Back to Journals » Patient Preference and Adherence » Volume 11
Evaluating the patient experience after implantation of a 0.4 mg sustained release dexamethasone intracanalicular insert (DextenzaTM): results of a qualitative survey
Authors Gira JP, Sampson R, Silverstein SM , Walters TR , Metzinger JL, Talamo JH
Received 1 November 2016
Accepted for publication 11 January 2017
Published 8 March 2017 Volume 2017:11 Pages 487—494
DOI https://doi.org/10.2147/PPA.S126283
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Joseph P Gira,1 Reginald Sampson,2 Steven M Silverstein,3 Thomas R Walters,4 Jamie Lynne Metzinger,5 Jonathan H Talamo5
1Ophthalmology Consultants, St Louis, MO, 2Montebello Medical Center, Inc., Montebello, CA, 3Silverstein Eye Centers, Kansas City, MO, 4Texan Eye, Austin, TX, 5Ocular Therapeutix, Inc., Bedford, MA, USA
Purpose: The purpose of this study is to evaluate the patient experience of sustained release dexamethasone intracanalicular insert (Dextenza™) following cataract surgery as part of a Phase III clinical trial program.
Methods: This cross-sectional, qualitative evaluation involved individual interviews lasting approximately 45 minutes. Patients from four US investigational study sites who had previously received an insert were enrolled. There were no predesignated end points; this was a qualitative survey seeking a deeper understanding of patient experience.
Results: Twenty-five patients were interviewed. Most patients (92%) reported the highest level of satisfaction grade with regard to overall product satisfaction. All patients described the insert as comfortable. Most patients (96%) described their overall experience with the insert as very convenient or extremely convenient. Twenty-two of 23 (96%) participants rated their experience with the insert as “very” or “extremely convenient”, compared to previous topical therapy, and 88% of patients stated that if they were to undergo cataract surgery again, they would request the insert. When asked if they would recommend the insert to family members or friends, 92% stated they would. The survey found that 84% of participants would be willing to pay more for the insert than for eye drop therapy.
Conclusion: The dexamethasone insert was found by patients to be highly favorable with regard to overall satisfaction, convenience, and comfort. The insert was well received and largely preferred over topical therapy alternatives following surgery. More extensive evaluation of the patient experience is warranted, and future studies should help inform design of the next generation of sustained release drug delivery systems.
Keywords: dexamethasone, cataract, sustained release, drug delivery, corticosteroid, Dextenza
Introduction
Untreated cataract is the leading cause of vision loss in the US, and as the size of the aging population increases, the problem is expected to intensify.1,2 According to US Census data, by the year 2020, it is estimated that the number of Americans diagnosed with cataract is expected to rise to 30.1 million, representing a 31.9% increase over current prevalence estimates.1 Given these numbers, it is not surprising that cataract removal is the most commonly performed surgical procedure in the US Medicare-eligible population.3 Approximately 3.6 million cataract surgeries were performed in the US in 2015, and >20 million surgeries were performed worldwide.4 In parallel with the steadily rising surgical volume is the ever-present requirement for safe and effective outcomes, driven by not only operative techniques but also appropriate pre- and postoperative care.
Significant variability exists among health care providers when it comes to medical therapy regimens used for cataract surgery. With a diverse and rapidly growing patient population undergoing cataract surgery at earlier stages of disease severity, expectations for outcomes are high. Surgical outcomes may be influenced by the timing, frequency, and route of administration of anti-inflammatory medications, but no standardized guidelines are widely accepted. Despite excellent results in a large majority of cases, postsurgical inflammation can have damaging, lasting effects on visual acuity and cause permanent structural and functional damage to ocular structures essential for good vision such as the cornea, macula, and optic nerve. In addition, although the effects are more transient, pain control and visual recovery time may be exacerbated and prolonged by inadequate control of intraocular inflammation following surgery.
