Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 8
Evaluating the impact of type 2 diabetes mellitus on cardiovascular risk in persons with metabolic syndrome using the UKPDS risk engine
Authors Ogedengbe OS, Ezeani I , Chukwuonye I , Anyabolu EN , Ozor I, Eregie A
Received 11 June 2014
Accepted for publication 9 January 2015
Published 15 September 2015 Volume 2015:8 Pages 437—445
DOI https://doi.org/10.2147/DMSO.S69199
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
O Stephen Ogedengbe,1 Ignatius U Ezeani,2 Ijezie I Chukwuonye,3 Ndukaife Anyabolu,4,5 Ikemefuna I Ozor,6 Aihanuwa Eregie1
1Department of Internal Medicine, University of Benin Teaching Hospital, Benin City, 2Division of Endocrinology, Diabetes and Metabolism, Department of Internal Medicine, Federal Medical Center, Umuahia, 3Division of Nephrology, Department of Internal Medicine, Federal Medical Center, Umuahia, 4Division of Nephrology, Department of Internal Medicine, Imo State University Teaching Hospital, Orlu, 5Division of Nephrology, Department of Internal Medicine, Federal Medical Centre, Owerri, 6Division of Neurosurgery, Department of Surgery, University of Nigeria Teaching Hospital, Enugu, Nigeria
Background: The aim of this study is to evaluate the impact of coexistence of metabolic syndrome (MS) and type 2 diabetes mellitus (T2DM) on the estimated cardiovascular risk as calculated using the United Kingdom Prospective Diabetic Study risk engine (UKPDS-RE) and also to determine the impact of the coexistence of MS and T2DM on the 10-year risk of developing coronary heart disease and stroke.
Methodology: This is a cross-sectional study in which convenience sampling technique was used to recruit 124 consecutive persons with T2DM and 96 controls using a questionnaire administered technique. The World Health Organization (WHO) criterion was used to define MS and the UKPDS-RE was used to identify persons with increased risk for stroke and those with increased risk for coronary heart disease. The data obtained were analyzed using SPSS version 16. Statistical comparisons were made with chi-square for comparison of proportions. A P-value of less than 0.05 was taken as statistically significant.
Results: Fifteen subjects were identified as having an increased 10-year risk for stroke and ten as having an increased risk for a coronary event. The odds of a T2DM subject with MS having an increased risk for stroke compared with a T2DM subject without MS was 0.9579≈1 while the odds of a T2DM subject with MS developing an increased risk for a coronary event compared with a T2DM subject without MS was =3.451≈3.
Conclusion: MS was more common in subjects with T2DM compared with controls (irrespective of the diagnostic criteria used) and MS appears to increase the risk of a coronary event in subjects with T2DM by threefold. Also from this study, MS did not appear to cause an additional increase in the risk of stroke in subjects with T2DM.
Keywords: diabetes mellitus, metabolic syndrome, coronary heart disease, cardiovascular disease, UKPDS risk engine
Introduction
Metabolic syndrome (MS) is a cluster of cardiovascular risk factors such as diabetes mellitus (DM), pre-diabetes, central obesity, dyslipidemia, and high blood pressure (BP).1–3 It is estimated that approximately one quarter of the world’s population have the syndrome and people with this condition are likely to have a heart attack or stroke compared with people without it.2 Even more alarming is the fact that the risk of developing DM is fivefold greater in people with this syndrome.1
In 1976, Wellborn and Wearne4 established the link between type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD). Pyorala5 showed strong links between glucose intolerance, hyperinsulinemia, and coronary heart disease (CHD) and this link was extended in the 1980s by Modan et al6 to include obesity, hypertension, and CVD.
