0.030). GC cases with high psTIL tended to have longer OS. Detailed data are shown in Table 3.
 |
Table 3 Analyses of major clinic-pathological factors and psTIL regarding GC OS |
Multivariate and ROC analyses
To further clarify whether psTIL could have a prognostic value, univariate and multivariate survival analyses were performed. The univariate analyses revealed that high psTIL (>10%), histological grade, serosa invasion, lymph node metastasis, distant metastasis, and TNM stage were significantly associated with postoperative outcome (P<0.05 for all). In multivariate analyses, high psTIL (HR=0.734, P=0.047) was identified as an independent better prognostic factor. On the contrary, characteristics included in the TNM system (pT, pN status, and distant metastasis) and histological grade were identified as independent worse prognostic factors (Table 4).
 |
Table 4 Univariate and multivariate analyses of factors associated with GC OS |
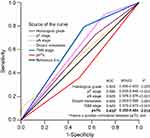
Predictive values of the above-mentioned 5 independent factors (histological grade, pT, pN status, distant metastasis, and psTIL) were further studied by ROC analysis. TNM stage was also compared. Among the tested factors, distant metastasis was the weakest risk factor for death. psTIL performed well in predicting the clinical outcomes of GC patients. Compared with other factors, psTIL was a beneficial prognostic factor, in a linear manner with increasing TILs infiltrates associated with improved survival (area under the curve: 0.432 [95%CI: 0.380–0.484], P=0.012) (Figure 5).
Discussion
The assessment of immune infiltrate in tumors, most commonly referred to as TILs, is gaining importance in the current quest for optimal biomarkers.18 TILs are mononuclear immune cells that infiltrate into tumor tissues. The presence of TILs reflects an individual immunological response state and contains potential clinical validity.19 In this study, we validated the usage of the TILs evaluating methods in BC, as well as the appropriate implementation in clinical practice for GC. In addition, the prognostic effects of psTIL were explored and stratified using the median value were further investigated in GC patients.
Theoretically, the complexity and heterogeneity of TILs originate from specific phenotypes of the cellular surface molecules.20,21 Hence, TILs can be evaluated by analyzing various subgroups through immunohistochemistry (IHC) methods.22,23 However, it remains unclear whether IHC method allows more accurate analysis, and different TILs subtypes may indicate controversial prognostic relevance.24 In research settings, many different methods are being used to investigate the host immune response to tumors. IHC and multiplexed fluorescent IHC with multispectral imaging allow the definition of the majority of immune cell subsets that can be refined by combinations of markers, including CD8+ cytotoxic T cells, CD4+ T helper cells, FOXP3+ Tregs, B cells, macrophages, and dendritic cells. But those methods require cell surface identification and a significant investment in initial optimization, has complex technology, analysis and storage requirements, and is yet to become routine. Compared with the IHC method, H&E staining–based TILs evaluation has been demonstrated to be standardized and reproducible.13 Because of its low cost, availability, convenience, and importance in prognosis, H&E-based evaluation method is more suitable in clinical practice and could easily be implemented in routine pathology diagnostics. In this study, H&E images were with vibrant colors, excellent contrast, and appropriate for direct visualization and measurement. In this study, our results showed that TILs could be demonstrably distinguished from other stromal areas and surrounding tumor nests, either in BC or in GC, which was essential for subsequent comparability. More importantly, it is reproducible, easy to determine, and quickly performed.