A sustained release hydrogel intracanalicular insert containing 0.4 mg of dexamethasone insert (Dextenza™; Ocular Therapeutix, Inc., Bedford, MA, USA) is currently in development for the treatment of ocular inflammation and pain following cataract surgery.5 It is a fluorescent yellow, 3 mm cylindrical-shaped preservative-free drug product. A single sustained release insert was delivered through insertion into the lacrimal canaliculus by the physician immediately following ophthalmic surgery to provide corticosteroid therapy of 1-month duration in two prospective, multicenter, randomized, double-masked, placebo-controlled Phase III trials.5 The dexamethasone insert promptly swells on contact with moisture from the tear fluid or balanced salt solution and continues to expand until firmly secured in the canaliculus; the proprietary hydrogel delivery vehicle is designed to remain in the vertical canaliculus for 30 days and beyond in order to ensure retention throughout the drug delivery period. Through hydrolysis, the dexamethasone insert softens, liquefies, and is cleared through the nasolacrimal duct, obviating the requirement for removal. A combination of objective and subjective measures (anterior chamber cells using the Standardization of Uveitis Nomenclature criteria, and a 10-point pain scale) was collected to evaluate the proportion of patients with an absence of cells in the anterior chamber and the proportion of patients with an absence of pain following surgery.5 A significantly greater percentage of patients receiving the sustained release dexamethasone insert were found to be free of ocular pain (score of 0) at 8 days after surgery in both studies (Study 1, 80.4% [131/164] vs 43.4% [36/83], P<0.0001; Study 2, 77.5% [124/161] vs 58.8% [47/80], P=0.0025), and a greater percentage of patients were found to have no inflammation (defined as the absence of anterior chamber cells) at day 14 in the first study (33.1% [54/164] vs 14.5% [12/83], P=0.0018); however, in the second study, this difference did not achieve statistical significance (39.4% [63/161] vs 31.3% [25/80], P=0.2182).5 Despite missing the primary end point for inflammation in one study, other clinical assessments of inflammation, mean anterior chamber cell score, and absence of anterior chamber flare, which were collected and analyzed as secondary end points, supported the anti-inflammatory efficacy of the dexamethasone insert.5 At days 8 and 14, patients in the dexamethasone group had significantly lower mean anterior chamber cell scores, as compared to patients in the placebo group in both studies, and absence of anterior chamber flare was also more prevalent in the dexamethasone group versus patients receiving placebo at days 8 and 14 in both studies.5 Rescue medication rates were significantly higher at days 8 and 14 for both studies in the placebo group, as compared to patients receiving the dexamethasone insert. Similar proportions of patients in each group experienced ocular and nonocular adverse events, and there were no serious adverse events related to treatment.5
Patient-reported outcome studies are a useful, ancillary tool for key stakeholders (regulators, providers, payers, drug developers, and patients) to consider when interpreting how clinical trial results are clinically meaningful. Aggregate data from patient-reported outcome surveys can highlight relevant gaps in treatment efficacy as defined by patient-perceived levels of functionality and well-being, thus identifying potential improvements for both individual products and the health care system as a whole.6 In clinical practice, this mismatch between physician and patient perception of the patient experience may manifest itself in several ways: providers may know of efficacious therapies, but if patients are unable to comply with the regimen due to physical or mental limitations or inconvenience, are intolerant of side effects, or are noncompliant since they do not place value on the added benefit of the drug, therapy is not properly delivered or delivered at all.7 Concordance, defined as the concept of an equitable relationship between physician and patient, has come into great favor among many physicians prescribing medical therapy over the last decade.7 There is a need for more patient-reported outcomes research to better define the impact of concordance on perioperative care for cataract surgery. The dexamethasone insert, a novel modality, was designed to address numerous constraints of care following surgery, including physical or mental impairments limiting self-administration, errors in the amount of drug administered and contamination and/or damage of the dispenser or administration site by the patient.8 The aim of this survey was to understand and evaluate the patient experience and the perceived value, if any, of the sustained release dexamethasone intracanalicular insert following cataract surgery. Particular attention was given in the survey to overall satisfaction, convenience, comfort, comparison to standard of care (topical corticosteroid drops), cost, and likelihood of recommending treatment to family and friends.
Methods
This cross-sectional, single-arm, qualitative evaluation involved individual in-depth telephone interviews, each approximately 45 minutes in duration. Patients were selected from four US investigational study sites (out of 32 sites total across two Phase III trials) who had previously received a dexamethasone intracanalicular insert following cataract surgery as participants in a Phase III clinical trial (active treatment group) and who offered consent to participate in the interview. Sites were selected based on prior research experience and number of enrolled subjects and were trained by a third-party contract research organization (CRO) on the details of the protocol. Prior to initiation of any study procedures, institutional review board approval was sought and obtained (Salus Institutional Review Board, Austin, TX, USA). Written informed consent was obtained from all survey participants prior to enrollment, and the study adhered to the tenants of the Declaration of Helsinki. Once consented, patients were interviewed and audiorecorded by trained CRO staff.