Although the pathogenesis is not fully understood, it has been postulated that genetically determined insulin resistance in a setting of suitable environmental factors is the pivotal pathogenic mechanism underlying MS.7 Lipoprotein lipase deficiency largely accounts for the lipid abnormalities seen in MS,8 while hypertension is due to enhanced sympathetic activities, salt sensitivity, and increased transmembrane cation transport.9,10 The role of TNF in obesity and insulin resistance has also been described.11
Developing nations are witnessing rapid industrialization, urbanization, and increased economic prosperity and the resulting acquisition of the western lifestyle characterized by calorie excess and physical inactivity would provide a suitable milieu for the development of MS in genetically predisposed individuals. The prevalence of MS is known to vary across the world: age and ethnicity of the populations being studied are also key issues influencing the prevalence.12
CVD is unanimously recognized as the major burden in T2DM in terms of mortality and morbidity.13,14 Unfortunately, myocardial infarction, stroke, and non-ischemic CVD are the causes of death in up to 80% of patients with T2DM.15 It is worthy to note that the constellation of metabolic derangements often seen in people with insulin resistance and T2DM is individually associated with an increased risk of a CVD.16
The relationship between pre-diabetes and a family history of DM and cardiovascular risk has been increasingly emphasized. Several hypotheses have been generated in order to explain such relationship between family history of diabetes and cardiovascular risk profile of individuals.17–24 Family history of diabetes increases per se the risk of CHD even in non-diabetic subjects.21 This may be due to an increased prevalence of abdominal fat content in such subjects,22 elevated systolic BP (SBP), higher triglyceride and cholesterol plasma concentration,23 and higher PAI-1 activity.24 All these conditions could increase the cardiovascular risk profile of individuals and lead to them being considered as an “at risk population” even considering their apparently “healthy” general clinical condition. Family history of DM is not only related to a pure increase in metabolic alterations predisposing to the onset of overt diabetes but it seems to overcome the endocrinology alterations and to provoke early lesions even in the vascular walls. Thus, family history of diabetes increases cardiovascular risk profile of individuals. Although this is not supported by strong scientific evidences, many observational studies revealed its relationship with increased cardiovascular risk. Although literature is poor about human protocols demonstrating the effects of pre-diabetes and diabetes on direct evidence of cardiac myocyte hypertrophy, some evidences can be outlined.25–27
Among Turkish people, a Coronary Heart Disease Study revealed that two out of every three cases originate from MS28 and according to the World Health Organization (WHO); “CHD is developing in developing countries” it is a plague to avoid.29 CHD was thought to be uncommon in Nigeria but the prevalence appears to be rising in keeping with urbanization, westernized diet, and increasing levels of physical inactivity. Though the current prevalence is not known, it seems reasonable to expect an increase in its frequency as the lifespan of the population increases, particularly with the increasing prevalence of T2DM.30 In Eastern Nigeria, Dagogo-Jack and Odia31 reported six cases of myocardial infarction in Port-Harcourt in 1990 and four out of the six cases had T2DM. Out of three cases of myocardial infarction reported by Danbauchi and Onyemelukwe in Zaria, Northern Nigeria, two of them had T2DM.32 Thus it can be said that the cardiovascular complications of DM (which are also a leading cause of blindness, amputation, and renal failure) account for much of the social and financial burden of the disease.1
With MS driving the twin global epidemics of T2DM and CVD, there is an overwhelming moral, medical, and economic imperative to identify people with this syndrome early so they can benefit from lifestyle interventions and treatment that may alter the course of the disease.
Diagnosing MS will help in identifying individuals at increased risk of CVDs: a similar risk is known to exist in individuals with T2DM. The question this study will also attempt to answer is “to what extent is CVD risk altered in T2DM persons with MS?” The United Kingdom Prospective Diabetic Study risk engine (UKPDS-RE) is a computer based estimation of cardiovascular risk in patients with T2DM: it will be used to estimate the cardiovascular risk in T2DM. The effect of MS on the estimated cardiovascular risk in these subjects will also be analyzed. This is the first study in Benin City that has assessed the impact of T2DM on cardiovascular risk in subjects with MS using the UKPDS-RE. To the best of our knowledge, there has not been any previous study on this group of subjects.
Aims and objectives
This study sets out to evaluate the impact of the coexistence of MS and T2DM on the estimated cardiovascular risk as calculated using the UKPDS-RE and also to determine the impact of the coexistence of MS and T2DM on the 10-year risk of developing CHD and stroke.
Methodology
Ethical considerations
Ethical approval was obtained from the Ethics and Research Committee of the University of Benin Teaching Hospital (UBTH) before the commencement of the study and consent was obtained from study subjects.
This was a cross-sectional study carried out at the Diabetes Clinic of the UBTH, a 500-bed Federal Government tertiary hospital in Benin City, Edo State in the South-south geopolitical region of Nigeria. The UBTH receives referral cases from Edo State and neighboring states like Delta, Ondo, Ekiti, and Kogi States and the Federal Capital Territory, Abuja. A total of 124 subjects were recruited from the Diabetes Clinic of the UBTH and the inclusion criteria includes: T2DM patients presenting to UBTH within the last 24 months using the 1999 WHO criteria,29 people aged 30 years and above, on treatment with oral hypoglycemic drugs plus or minus non-pharmacological therapy and not requiring insulin for survival, and finally subjects who consented to participate in the study. The exclusion criteria included subjects diagnosed with other types of DM, with T2DM and age <30 years, and those who declined.