Before the recommendation has been proposed, the extent of TILs infiltration assessed by simple morphological evaluation of H&E-stained views has been shown to have predictive and prognostic values for BC.25,26 Luen and his colleagues showed that patients in certain subtype with advanced HER2-positive BC treated with docetaxel, trastuzumab, and pertuzumab or placebo should consider TILs as a stratification factor and investigate whether therapies that can augment immunity could potentially further improve survival.27 In addition, further studies have shown that there is a strong linear relationship between the increase in TILs and improved DFSl for triple-negative and HER2-positive disease.28 In this study, the prognostic value of TILs in BC has been explored, which is similar to the previous studies across all BC subtypes that have uncovered the prognostic significance of TILs.29 All results revealed the high heterogeneous of BC, suggesting different biology of the immunological infiltration in different BC subtypes.30
Interesting, this study showed the prognostic value of psTIL in all GC but not certain subtypes by the H&E-based evaluation. Considering the higher morbidity and mortality of GC in China, a total of 4,185 patients were enrolled in a recent meta-analysis to evaluate the prognostic significance of TILs subsets in GC.31 TILs were summarized as potential prognostic biomarkers in GC; however, contradictory findings existed. Some studies had shown associations between higher quantities of TILs and greater proportions of patients achieving longer survival time,32,33 whereas some subtypes showed a bidirectional prognostic role or predicted worse prognosis.34 As illustrated early, the difficulty of comparability among previous studies may be attributable to different methods, TILs subsets, and stratification decisions. In fact, few studies were conducted from the aspect of the percentage index according to the recommendation. However, further work is needed to ensure that the valuable information that could be obtained from TILs assessment is not lost due to issues of resource commitments, methodology, or lack of standardization. Notwithstanding, there is no current consensus on the best TILs distribution for predicting survival in GC.35
In this study, GC cases with low psTIL were at high risk of serosa invasion, advanced stages, and poor survival. This result is in agreement with a retrospective analysis of a randomized phase 3 study,27 suggesting TILs as a stratification parameter in clinical practice. Furthermore, psTIL was associated with GC OS in a linear manner with increasing TILs infiltrates associated with improved survival. Some studies observed TILs as a better predictor of patient survival at earlier stages or advanced stages of lung cancer.36,37 This result might originate from differing biological behaviors during GC progression, which was best conceptualized as cancer immunoediting.38 During this process, TILs subgroups may play two-way regulating roles, with a predominant contribution to either protumor or antitumor.12 Generally, TILs exist in intratumoral areas recruited by critical factors,39 exerted antitumor immunity and thus high psTIL exhibited favorable prognosis. However, recent evidence also suggested that TILs subgroups like CD4+ regulatory T cells (Treg) might be responsible for the failure of host immunological surveillance by inhibiting local inflammatory processes.40 Therefore, the functional role of psTIL differed in terms of GC clinical status.
Our study has some limitations. It was a retrospective study with limited generalizability as all specimens were obtained from patients in China, and the distribution of clinical characteristics might be different in other areas. However, the significant contribution of China to the worldwide burden of GC supports the performance of an initial investigation of the importance of H&E-based TILs evaluation in a Chinese population. Thus, a prospective, international, multicenter clinical trial will be needed to further validate our findings.
Conclusions
In summary, this study comprehensively analyzed the roles of psTIL in solid tumor and demonstrated that H&E staining–based semi-quantitative evaluation obtains a pragmatic, reproducible and easy assessment of stromal TILs. To our knowledge, the parameter psTIL could be served as an independent, favorable prognostic factor of GC. The classification of GC via stratification of psTIL could help predicting clinical outcomes and fine-tuning therapies in future practice.
Synopsis
In this study, the percentage of stromal TILs (psTIL) was reported semi-quantitatively by H&E staining-based evaluation. Two independent cohorts of BC (n=240) and GC (n=481) were included for evaluating the relationship between psTIL and clinic-pathological features, as well as OS. A beneficial OS of high psTIL was found in a linear manner with increasing TILs infiltration associated with improved OS [Kaplan–Meier survival curve: P=0.030; univariable (HR: 0.734, P=0.047) and multivariate analyses (HR: 0.722, P=0.030)]. Therefore, the parameter psTIL could be served as an independent, favorable prognostic factor of GC and certain subtypes of BC.
Acknowledgments
This work was supported by Science Fund of the National Natural Science Foundation of China (No. 81401515; 81230031), the Fundamental Research Funds for the Central Universities of Ministry of Education of China (No. 2042014kf0096; No. 2042019kf0141), and Zhongnan Hospital of Wuhan University Science, Technology and Innovation Seed Fund (znpy2018030; znpy2018048). This work was also funded by “351 talent project (Luojia Young Scholars)” of Wuhan University and Training project for young and middle-aged medical key personnel of Wuhan City.
