Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 14
Evaluating the Impact of CYP3A5 Genotype on Post-Transplant Healthcare Resource Utilization in Pediatric Renal and Heart Transplant Recipients Receiving Tacrolimus
Authors Pasternak AL , Marshall VD , Gersch CL, Rae JM, Englesbe M, Park JM
Received 10 October 2020
Accepted for publication 11 January 2021
Published 12 March 2021 Volume 2021:14 Pages 319—326
DOI https://doi.org/10.2147/PGPM.S285444
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Amy L Pasternak,1 Vincent D Marshall,1 Christina L Gersch,2 James M Rae,2 Michael Englesbe,3 Jeong M Park1
1Department of Clinical Pharmacy, University of Michigan College of Pharmacy, Ann Arbor, MI, 48109, USA; 2Department of Internal Medicine, Michigan Medicine, Ann Arbor, MI, 48109, USA; 3Department of Surgery, Michigan Medicine, Ann Arbor, MI, 48109, USA
Correspondence: Amy L Pasternak Email [email protected]
Purpose: CYP3A5 genotype is a significant contributor to inter-individual tacrolimus exposure and may impact the time required to achieve therapeutic concentrations and number of tacrolimus dose adjustments in transplant patients. Increased modifications to tacrolimus therapy may indicate a higher burden on healthcare resources. The purpose of this study was to evaluate whether CYP3A5 genotype was predictive of healthcare resource utilization in pediatric renal and heart transplant recipients.
Patients and Methods: Patients < 18 years of age with a renal or heart transplant between 6/1/2014– 12/31/2018 and tacrolimus-based immunosuppression were included. Secondary use samples were obtained for CYP3A5 genotyping. Clinical data was retrospectively collected from the electronic medical record. Healthcare resource utilization measures included the number of dose changes, number of tacrolimus concentrations, length of stay, number of clinical encounters, and total charges within the first year post-transplant. Rejection and donor-specific antibody (DSA) formation within the first year were also collected. The impact of CYP3A5 genotype was evaluated via univariate analysis for the first year and multivariable analysis at 30, 90, 180, 270, and 365 days post-transplant.
Results: Eighty-five subjects were included, 48 renal transplant recipients and 37 heart transplant recipients. CYP3A5 genotype was not associated with any outcomes in renal transplant, however, a CYP3A5 expresser phenotype was a predictor of more dose changes, more tacrolimus concentrations, longer length of stay, and higher total charges in heart transplant recipients. CYP3A5 genotype was not associated with rejection or DSA formation. Age and induction therapy were associated with higher total charges.
Conclusion: CYP3A5 genotype may predict healthcare resource utilization in the first year post-transplant, although this may be mitigated by differences in tacrolimus management. Future studies should evaluate the impact of genotype-guided dosing strategies for tacrolimus on healthcare utilization resources.
Keywords: pharmacogenetics, cost of care, pediatric transplant
Introduction
Tacrolimus is the calcineurin inhibitor used in greater than 90% of pediatric transplant recipients in the U.S.1,2 Due to the narrow therapeutic index and variable pharmacokinetics of the drug, tacrolimus requires extensive therapeutic drug monitoring to ensure safe and effective therapy.3 A number of clinical characteristics have been shown to influence tacrolimus concentrations including: CYP3A5 genotype, age, hematocrit, weight, and donor status (living vs deceased).4–6 CYP3A5 genotype is estimated to explain upwards of 50% of the inter-patient tacrolimus variability; however, the utilization of CYP3A5 genotype-guided personalized dosing strategies for tacrolimus has not been routinely implemented in clinical practice.