Control subjects
Ninety-six control subjects were recruited from among the staff of UBTH and healthy relatives of non-diabetic patients. The inclusion criteria includes: non-diabetic age- and sex-matched adult with normal fasting blood sugar less than 110 mg/dL while the exclusion criteria includes: non-diabetics less than 30 years of age, first degree relative of type 2 diabetic, non-diabetics who declined being a part of the study.
A convenience sampling technique was used to recruit 124 subjects with T2DM and 96 controls using a questionnaire administered technique. The following were assessed: anthropometric indices, BP, serum lipid profile, fasting blood sugar, proteinuria, and microalbuminuria.30,31 The WHO criterion was used in this study to define MS (Table 1). In order to determine the sensitivity and specificity of these diagnostic criteria in persons living with T2DM, the UKPDS-RE was used to identify persons with increased risk for stroke and those with increased risk for CHD. Individuals with stroke risk <15% (in the green zone) were considered normal (Figure 1). Those with stroke risk ≥15% (outside the green zone) were considered as having an increased risk for stroke. Similarly, persons with risk of <15% for CHD were considered normal while those with CHD risk ≥15% were considered as having an increased risk for a coronary event. The data obtained were analyzed using the SPSS version 16. Statistical comparisons of proportions were made with chi-square. A P-value of less than 0.05 was taken as statistically significant.
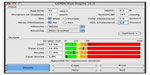  | Figure 1 UKPDS risk engine. |
Anthropometric measurements of weight, height, waist circumference (WC), and hip circumference (HC) were measured for each subject as follows: the weight was measured with subjects in light clothing, without shoes using a weighing scale and recorded in kilograms measured to the nearest 0.1 kg. The height was measured, without shoes and the subject standing upright and looking straight ahead (along the coronal plane) using a stadiometer and was recorded to the nearest 0.1 m. The body mass index (BMI) was calculated by the formula: BMI = weight (kg)/Height2 (m). The WC was taken at the point midway between the inferior margin of the rib cage and the iliac crest to the nearest 0.1 cm using measuring tape. The HC was measured at the level of the maximal gluteal circumference (along the greater trochanter) to the nearest 0.1 cm with subjects standing erect, hands at the sides, and feet together. The waist-hip ratio (WHR) was thereafter determined as the WC divided by the HC. The BP was measured to the nearest 2 mmHg using a standard mercury sphygmomanometer with subjects in the sitting position and the arms resting on the arms of a chair and the sphygmomanometer at the level of the heart using the first and fourth Korotkoff sounds for the SBP and diastolic BP (DBP) respectively.
Laboratory investigations
All subjects were instructed to observe an overnight fast for 8–10 hours before the day of sample collection. Approximately 20 mL of blood was collected from the ante-cubital vein using sterile disposable needles and syringes, for the following investigations:
- Fasting blood sugar: 2 mL of blood was collected in fluoride oxalate bottles and then analyzed for plasma glucose within 1 hour by the glucose oxidase method.
- Serum lipid profile: blood was collected in plain bottles, allowed to clot and the serum separated and stored at −20°C until analyzed. The assay was done by enzymatic method using Randox Kit.
- Approximately 1 mL of blood was collected for glycated hemoglobin assay.
- Urinalysis testing for proteinuria using Combi 10 test strips and microalbuminuria using Micral test kit.
How the UKPDS-RE works
The UKPDS-RE is a type 2 diabetes specific risk calculator based on 53,000 patient years of data from the UK Prospective Diabetes Study, which also provides an approximate “margin of error” for each estimate. First release of the UKPDS-RE program and Excel spreadsheet was on March 26, 2002. It provides risk estimates and 95% confidence intervals, in individuals with type 2 diabetes not known to have heart disease, for: non-fatal and fatal CHD, fatal CHD, non-fatal and fatal stroke, fatal stroke. These can be calculated for any given duration of type 2 diabetes based on current age, sex, ethnicity, smoking status, presence or absence of atrial fibrillation, and levels of HbA1c, SBP, total cholesterol, and high-density lipoprotein (HDL) cholesterol.
Results
This study was conducted between February 2011 and July 2011. Two hundred and twenty-three subjects were enrolled: 125 subjects living with T2DM, 98 non-diabetic control subjects (without a history of DM in a first degree relative). Out of the 125 subject living with T2DM, 124 met the requirements for inclusion in the analysis stage. The only subject excluded had an incomplete result and an incomplete questionnaire. Out of the 98 non-diabetic control subjects, two were dropped for the same reason. One hundred and twenty-four subjects with T2DM and 96 controls who met all the requirements were included. Therefore, a total number of 220 subjects participated in this study.