Patients were deemed eligible if they were enrolled in one of two Phase III clinical trials (OTX-13-002 or OTX-14-003), received active treatment (as opposed to placebo vehicle), and were willing to participate in the telephone interview. Patients with a history of cognitive deficit were excluded from participation. The semistructured interview consisted of approximately 15 questions: three to five questions were open ended, with an opportunity to collect in-depth responses through probing, followed by eight to ten closed-ended survey questions on patient experience with the dexamethasone insert. A synopsis of the discussion guide is outlined in Table 1. During the interviews, patients were initially asked to provide demographic information (gender, age, ethnicity, prior cataract surgery location and date, prior eye drop use, and experience), then discuss their pain and symptoms following cataract surgery, their recent experience with the dexamethasone insert, and their willingness to use the product in the future. The dexamethasone insert was referred to as the “dexamethasone punctum plug” or “plug” during the interview process.
  | Table 1 Abridged discussion guide |
There were no predesignated primary or secondary end points. This was a qualitative survey seeking a deeper understanding of the patient experience with the dexamethasone insert. Qualitative and descriptive statistics were used to analyze the results, report substantive content themes, and summarize findings. The survey further served to direct future hypothesis generation for subsequent patient-based research.
Results
Twenty-five patients were enrolled and interviewed during March and April 2016 from four sites in the US; two patients withdrew consent prior to completing the survey (one patient, personal conflict; one patient, financial concerns). Patient demographic information is presented subsequently (Table 2). The average age of participants was 70.88 years (range: 53–86 years), and 48% (12/25) of respondents were male. At the time of the survey, cataract surgery had been performed in both eyes of 92% (23/25) of patients (note: only one eye was treated as part of the Phase III protocol). Previous experience with eye drops following cataract surgery was reported by all 23 of these patients. In 70% (16/23) of patients, this prior experience had occurred within the last 24 months.
  | Table 2 Participant demographics |
Global experience with insert
Patients were evaluated on their overall experience with regard to satisfaction, comfort, and convenience of the insert.
A majority of patients (23/25, 92%) reported the highest level of satisfaction (“very satisfied”), with regard to overall satisfaction with the dexamethasone insert. Patients cited convenience, ease of use compared to topical eye drop therapy, comfort, absence of pain, (perceived) improved healing time, fulfillment of expectations, and improvement in compliance as reasons supportive of their responses. Two patients rated their experiences with the dexamethasone insert as unsatisfactory (1) or neutral (1). In one case, the patient attributed this dissatisfaction to adverse events during treatment: increased healing time when compared to the contralateral eye and epiphora following surgery which required the use of warm compresses. In the other case, the patient felt the clinical trial study requirement of frequent in-office follow-up was inconvenient.
All patients (25/25) described the insert as comfortable, with seven patients (28%) rating their experience as “extremely comfortable”, 17 patients (68%) rating their experience as “very comfortable”, and one patient (4%) rating their experience as “somewhat comfortable”. Most patients were not aware of the insert (21/25, 84%), and two patients reported awareness to some degree (“somewhat aware”) or were unsure. When the issue of “awareness” was explored further, most patients said that they could not see or feel the insert (19/25, 76%); two patients reported awareness in the initial stages of the clinical study (days 1–3, “slight tenderness”, “sensation, but very small”); and two patients reported a more pronounced, temporary awareness of something in their periphery.
All patients (25/25) reported that the level of convenience of the insert compared to using eye drops after surgery was “somewhat”, “extremely”, or “very convenient”. One patient felt that the dexamethasone insert was only somewhat convenient because her experience did not differ from a prior experience requiring eye drops after surgery and reported that no additional convenience was provided by the dexamethasone insert. In this case, she cited the continued requirement of topical antibiotic eye drops as the reason, and it should be noted that all patients per protocol were required to administer topical antibiotic after surgery.
Relief of symptoms
With regard to symptom relief, results were similar to overall treatment satisfaction, with 22 patients very satisfied with the efficacy of the dexamethasone insert’s symptom alleviation (Figure 1). Twenty-three patients responded that they felt the insert was working throughout therapy (92%), one patient responded it worked some of the time (4%), and one patient responded he/she felt the insert did not work (4%).