Although the associations of CYP3A5 genotype with tacrolimus exposure have been demonstrated in many retrospective or observational studies, only four trials prospectively assessed the impact of genotype-guided dosing on outcomes.7–10 These studies were primarily conducted in renal transplant recipients, two were in adults and two in pediatric populations. The adult studies were conflicting, with one showing CYP3A5 genotype-guided dosing decreased the time to achieve therapeutic trough concentrations while the other did not.9,10 The pediatric studies used CYP3A5 genotype, in addition to other patient characteristics, to personalize the final tacrolimus dose. The study that utilized a genotype and age-based dosing algorithm found a reduced time to achieving therapeutic concentrations,8 while the study using a genotype-based pharmacokinetic model did not adequately predict tacrolimus exposure and was terminated early.7 One study also identified a reduction in the number of tacrolimus dose modifications when utilizing genotype-guided dosing, although this was not confirmed in others.9 None of these studies identified an association between the personalized dosing strategy and improved short-term nor long-term clinical outcomes, such as acute rejection rates in the first 30–90 days post-transplant.11
Whether CYP3A5 genotypes affect resource utilization is a key question, as clinical implementation requires a careful assessment of finances. Two of the prior prospective studies observed differences in number of dose adjustments and time to reach therapeutic concentrations, which suggest that healthcare resource utilization among transplant recipients could be influenced by pharmacogenetics.8,9 A study from Thailand in adult renal transplant identified significant differences in the cost of care associated with tacrolimus monitoring and hospitalization post-transplant among CYP3A5 genotypes.12 Additional data is needed to more clearly determine whether CYP3A5 genotype contributes to healthcare resource utilization in other populations and practice models, as this could have significant implications on the perceived utility of employing personalized dosing in routine practice. This study was conducted to evaluate the impact of CYP3A5 genotype on post-transplant healthcare resource utilization and transplant outcomes within the first year post-transplant in pediatric renal and heart transplant recipients.
Patients and Methods
This single-center, retrospective cohort study was approved by the University of Michigan Investigational Review Board and a waiver of informed consent was provided for secondary use research (HUM00155810). All patient data access complied with relevant data protection and privacy regulations, in compliance with the Declaration of Helsinki. All organ donations included written informed consent and were conducted in accordance with the Declaration of Istanbul.
Data Collection
Patients were included if they received a renal or heart transplant between June 1, 2014 and December 31, 2018, were <18 years of age at the time of transplant, and had a stored DNA sample from their transplant workup available for analysis. Patients were excluded if they did not receive tacrolimus as part of the initial immunosuppression regimen or if they experienced primary graft non-function. In kidney transplant, protocol-based tacrolimus dosing was recommended as 0.1 mg/kg q 12 hours orally, with consideration of q8 hour dosing if the patient was <40 kg, and tacrolimus concentrations were assessed daily. In heart transplant, protocol-based tacrolimus dosing was recommended as 0.05 mg/kg q 12 hours orally and tacrolimus concentrations were measured after at least 3 stable doses. Discrete clinical variables, including tacrolimus administration and dosing, concomitant immunosuppression regimen (eg, standard vs steroid avoidant), induction therapy, laboratory data, patient demographic characteristics, and encounter information and encounter associated charges were queried from the electronic medical record via Data Direct, an internal self-service data tool. Any non-discrete data variables, such as biopsy results, were manually extracted from the electronic medical record using the EMERSE search engine.13 All data were stored in a secure RedCap database.14
Genotyping
Secondary use samples of extracted DNA were obtained from the University of Michigan histocompatibility laboratory for patients who met the inclusion criteria. Commercial Taqman assays were used as described previously, with minor modifications.15 Briefly, reactions were carried out using 10 ng of DNA with Genotyping Master Mix (Applied Biosystems) in an iCycler real-time thermocycler (BioRad) for 40 cycles. Genotyping was performed for the following single nucleotide polymorphisms (SNP) CYP3A5*3 (rs776746), CYP3A5*6 (rs10264272), and CYP3A5*7 (rs41303343) (Thermo Fisher Scientific, Waltham, MA). Call rates for all SNPs were >99%; 10% of samples were retested and results were 100% concordant. CYP3A5 genotypes were grouped into the corresponding clinical phenotype for analysis: CYP3A5 expressers (those with 1 or zero variant alleles) and CYP3A5 non-expressers (those with 2 variant alleles).