Of the 96 controls, 38 (39.6%) were males while 58 were females (60.4%) and their mean age was 58.6±11.2 years while the age range was 31–83 years. The majority (over 60%) of the control subjects were within the age range of 50–69 years (Table 2). The mean age (standard deviation) of the subjects with T2DM was 58.6±11.2 years. No significant age and sex difference was observed in these groups and this is suggestive of a study with subjects well matched for age and sex. For subjects with T2DM, males constituted 40.3% of the population while the females accounted for 59.7%. The statistical analysis comparing the age group distribution yielded no significant difference (χ2=2.107, degrees of freedom [df] =8, and P=0.97). Similarly, no significant sex difference was observed ((χ2=0.017, df =8, and P=0.99).
Prevalence of MS
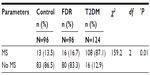
Table 3 shows the prevalence of MS using the WHO diagnostic criteria. Using the WHO as the gold standard criteria for diagnosis, the prevalence of MS in the control and T2DM groups were 13.5% and 87.1% respectively.
A group by group comparison of proportions of subjects with MS showed a statistically significant higher proportion of persons with MS in the T2DM group than in the control (χ2=1.183, df =1, and P=0.000).
A statistically significant difference in the means of WC (98.10±11.94 cm and 84.81±12.43 cm), BMI (27.29±5.04 kg/m2 and 23.10±2.26 kg/m2), SBP and DBP (137.85±17.10 mmHg/126.56±9.42 mmHg and 86.60±9.92 mmHg/78.01±6.24 mmHg) respectively of T2DM subjects with MS and those without MS was observed (P=0.01, 0.01, 0.01, 0.01 respectively). These means were higher in T2DM subjects with MS. There was no significant difference observed in the mean WHR of T2DM subjects with or without MS even though the mean WHR was higher in subjects with MS. There was a statistically significant difference in the mean of the triglycerides, SBP, DBP, FBS, and HbA1c in T2DM subjects with MS compared to T2DM subjects without MS (P=0.02, 0.01, 0.01, 0.01, 0.01 respectively). However, no significant difference in total cholesterol levels, HDL, and low-density lipoprotein (LDL) levels was observed (Table 4).
Of the 124 subjects with T2DM, 15 (12.10%) were identified by the UKPDS-RE version 2.0 as having an increased risk for stroke while ten (8.06%) were identified as having an increased risk for a coronary event. Seven subjects (5.65%) had increased risk for both conditions. The ability of the WHO criteria to identify these individuals with T2DM who have significant cardiovascular risk was assessed and odds of developing a stroke and that of developing a coronary event was calculated to determine the possible impact of MS in the lives of T2DM subjects with MS. This implies that the odds of a T2DM subject with MS developing a stroke compared with that of a T2DM subject without MS developing a stroke are equal (0.9579≈1). Table 5 shows the odds associated with developing an increased risk for stroke while having MS.
This implies that the risk of a coronary event occurring in a T2DM subject with MS is not the same as that of a T2DM subject without MS. Persons with T2DM and MS have a threefold risk of having a coronary event when compared with persons with T2DM without MS. Table 5 also shows the odds associated with developing an increased risk for coronary as a result of having MS.
Discussion
The mean age of T2DM subjects in this study was (58.6±11.2 years) and there were more females (59.7%) than males (40.3%) with T2DM.
A comparative analysis of clinical and biochemical variables between T2DM patients with and without MS revealed that the mean BMI, SBP, DBP, FBS, HbA1c, total cholesterol, LDL cholesterol, and triglycerides were higher in T2DM subjects with MS; the mean BMI, SBP, DBP, FBS, HbA1c, and triglycerides were significant. On the other hand, the mean HDL cholesterol was higher in T2DM subjects without MS though this was not significant. This is despite the fact that the use of medications for both hypertension and hyperlipidemia was much more common in T2DM patients with MS. This finding was similar to that reported in Japan by Hirohito et al33 who observed that BP and serum triglycerides were significantly higher and HDL cholesterol was significantly lower in MS patients. Similarly, Carole et al34 reported that patients with MS were more likely to have higher HbA1c, total cholesterol, and LDL cholesterol.