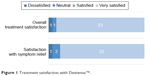  | Figure 1 Treatment satisfaction with Dextenza™. |
Convenience of insert
Most participants (24/25, 96%) felt that, when compared to using eye drops at home on a tapering schedule for 4 weeks or more, the insert was “very” or “extremely convenient”. Additionally, respondents were queried about the convenience of the dexamethasone insert versus their prior drop experience; this question was only administered to the 23 patients who had reported prior experience with perioperative topical therapy. Twenty-two participants (96%) rated their experience with the dexamethasone insert as very or extremely convenient, as compared to previous topical therapy (Figure 2).
  | Figure 2 Patient experience with Dextenza™. |
Symptoms experienced after cataract surgery
After undergoing cataract surgery and receiving the dexamethasone insert, some (11/25, 44%) patients reported experiencing eye pain, eye inflammation, vision problems, eye discomfort, dry eyes, and watery eyes (Table 3). Fourteen of 25 (56%) respondents reported no symptoms with regard to postcataract surgery follow-up.
  | Table 3 Symptoms reported after cataract surgery |
Preference for product and recommendation to others
Patients were surveyed further regarding the theoretical likelihood of requesting and/or recommending the insert to others in the future for the treatment of inflammation and cataract symptoms following surgery (Figure 2). Eighty-eight percent (22/25) of patients stated that if they were to undergo cataract surgery again, they would request the dexamethasone insert. When asked if they would recommend the insert to family members or friends, 92% (23/25) would recommend, with convenience cited as the key reason for this recommendation. Additional sources for these responses included personal preference, removal of the task of remembering to administer eye drops, ease of use, lack of interference on daily life activities, and less discomfort overall.
Willingness to pay
Patients were surveyed on measures surrounding out-of-pocket costs for the insert as well. An equal number of respondents (10/25, 40%) felt that eye drops should cost the same amount or less than the insert, while five respondents (20%) expected eye drops to cost less than the dexamethasone insert. Eighty-four percent (21/25) of survey participants would be willing to pay more for the insert (Figure 3). Principal reasons for the willingness to pay more for the insert included convenience, less discomfort throughout the perioperative period, less energy spent determining which drops to administer at what frequency (overall ease of use), and satisfaction with treatment (including efficacy and painlessness). Two patients (2/25, 8%) voiced concerns over the uncertainty of their insurance coverage and their fixed-income status, which might preclude them from paying substantial out-of-pocket costs.
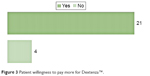  | Figure 3 Patient willingness to pay more for Dextenza™. |
Discussion
In this survey, we evaluated the patient experience and the perceived value of the sustained release The dexamethasone insert intracanalicular insert following cataract surgery as part of a Phase III clinical trial program. The dexamethasone insert is a novel extended release corticosteroid insert for ophthalmic use and provides a self-tapering, consistent release of drug. A one-time administration of the dexamethasone insert replaces the complex topical corticosteroid dosing regimen that affects consistent patient outcomes. With regard to overall satisfaction, convenience, and comfort, most subjects rated the dexamethasone insert experience as extremely favorable or very highly favorable in these categories, and a large proportion of the cohort expressed their willingness to pay more for these attributes.
Survey participants, when given the opportunity to voice final feedback, said that they viewed the insert technology as ideal for themselves and their peers, primarily due to convenience and the removal of the treatment burden of additional topical eye drops (both the task of administration and remembering to administer) while achieving equivalent and/or better outcomes postsurgery. Participants who had prior experience with drops explicitly preferred a regimen that included the dexamethasone insert, lessening the overall number of topical drops required for perioperative care.
Few publications have examined patient-reported outcomes of satisfaction with cataract surgery, but many clinicians are of the opinion that patient satisfaction is associated with patient expectations of minimal discomfort and visual function improvement.9 This assessment is the first, to the authors’ knowledge, that assesses patient-reported outcomes after cataract surgery using a novel, sustained release treatment modality to deliver topical steroid therapy. Overall treatment satisfaction, symptom relief, and overall comfort with the dexamethasone insert were apparent in this population. Patients reported very few symptoms or problems retrospectively and were highly likely to recommend the insert to others or request its use for future surgical procedures.