Analysis
The number of tacrolimus dose changes, number of tacrolimus concentrations, number of clinical encounters, for all types and outpatient only, and total charges for all encounters were calculated for each subject at 30, 90, 180, 270, and 365 days post-transplant. Biopsy proven acute rejection (BPAR) was defined as a T-cell mediated rejection grade ≥1A for renal transplant or acute cellular rejection grade ≥1 for heart transplant.16,17 De novo donor-specific antibody (DSA) was defined as the first positive DSA result post-transplant in patients who had negative baseline DSA. Induction therapy was categorized as rabbit anti-thymocyte globulin (rATG) versus basiliximab/no induction.
Demographic characteristics and calculated variables were evaluated descriptively for each transplant type at 365 days and then compared between transplant types with univariate regressions for both categorical variables and continuous variables. The univariate impact of CYP3A5 genotype on number of tacrolimus dose changes, number of tacrolimus concentrations, and number of clinical encounters at 365 days were evaluated via linear regression. The univariate impact of CYP3A5 genotype on length of stay (LOS), intensive care unit LOS (ICU LOS) for transplant admission and total charges at 365 days were assessed with quasipoisson regression. Multivariable analysis of the impact of CYP3A5 genotype was assessed for these outcomes while also controlling for patient age (continuous), induction agent, and sex.
The impacts of CYP3A5 phenotype on BPAR, de novo DSA, and the composite outcome of BPAR or de novo DSA, whichever occurred first were evaluated with Kaplan-Meier analyses. Cox proportional hazards models evaluated the impact of CYP3A5 genotype on time to event controlling for patient’s age, sex, and rATG induction therapy. All data analysis were performed in R (v.3.6.3).18
Results
Eighty-eight pediatric patients received a renal or heart transplant between June 1, 2014–December 31, 2018; three patients were subsequently excluded for no tacrolimus administration or primary graft non-function (Figure 1) resulting in a final cohort of 48 kidney transplant recipients and 37 heart transplant recipients (n=85). The average age at the time of transplant was 9.7 ± 5.9 years. The majority of patients were Caucasian (76.5%), were CYP3A5 non-expressers (77.6%), and received a kidney transplant (56.5%). The most common induction therapy was rATG (50.6%) and immunosuppression regimen was tacrolimus with mycophenolate (96.4%). Demographic characteristics by transplant type are shown in Table 1. Characteristics did not differ significantly between transplant types except for induction therapy, where significantly more renal transplant recipients received induction therapy compared to heart transplant recipients (p<0.001). Fifty-four percent of renal transplant recipients received a steroid-avoidant immunosuppression regimen. Two patients died within the first year post-transplant, both heart transplant recipients.
 |
Table 1 Patient Demographics by Transplant Type |
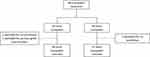 |
Figure 1 Inclusion of eligible patients. |
The calculated healthcare utilization measures differed significantly between transplant types (Supplemental Table 1). Renal transplant recipients had a significantly higher number of tacrolimus dose changes and outpatient visits. Heart transplant recipients had a longer ICU LOS, LOS, and higher total charge. The median tacrolimus starting dose was 0.2 mg/kg/day (IQR:0.19–0.22 mg/kg/day) in renal transplant, with the majority of patients (n=43) receiving every 12-hour dosing; the median tacrolimus starting dose was 0.09 mg/kg/day (IQR: 0.065–0.1 mg/kg/day) in heart transplant with all patients receiving every 12-hour dosing. Secondary to these differences, all subsequent analyses of these outcomes were stratified by transplant type.