Diabetic patients are known to be at greater risk for CVD than non-diabetic subjects,35 and it has been suggested that MS is responsible for the increased prevalence of CHD seen in diabetic patients.36 To the best of our knowledge, there have been few cohort studies specifically targeting diabetic patients to determine the relative risk of MS on the incidence of CVD36–38 and mortality due to CVD.39
The UKPDS-RE is a risk model used only for diabetes patients. Van Der Heijden et al40 reported that the UKPDS risk function was more accurate in predicting CHD in a Hoorn study cohort of newly diagnosed T2DM patients than were the Framingham and SCORE methods. The UKPDS-RE version 2.0 was used to identify T2DM persons at increased risk for stroke and coronary events. Fifteen subjects were identified in our study as having an increased 10-year risk for stroke and ten subjects had an increased risk for a coronary event. Using the WHO criteria to screen for persons with MS who are likely to present with these events, the odds ratio for the events was calculated. The odds of a T2DM person with MS having an increased risk for stroke compared with that of a T2DM person without MS was 0.9579≈1. This implies that a T2DM person with MS and a T2DM person without MS have equal risk of developing an increased risk for stroke. Thus, MS from this study does not appear to cause an additional increase in the risk of stroke in persons with T2DM, however DM is clearly one of the most important risk factors for stroke.41 Commonly, the relative risk of stroke in diabetic patients is higher than non-diabetic, but the rate of relative risk is wide and varies in different studies.42 In black diabetic Americans, the peak rate of risk for stroke (eight to ten times higher) is at the age of 34–45 years, but in the same age range, whites have a 2.6–5.3 relative risk for stroke.42 The highest risk for stroke for white diabetic Americans is at the age of 45–64 years. Approximately 37%–42% of all incidences of stroke are associated with diabetes alone or in combination with hypertension43 and one-third of all acute stroke patients may have diabetes. For patients presenting with post-stroke hyperglycemia, impaired glucose tolerance or diabetes is present in two-thirds of survivors.44 Japanese with diabetes have two to five times the risk for stroke compared to non-diabetics.45 The risk of stroke in Japanese men in Hawaii increased with age for diabetics and non-diabetics. The risk was substantially higher among diabetic compared with non-diabetic individuals at almost all ages.45,46 Women with diabetes and no history of CVD have a threefold increased fatal stroke risk compared with non-diabetic women without CVD.47
The odds of a T2DM subject with MS developing an increased risk for a coronary event compared with that of a T2DM subject without MS was 3.451≈3. This implies that the risk of having an increased risk for a coronary event in a T2DM subject with MS is not equal to the risk of a T2DM subject developing a coronary event and it appears that T2DM subjects with MS are more likely to suffer a coronary event than T2DM subjects without MS. It has been shown that MS can increase (by three to four times) the risk of death due to ischemic heart disease by the Kuopio Ischaemic Disease Risk Factor study in Finland.48 Qiao et al48 reported in Finnish and Swedish cohorts that MS and its components predicted the incidence of stroke and CHD equally well. Bruno et al35 in the Casale Monferrato Study, reported that diabetic subjects with even only one component of MS have more than twofold higher risk of cardiovascular mortality than subjects with diabetes only, independent of age, sex, smoking, total cholesterol level, CHD, and cumulative HbA1c. Similarly Guzder et al reported that MS at baseline is associated with an increased risk of CVD incident in the 5 years following diagnosis of T2DM.49 The CVD-free survival rates declined incrementally as the presence of MS features increased. Though there are few studies that have compared cardiovascular mortality amongst T2DM patients with and without MS, there are several studies that have looked at the cardiovascular outcomes in patients with T2DM. In a study by Seon et al50 the 10-year CHD risk and 10-year stroke risk were 14.92% and 4.03%, respectively, calculated using the UKPDS-RE. The 10-year CHD risk was relatively high, and the risk of CHD tended to increase after onset of T2DM: although they did not study patients with MS like in our study, it is worthy to note that patient outcome was similar to what we reported. The UKPDS-RE has recently become controversial in its reliability to predict CHD or stroke. Bannister et al51 noted that the relatively poor performance of the UKPDS-RE may be explained, at least in part by the differences in the baseline profiles of the UKPDS and Clinical Practice Research Datalink populations. These plausible explanations include the epidemiological setting, changes in life expectancy, changes in smoking habits, the presence or absence of comorbidities, temporal changes in diabetes management, and changes in the general quality of care. Other plausible explanations include the possible harm of overly aggressive treatment with sulfonylureas and insulin in the early stages of the disease.