Disclosure
The authors declare no conflict of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Noh SH, Park SR, Yang HK, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(12):1389–1396. doi:10.1016/S1470-2045(14)70473-5
3. Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379(9813):315–321. doi:10.1016/S0140-6736(11)61873-4
4. Peng C, Liu J, Yang G, Li Y. The tumor-stromal ratio as a strong prognosticator for advanced gastric cancer patients: proposal of a new TSNM staging system. J Gastroenterol. 2018;53(5):606–617. doi:10.1007/s00535-017-1379-1
5. Mlecnik B, Tosolini M, Kirilovsky A, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29(6):610–618. doi:10.1200/JCO.2010.30.5425
6. Galon J, Mlecnik B, Bindea G, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232(2):199–209. doi:10.1002/path.4287
7. Saito T, Nishikawa H, Wada H, et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med. 2016;22(6):679–684. doi:10.1038/nm.4086
8. Brambilla E, Le Teuff G, Marguet S, et al. Prognostic effect of tumor lymphocytic infiltration in resectable non-small-cell lung cancer. J Clin Oncol. 2016;34(11):1223–1230. doi:10.1200/JCO.2015.63.0970
9. Ward MJ, Thirdborough SM, Mellows T, et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br J Cancer. 2014;110(2):489–500. doi:10.1038/bjc.2013.639
10. Perez EA, Ballman KV, Tenner KS, et al. Association of stromal tumor-infiltrating lymphocytes with recurrence-free survival in the N9831 adjuvant trial in patients with early-stage Her2-positive breast cancer. JAMA Oncol. 2016;2(1):56–64. doi:10.1001/jamaoncol.2015.3239
11. Swisher SK, Wu Y, Castaneda CA, et al. Interobserver agreement between pathologists assessing tumor-infiltrating lymphocytes (TILs) in breast cancer using methodology proposed by the International TILs Working Group. Ann Surg Oncol. 2016;23(7):2242–2248. doi:10.1245/s10434-016-5173-8
12. Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259–271. doi:10.1093/annonc/mdu450
13. Dieci MV, Radosevic-Robin N, Fineberg S, et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: a report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin Cancer Biol. 2018;52(Pt 2):16–25. doi:10.1016/j.semcancer.2017.10.003
14. Hendry S, Salgado R, Gevaert T, et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the International Immuno-Oncology Biomarkers Working Group: part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv Anat Pathol. 2017;24(6):311–335. doi:10.1097/PAP.0000000000000161
15. Kang BW, Kim JG, Lee IH, Bae HI, Seo AN. Clinical significance of tumor-infiltrating lymphocytes for gastric cancer in the era of immunology. World J Gastrointest Oncol. 2017;9(7):293–299. doi:10.4251/wjgo.v9.i7.293
16. Harris LN, Ismaila N, McShane LM, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2016;34(10):1134–1150. doi:10.1200/JCO.2015.65.2289
17. Jiang D, Liu Y, Wang H, et al. Tumour infiltrating lymphocytes correlate with improved survival in patients with esophageal squamous cell carcinoma. Sci Rep. 2017;7:44823. doi:10.1038/srep44823
18. Pruneri G, Vingiani A, Bagnardi V, et al. Clinical validity of tumor-infiltrating lymphocytes analysis in patients with triple-negative breast cancer. Ann Oncol. 2016;27(2):249–256. doi:10.1093/annonc/mdv571
19. Savas P, Salgado R, Denkert C, et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol. 2016;13(4):228–241. doi:10.1038/nrclinonc.2015.215
20. Berghoff AS, Fuchs E, Ricken G, et al. Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunology. 2015;5(1):e1057388. doi:10.1080/2162402X.2015.1057388
21. Gu-Trantien C, Loi S, Garaud S, et al. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. 2013;123(7):2873–2892. doi:10.1172/JCI67428
22. Brown JR, Wimberly H, Lannin DR, Nixon C, Rimm DL, Bossuyt V. Multiplexed quantitative analysis of CD3, CD8, and CD20 predicts response to neoadjuvant chemotherapy in breast cancer. Clin Cancer Res. 2014;20(23):5995–6005. doi:10.1158/1078-0432.CCR-14-1622
23. Liu JY, Yuan JP, Geng XF, Qu AP, Li Y. Morphological study and comprehensive cellular constituents of milky spots in the human omentum. Int J Clin Exp Pathol. 2015;8(10):12877–12884.