Kidney Transplant
In the kidney transplant cohort, no associations were identified between CYP3A5 phenotype and any healthcare utilization measures at 1-year post-transplant in the univariate analysis (Table 2). CYP3A5 phenotype was also not associated with any outcome at any time point after controlling for age, sex, and rATG induction therapy. Induction therapy was associated with a higher number of visits, higher total charges, and a higher number of tacrolimus concentrations at each of the evaluated time points (Supplemental Table 2). Age was directly associated with higher total charges at all evaluated time points, and was inversely associated with the number of tacrolimus concentrations at 30 [β= −0.3, 95% CI (−0.53, −0.07)], 90 [β=−0.42, 95% CI (−0.73, −0.11)], and 270 days [β= −0.79, 95% CI (−1.44, −0.13)]. A post hoc analysis was performed to evaluate the impact of CYP3A5 genotype on the number of dose changes that occurred only during outpatient visits due to concern for confounding by patient location. These multivariable analyses also controlled for age, sex, and induction treatment; no variables in these models were associated with the outcomes at any study time point.
 |
Table 2 Impact of CYP3A5 Phenotype on Healthcare Resource Utilization Measures During First Year Post-Transplant in Kidney Transplant Recipients |
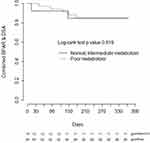
CYP3A5 phenotype was not associated with the time to developing BPAR (n=8), de novo DSA (n=15), or the composite of BPAR or de novo DSA alone (n=17), nor when controlling for age, sex, and induction regimen (Figure 2).
Heart Transplant
In the heart transplant cohort, CYP3A5 phenotype was significantly associated with an increased number of tacrolimus dose changes and increased number of tacrolimus concentrations, but not with other measures of healthcare utilization, in the univariate analysis of 1-year post-transplant (Table 3). After controlling for age, sex, and rATG induction, CYP3A5 phenotype remained a significant predictor of number of dose changes and number of tacrolimus concentrations for all evaluated time points within the first year post-transplant (Supplemental Table 3). The CYP3A5 expresser phenotype was also determined to be associated with increased ICU LOS, LOS, and total charges. rATG induction therapy was also associated with an increased ICU LOS, LOS, and total charges for all time points. Age was directly associated with ICU LOS and LOS. Age was also directly associated with the total charge at 90, 180, and 270 days post-transplant and inversely associated with the number of dose changes for all time points except 30 days (Supplemental Table 3).
 |
Table 3 Impact of CYP3A5 Phenotype on Healthcare Resource Utilization Measures During First Year Post-Transplant in Heart Transplant Recipients |
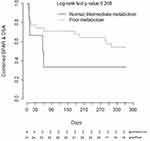
CYP3A5 phenotype was not significantly associated with time to developing BPAR (n=7), de novo DSA (n=20), or the composite of BPAR or de novo DSA (n=23) within the first year post-transplant alone nor after controlling for age, sex, or induction regimen (Figure 3).
Discussion
This study identified that CYP3A5 phenotype was a significant predictor of post-transplant resource utilization in the pediatric heart transplant but not the pediatric renal transplant population. Age and use of rATG induction therapy were associated with higher total charges within the first year post-transplant in both populations. These findings suggest the impact of CYP3A5 phenotype on these resource outcomes is likely significantly impacted by clinical practice in tacrolimus management. At our institution, tacrolimus therapeutic drug monitoring (TDM) protocols differ significantly between renal and heart transplant programs. In renal transplant, the TDM protocol recommends daily tacrolimus concentrations while the patient is admitted post-transplant, weekly for the first 3 months, every other week months 3–6, bimonthly months 6–9, and monthly thereafter. In heart transplant, TDM is recommended after 3 stable doses have been administered after tacrolimus dose changes while inpatient, within 7–10 days from discharge, monthly for the first 2 months, every 1.5 months until 9 months and at 12 months. Additionally, the target tacrolimus concentration for renal transplant was reduced approximately every 3 months over the first transplant year, while it remained the same for the first 12-months post heart transplant.