In the external validation of the UKPDS-RE in patients with T2DM study by Van Dieren et al;52 they observed that their mean follow-up time was 8 years; therefore, they could not validate 10-year CVD and CHD risks. However, the UKPDS-RE is in principle, designed for all risk periods including periods shorter than 10 years. Secondly, their population consisted of all diabetes cases, not just individuals newly diagnosed with diabetes. Therefore, they could only validate the use of the UKPDS-RE for patients who have been diagnosed with diabetes for some time. Finally, they had some missing values in the baseline factors. There are several explanations for the poor to moderate performance of the UKPDS-RE to predict CHD and CVD risk in this population. First, the UKPDS-RE was developed from a cohort that started including patients in 1977. Treatment of T2DM and prevention of CVD has improved since 1977 and the risk of developing CVD has declined with better treatment of T2DM. Also, as diabetes is now detected at an earlier stage, therapeutic intervention can be initiated earlier, reducing CVD risk even further. Altogether, this may likely explain the large differences in predicted and observed absolute risks that have led to poor calibration. A major strength of this study is that it is the first study in Benin City that assessed the impact of T2DM on cardiovascular risk in subjects with MS using the UKPDS-RE. To the best of our knowledge, there has not been any previous study on this group of subjects. This was a small study with notable limitations which include: 1) the original UKPDS model tended to overestimate event rates across studies, therefore a modified UKPDS model which includes adjustments for prior cardiovascular history has the potential for use as a tool for benchmarking and may be useful for predicting cardiovascular rates in clinical studies. This modification could be further evaluated, recalibrated, and validated using patient-level information derived from prospective clinical studies to yield greater predictability. 2) We did not consider medication use in the diagnosis of MS in this study. 3) Mortality was not analyzed because we did not have sufficient occurrences at this stage of the study. A larger and specifically designed study is needed to evaluate the effects or impact of the MS on the cardiovascular status of Nigerians with T2DM, as cardiovascular events are known to be a major cause of mortality in this group of persons worldwide.
Conclusion
In conclusion, the increased risk of CVD at the onset of type 2 diabetes observed in this study is consistent with previous studies. This study has demonstrated that the MS appears to increase the risk of a coronary event in a person with T2DM by threefold.
Acknowledgments
We thank Dr Aihanuwa Eregie and Dr Andrew Edo who contributed toward the article by making substantial contributions to conception and revision of manuscript for important intellectual content.
Disclosure
The authors have no conflicts of interest to disclose.
References
Alberti KG, Zimmet P, Shaw J; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome: a new worldwide definition. Lancet. 2005;366(9491):1059–1062. | |
International Diabetes Federation. The Idf Consensus Worldwide Definition of the Metabolic Syndrome. International Diabetes Federation; 2006. Available from: http://www.idf.org/webdata/docs/idf_meta_def_final.pdf. Accessed July 19, 2015. | |
Alberti KG, Zimmet P, Shaw J. Metabolic syndrome: a new world wide definition. A Consensus Statement from the International Diabetes Federation. Diabetes Med. 2006;23(5):460–69. | |
Wellborn TA, Wearne K. Coronary heart disease incidence and cardiovascular mortality in Busselton with reference to glucose and insulin concentrations. Diabetes Care. 1976;2(2):154–160. | |
Pyorala K. Relationship of glucose tolerance and plasma insulin to the incidence of coronary heart disease: results from two population studies in Finland. Diabetes Care. 1979;2(2):131–141. | |
Modan M, Halkin H, Karasik A, Lusky A. Elevated serum uric acid: a facet of hyperinsulinaemia. Diabetologia. 1987;30(9):713–718. | |
Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–1607. | |
Verges BL. Dyslipidemia in diabetes mellitus. Review of the main lipoprotein abnormalities and their consequences on the development of atherogenesis. Diabetes Metab. 1999;25 Suppl 3:32–40. | |