Beyond the convenience conveyed by reduced eye drop treatment burden, the greater satisfaction experienced by patients may be attributed to several key attributes of the dexamethasone insert. Removing the topical corticosteroid therapy liberates energy expended on properly executing a 4-week taper, which is arguably more tedious than a once- or twice-a-day topical antibiotic or nonsteroidal anti-inflammatory drop. Immediately after placement, the insert hydrates and expands to occlude the canaliculus and reduce the rate of tear film clearance from the ocular surface, which may support the health of the ocular surface in an elderly patient population where decreased tear production is common. A reduced tear volume often results in an exacerbation of ocular surface disease (dry eye and blepharitis) and the attendant symptoms of discomfort after cataract surgery, such as foreign body sensation, dryness, soreness, and fluctuating vision. The absence of preservatives in the product may also act to improve comfort, especially in patients who compared their use of the dexamethasone insert with prior drop experience, as the majority of topical corticosteroid formulations are not preservative free. Preservatives in topical ophthalmic therapies have toxic effects and are known to cause superficial punctate keratitis and exacerbate the signs and symptoms of other ocular surface disorders. The steady levels of steroid medication in the tear film provided by the dexamethasone insert, as compared to short bursts of medication delivered via topical drop therapy, may also further explain the high patient-reported comfort scores. In the case of standard of care, initiation of corticosteroid drops after surgery may be delayed until a prescription is filled or due to recovery from anesthesia, while the dexamethasone insert is inserted at the completion of cataract surgery; this difference in time to initiation of therapy may be noteworthy as well, as pain relief in the dexamethasone insert clinical trials was found to be both rapid and pronounced.5 In aggregate, these seemingly minor differences in product profile may amount to major differences in outcomes from the patient perspective.
This survey is limited by a small sample size, which may limit the extrapolation of its findings to a broader population. A majority of our self-reported findings rely on retrospective recall in an elderly population, and there may have been selection bias in inclusion of participants. Participation was voluntary, and only four sites were selected to participate in the wider multicenter Phase III trial investigator base. However, more than one patient was unable to differentiate the requirements of trial participation from the actual experience with the dexamethasone insert into their responses, and it may have factored into our final findings, such as decreased convenience due to additional clinic visits (per the study protocol). This survey was performed as an adjunct to a rigorously conducted pivotal Phase III clinical trial and aimed to gather data on the patient experience surrounding the use of this product; it was not designed to support any other labeling claims about the dexamethasone insert.
Conclusion
While no definitive conclusions about patient preferences regarding the dexamethasone insert from the findings of this small survey can be derived, results suggest that the patient experience of the cataract patient using new therapeutic modalities may be worthy of further investigation. Improvements in the area of patient satisfaction should not be trivialized. More rigorous, controlled, appropriately powered research on the patient experience with sustained release intracanalicular inserts will be helpful to develop products that further medical progress while improving the patient experience.
Acknowledgments
This research was sponsored by Ocular Therapeutix, Inc., Bedford, MA, USA. This research has not been published elsewhere previously, and it is not being considered for publication simultaneously for any other publication.
Disclosure
JLM and JHT are employees of Ocular Therapeutix, Inc. The other authors received a grant to complete this research, but report no other proprietary interests. The authors report no other conflicts of interest in this work.
References
Congdon N, Vingerling JR, Klein BE, et al. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122(4):487–494. | ||
Gollogly HE, Hodge DO, St Sauver JL, Erie JC. Increasing incidence of cataract surgery: population-based study. J Cataract Refract Surg. 2013;39(9):1383–1389. | ||
Schein OD, Cassard SD, Tielsch JM, Gower EW. Cataract surgery among Medicare beneficiaries. Ophthalmic Epidemiol. 2012;19(5):257–264. | ||
Lindstrom R. Thoughts on cataract surgery: 2015. Rev Ophthalmol. 2015. | ||
Walters TR, Bafna S, Vold S, et al. Efficacy and safety of sustained release dexamethasone for the treatment of ocular pain and inflammation after cataract surgery: results from two phase 3 studies. J Clin Exp Ophthalmol. 2016;7(4). | ||
Mollazadegan K, Lundstrom M. A study of the correlations between patient-reported outcomes and clinical outcomes after cataract surgery in ophthalmic clinics. Acta Ophthalmol. 2015;93(3):293–298. | ||
Fraser S. Concordance, compliance, preference or adherence. Patient Prefer Adherence. 2010;4:95–96. | ||
An JA, Kasner O, Samek DA, Levesque V. Evaluation of eyedrop administration by inexperienced patients after cataract surgery. J Cataract Refract Surg. 2014;40(11):1857–1861. | ||
Garcia-Gutierrez S, Quintana JM, Aguire U, et al. Impact of clinical and patient-reported outcomes on patient satisfaction with cataract extraction. Health Expect. 2014;17(6):765–775. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