These differences in program-specific TDM schedule likely explain the substantial difference in the number of tacrolimus concentrations between our two transplant cohorts. Daily tacrolimus monitoring may supersede any impact of CYP3A5 phenotype on tacrolimus TDM, because all patients have the same baseline monitoring frequency. Additionally, as is suggested by our increased number of dose changes in the kidney transplant cohort, patients with daily tacrolimus monitoring may be titrated more quickly than those with more time between monitoring as a clinician may inherently want to act on the drug concentration. However, there are important considerations for whether this is advantageous for all patients. For narrow therapeutic index medications such as tacrolimus, earlier monitoring may be warranted, especially around times of clinical changes, to ensure supra-therapeutic concentrations are not occurring. However, daily dose changes, particularly in response to sub-therapeutic concentrations, may be more likely to overshoot the target concentration because there is insufficient time for the drug to reach steady state between dose changes, which for tacrolimus is after two to three days of therapy.19 This potential for over adjustment may put patients at higher risk for non-therapeutic concentrations. Differences in concomitant immunosuppressive regimen, such as the increased use of T-cell depleting induction in renal transplant, may also contribute to increased monitoring for close assessment of potentially shared adverse events such as leukopenia.
Another significant concern with tacrolimus monitoring is the risk for inappropriately drawn tacrolimus trough concentrations relative to drug administration, which has been shown to happen frequently in tacrolimus monitoring and may have significant implications on drug management.20 If clinicians are adjusting doses based on a non-trough tacrolimus concentration, there is a higher risk the dose adjustments will likely be inappropriate for the patient’s target concentration. Although the appropriateness of the concentration was not directly assessed in our study, since we were assessing the total number of tacrolimus concentrations obtained, this may also significantly influence overall resource utilization.
Consistent with the prior prospective studies we did not identify a significant association of rejection-associated outcomes, such as BPAR and de novo DSA formation, with CYP3A5 phenotype. However, the small number of events within our cohort indicate this study was likely underpowered to detect differences among these groups. Interestingly, there is a visible separation on the Kaplan-Meier plot between the CYP3A5 phenotypes in heart transplant, but not on the renal transplant plot. A major baseline difference between these cohorts was the use of induction therapy, with 100% of renal transplant recipients receiving an induction agent (rATG or basiliximab) compared to only 38% of heart transplant recipients. Induction therapy is utilized to decrease the incidence of acute rejection in transplant recipients, and the use of T-cell depleting therapy has increased over the past decade.1,2 In the prior prospective studies that assessed impact of personalized tacrolimus dosing on early clinical outcomes, all study participants received induction therapy.8–10 Earlier studies, where ~50% or less of patients received induction therapy, primarily with IL-2 receptor blockers, did find associations between CYP3A5 genotype and early acute rejection outcomes.21–25 Although there are also negative studies, a meta-analysis of this data identified a significant association between CYP3A5 genotype and acute rejection when controlling for induction therapy.26 Our findings, as well as the previous literature, suggest CYP3A5 phenotype may be a more impactful covariate for early clinical outcomes in patients receiving tacrolimus-based immunosuppression without T-cell depleting induction therapy, although this hypothesis needs further validation.
An increased number of tacrolimus dose changes may have important implications for clinical and healthcare resource utilization beyond what was assessed within this study. Increased intra-patient variability of tacrolimus dose, measured as the coefficient of variation (CV), was associated with increased rates of acute rejection in the first 6 months post-transplant in a large cohort of adult kidney transplant recipients.27 In their study, CYP3A5 non-expression was associated with higher CV for tacrolimus doses, while CYP3A5 expression was associated with higher CV of tacrolimus trough concentrations. The authors discuss that while increased CV of tacrolimus doses may be a surrogate for a difficult post-transplant course and not a direct indicator for rejection, their data suggest dose adjustments may not be beneficial for trough fluctuations that are close to the target range. An additional consideration for resource utilization that we were unable to capture within this study was the clinician time required to determine, implement, and communicate a tacrolimus dose change for a patient. Frequently the patient may have already been discharged from their appointment prior to the clinician being able to assess the trough result. There is therefore the additional resource of clinician time outside of a clinic visit to act on these results and ensure the patient understands the dosing changes.
The findings of this study are limited by the small sample size. There are many clinical factors that can influence the monitoring frequency of tacrolimus, such as acute kidney injury, changes to concomitant medications, or adverse events that we were unable capture in this retrospective study. Further evidence is needed to more thoroughly investigate these associations in larger cohorts.