Rosolova H. [The sympathetic nervous system and insulin resistance]. Vintr Kek. 2003;49(1):61–65. Czech. | |
Ginner V, Coca A, de la Sierra A. Increased insulin resistance in salt-sensitive hypertension. J Hum Hypertens. 2001;15(7):481–485. | |
Hotamisligil GS. The role of TNF-alpha and TNF receptors in obesity and insulin resistance. J Intern Med. 1999;245(6):621–625. | |
Robert H. Eckel. The metabolic syndrome. In: Fauci AS, Braunwald E, Kasper DL, Hauser SI, Longo DL, Jameson JL, Loscaizo J, editors. Harrison’s Principles of Internal Medicine. 17th ed. The McGraw-Hill; 2008:1509–1513. | |
Diabetes Atlas. 2nd edition. International Diabetes Federation; 2003. | |
Turner R, Cull C, Holman R. United Kingdom Prospective Diabetes Study 17: a 9-year update of a randomized, controlled trial on the effect of improved metabolic control on complications in non-insulin-dependent diabetes mellitus. Ann Intern Med. 1996;124(1 Pt 2):136–145. | |
Stern MP, Haffner SM. Dyslipidaemia in type II diabetes. Implications for therapeutic intervention. Diabetes Care. 1991;4(12):1144–1159. | |
Buse JB, Polonosky KS, Burant CF. Type 2 Diabetes Mellitus. In: Kronenberg, Melmed, Polonosky, editors. Williams Textbook of Endocrinology. 11th ed. Philadelphia, PA: Saunders Elsevier; 2008:1329–1372. | |
Balkau B, Shipley M, Jarrett RJ, et al. High blood glucose concentrations is a risk factor for mortality in middle-aged non diabetic men: 20-year follow-up in the Whitehall Study, the Paris Prospective Study, and the Helsinki Policemen Study. Diabetes Care. 1998;21(3):360–3677. | |
Balletshofer BM, Rittig K, Enderle MD, et al. Endothelial dysfunction is detectable in young normotensive first-degree relatives of subjects with type 2 diabetes in association with insulin resistance. Circulation. 2000;101(15):1780–1784. | |
Scuteri A, Tesauro M, Rizza S, et al. Endothelial function and arterial stiffness in normotensive normoglycemic first-degree relatives of diabetic patients are independent of the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2008;18(5):349–456. | |
Hopkins KD, Lehmann ED, Jones RL, Turay RC, Gosling RG. A family history of NIDDM is associated with decreased aortic distensibility in normal healthy young adult subjects. Diabetes Care. 1996;19(5):501–503. | |
Eschwege E, Richard JL, Thibult N, et al. Coronary heart disease mortality in relation with diabetes, blood glucose and plasma insulin levels: the Paris prospective study, ten years later. Horm Metab Res Suppl. 1985;15:41–46. | |
Groop L, Forsblom C, Lehtovirta M, et al. Metabolic consequences of a family history of NIDDM (the Botnia study): evidence for sex-specific parental effects. Diabetes. 1996;45(11):1585–1593. | |
Sarlund H, Pyörälä K, Penttilä I, Laakso M. Early abnormalities in coronary heart disease risk factors in relatives of subjects with non- insulin-dependent diabetes. Arterioscler Thromb. 1992;12(6):657–663. | |
Gürlek A, Bayraktar M, Kirazli S. Increased plasminogen activator inhibitor-1 activity in offspring of type 2 diabetic patients: lack of association with plasma insulin levels. Diabetes Care. 2000;23(1):88–92. | |
De Marco M, de Simone G, Roman MJ, et al. Cardiac geometry and function in diabetic or pre-diabetic adolescents and young adults: the Strong Heart Study. Diabetes Care. 2011;34(10):2300–2305. | |
Velagaleti RS, Gona P, Chuang ML, et al. Relations of insulin resistance and glycemic abnormalities to cardiovascular magnetic resonance measures of cardiac structure and function: the Framingham Heart Study. Circ Cardiovasc Imaging. 2010;3(3):257–263. | |
Lee S, Cowan PA, Wetzel GT, Velasquez-Mieyer P. Prediabetes and blood pressure effects on heart rate variability, QT-interval duration, and left ventricular hypertrophy in overweight-obese adolescents. J Pediatr Nurs. 2011;26(5):416–427. | |
Gemalmaz A, Aydin S, Basak O, et al. Prevalence of the metabolic syndrome in a rural Turkish population: comparison and concordance of two diagnostic criteria. Turk J Med Sci. 2008;38(2):1659–1165. | |
Katibi IA, Akande AA, Salami AK. Metabolic Syndrome among type 2 diabetes mellitus patients: Our experience in Ilorin, Nigeria. The Nigerian Journal of General Practice. 2003;7:1–5. | |
Muna WF. Cardiovascular disorders in Africa. World Health Stat Q. 1993;46(2):125–133. | |