24. Yu X, Zhang Z, Wang Z, Wu P, Qiu F, Huang J. Prognostic and predictive value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and meta-analysis. Clin Transl Oncol. 2016;18(5):497–506. doi:10.1007/s12094-015-1391-y
25. Dieci MV, Criscitiello C, Goubar A, et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol. 2014;25(3):611–618. doi:10.1093/annonc/mdt556
26. Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25(8):1544–1550. doi:10.1093/annonc/mdu112
27. Luen SJ, Salgado R, Fox S, et al. Tumour-infiltrating lymphocytes in advanced HER2-positive breast cancer treated with pertuzumab or placebo in addition to trastuzumab and docetaxel: a retrospective analysis of the CLEOPATRA study. Lancet Oncol. 2017;18(1):52–62. doi:10.1016/S1470-2045(16)30631-3
28. Wein L, Savas P, Luen SJ, Virassamy B, Salgado R, Loi S. Clinical validity and utility of tumor-infiltrating lymphocytes in routine clinical practice for breast cancer patients: current and future directions. Front Oncol. 2017;7:156. doi:10.3389/fonc.2017.00156
29. Luen SJ, Savas P, Fox SB, Salgado R, Loi S. Tumour-infiltrating lymphocytes and the emerging role of immunotherapy in breast cancer. Pathology. 2017;49(2):141–155. doi:10.1016/j.pathol.2016.10.010
30. Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50. doi:10.1016/S1470-2045(17)30904-X
31. Zheng X, Song X, Shao Y, et al. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: a meta-analysis. Oncotarget. 2017;8(34):57386–57398. doi:10.18632/oncotarget.18065
32. Kim KJ, Lee KS, Cho HJ, et al. Prognostic implications of tumor-infiltrating FoxP3+regulatory T cells and CD8+cytotoxic T cells in microsatellite-unstable gastric cancers. Hum Pathol. 2014;45(2):285–293. doi:10.1016/j.humpath.2013.09.004
33. Wang B, Xu DZ, Yu X, et al. Association of intra-tumoral infiltrating macrophages and regulatory t cells is an independent prognostic factor in gastric cancer after radical resection. Ann Surg Oncol. 2011;18(9):2585–2593. doi:10.1245/s10434-011-1609-3
34. Kawazoe A, Kuwata T, Kuboki Y, et al. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer. 2017;20(3):407–415. doi:10.1007/s10120-016-0631-3
35. Solinas C, Pusole G, Demurtas L, et al. Tumor infiltrating lymphocytes in gastrointestinal tumors: controversies and future clinical implications. Crit Rev Oncol Hematol. 2017;110:106–116. doi:10.1016/j.critrevonc.2016.11.016
36. Horne ZD, Jack R, Gray ZT, et al. Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1A non-small-cell lung cancer. J Surg Res. 2011;171(1):1–5. doi:10.1016/j.jss.2011.03.068
37. Feng W, Li Y, Shen L, et al. Prognostic value of tumor-infiltrating lymphocytes for patients with completely resected stage IIIA(N2) non-small cell lung cancer. Oncotarget. 2016;7(6):7227–7240. doi:10.18632/oncotarget.6979
38. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi:10.1126/science.1203486
39. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–209. doi:10.1038/nature13480
40. Nagase H, Takeoka T, Urakawa S, et al. ICOS+ Foxp3(+) TILs in gastric cancer are prognostic markers and effector regulatory T cells associated with Helicobacter pylori. Int J Cancer. 2017;140(3):686–695. doi:10.1002/ijc.30475
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.