Dagogo-Jack S, Odia OJ. Myocardial infarction in Nigerian Africans. Orient J Med. 1990;2:129–132. | |
Danbauchi SS, Onyemelukwe GC. Ischaemic Heart Disease in Nigerians. Report of two cases. Diabetes International. 2001;10:59–60. | |
Hirohito S, Sachiko M, Hitomi F, Yukio Y, Yoshimitsu Y, Shun I, Shigehiro K. Is the Diagnosis of Metabolic Syndrome Useful for Predicting Cardiovascular Disease in Asian Diabetic Patients? Analysis from the Japan Diabetes Complications Study. Diabetes Care. 2005. 28:1463–1471. | |
Cull CA, Jensen CC, Retnakaran R, Holman RR. Impact of the Metabolic Syndrome on Macrovascular and Microvascular Outcomes in Type 2 Diabetes Mellitus United Kingdom Prospective Diabetes Study 78. Circulation. 2007;116(19):2119–2126. | |
Bruno G, Merletti F, Biggeri A, et al. Metabolic Syndrome as a Predictor of All-Cause and Cardiovascular Mortality in Type 2 Diabetes: The Casale Monferrato Study. Diabetes Care. 2004;27(11):2689–2694. | |
Gimeno Oma JA, Lou Arnal LM, Molinero Herguedas E, Boned Julián B, Portilla Córdoba DP. [Metabolic syndrome as a cardiovascular risk factor in patients with type 2 diabetes]. Rev Esp Cardiol. 2004;57(6):507–513. Spanish. | |
Bonora E, Targher G, Formentini G, et al. Metabolic syndrome is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabet Med. 2000;21(1):52–58. | |
Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001; 24(4):683–689. | |
Bruno G, Merletti F, Biggeri A, et al. Casale Monferrato Study: Metabolic syndrome as a predictor of all-cause and cardiovascular mortality in type 2 diabetes: the Casale Monferrato Study. Diabetes Care. 2000;27(11):2689–2694. | |
Van Der Heijden AA, Ortegon MM, Niessen LW, Nijpels G, Dekker JM. Prediction of coronary heart disease risk in a general, pre-diabetic, and diabetic population during 10 years of follow-up: accuracy of the Framingham, SCORE, and UKPDS risk functions: The Hoorn Study. Diabetes Care. 2009;32(11):2094–2098. | |
Karsito, Soeatmadji DW. Diabetes and Stroke. Acta Med Indones. 2008;40(3):151–158. | |
Kisella BM, Khoury J, Kleindorfer D, et al. Epidemiology of ischemic stroke in patients with diabetes: the greater Cincinnati/Northern Kentucky Stroke Study. Diabetes Care. 2005;28(2):355–359. | |
Gray CS, Scott JF, French JM, Alberti KG, O’Connell JE. Prevalence and prediction of unrecognized diabetes mellitus and impaired glucose tolerance following acute stroke. Age Ageing. 2004;33(1):71–77. | |
Kuller LH. Stroke and diabetes. Diabetes in America. 2nd edition. Bethesda: The National Diabetes Information Clearinghouse (NDIC) of NIDDK; 1995:449–456. | |
Eguchi K, Kario K, Shimada K. Greater impact of coexistence of hypertension and diabetes on silent cerebral infarcts. Stroke. 2003;34(10):2471–2474. | |
Ho JE, Paultre F, Mosca L; Women’s Pooling Project. Is diabetes mellitus a cardiovascular disease risk equivalent for fatal stroke in women? Data from the women’s pooling project. Stroke. 2003;34(12):2812–2816. | |
Qiao Q, Pyörälä K, Tuomilehto J, Gao W, Hu G, Peltonen M. Comparison of three different definitions for the metabolic syndrome in non-diabetic Europeans. The British Journal of Diabetes and Vascular Disease. 2005;5:161–168. | |
Qiao Q, Laatikainen T, Zethelius B, et al. Comparison of different definitions of the metabolic syndrome in relation to the risk of developing stroke and coronary heart disease in Finnish and Swedish cohorts. Stroke. 2009;40(2):337–343. | |
Guzder RN, Gatling W, Mullee MA, Byrne DC. Impact of metabolic syndrome criteria on cardiovascular disease risk in people with newly diagnosed type 2 diabetes. Diabetologia. 2006;49(1):49–55. | |
Seon CS, Min KW, Lee YS, et al. Cardiovascular Risk Assessment with Vascular Function, Carotid Atherosclerosis and the UKPDS Risk Engine in Korean Patients with Newly Diagnosed Type 2 Diabetes. Diabetes Metab J. 2011;35(6):619–627. | |
Bannister CA, Poole CD, Jenkins-Jones S, et al. External Validation of the UKPDS Risk Engine in Incident Type 2 Diabetes: a need for new risk type 2 diabetes-specific risk equations. Diabetes Care. 2014;37(2):537–545. | |
Van Dieren S, Peelen LM, Nothlings U, et al. External validation of the UK Prospective Diabetes Study (UKPDS) risk engine in patients with type 2 diabetes. Diabetologia. 2011;54(2):264–270. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.