Conclusion
In this study, we identified that CYP3A5 phenotype can be an indicator of healthcare resource utilization post-transplant, although this appears to be strongly influenced by the tacrolimus monitoring protocol that is employed. T-cell depleting induction therapy and age were other covariates consistently associated with resource utilization measures. Additional studies are needed to evaluate whether personalized tacrolimus dosing strategies could normalize the post-transplant resource utilization between CYP3A5 phenotypes.
Disclosure
Dr Amy L Pasternak and Dr Jeong M Park report grant funding from the National Center for Advancing Translational Sciences (NCATS) to support this work under award number: UL1TR002240. The authors report no other conflicts of interest in this work.
References
1. Colvin M, Smith JM, Hadley N, et al. OPTN/SRTR 2018 annual data report: heart. Am J Transpl. 2020;20(Suppl s1):340–426. doi:10.1111/ajt.15676
2. Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2018 annual data report: kidney. Am J Transpl. 2020;20(Suppl s1):20–130. doi:10.1111/ajt.15672
3. Brunet M, van Gelder T, Asberg A, et al. Therapeutic drug monitoring of tacrolimus-personalized therapy: second consensus report. Ther Drug Monit. 2019;41(3):261–307. doi:10.1097/FTD.0000000000000640
4. Andrews LM, Hesselink DA, van Gelder T, et al. A population pharmacokinetic model to predict the individual starting dose of tacrolimus following pediatric renal transplantation. Clin Pharmacokinet. 2018;57(4):475–489. doi:10.1007/s40262-017-0567-8
5. Knops N, Herman J, van Dyck M, et al. Tacrolimus dose requirements in paediatric renal allograft recipients are characterized by a biphasic course determined by age and bone maturation. Br J Clin Pharmacol. 2017;83(4):863–874. doi:10.1111/bcp.13174
6. Zhao W, Elie V, Roussey G, et al. Population pharmacokinetics and pharmacogenetics of tacrolimus in de novo pediatric kidney transplant recipients. Clin Pharmacol Ther. 2009;86(6):609–618. doi:10.1038/clpt.2009.210
7. Andrews LM, de Winter BCM, Cornelissen EAM, et al. A population pharmacokinetic model does not predict the optimal starting dose of tacrolimus in pediatric renal transplant recipients in a prospective study: lessons learned and model improvement. Clin Pharmacokinet. 2020;59(5):591–603. doi:10.1007/s40262-019-00831-8
8. Min S, Papaz T, Lafreniere-Roula M, et al. A randomized clinical trial of age and genotype-guided tacrolimus dosing after pediatric solid organ transplantation. Pediatr Transpl. 2018;22(7):e13285. doi:10.1111/petr.13285
9. Thervet E, Loriot MA, Barbier S, et al. Optimization of initial tacrolimus dose using pharmacogenetic testing. Clin Pharmacol Ther. 2010;87(6):721–726. doi:10.1038/clpt.2010.17
10. Shuker N, Bouamar R, van Schaik RH, et al. A randomized controlled trial comparing the efficacy of Cyp3a5 genotype-based with body-weight-based tacrolimus dosing after living donor kidney transplantation. Am J Transpl. 2016;16(7):2085–2096. doi:10.1111/ajt.13691
11. Pallet N, Etienne I, Buchler M, et al. Long-term clinical impact of adaptation of initial tacrolimus dosing to CYP3A5 genotype. Am J Transpl. 2016;16(9):2670–2675. doi:10.1111/ajt.13788
12. Vannaprasaht S, Limwattananon C, Anutrakulchai S, Chan-On C. Effect of CYP3A5 genotype on hospitalization cost for kidney transplantation. Int J Clin Pharm. 2019;41(1):88–95. doi:10.1007/s11096-018-0750-5
13. Hanauer DA. EMERSE: the electronic medical record search engine. AMIA Annu Symp Proc. 2006;941.
14. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377–381. doi:10.1016/j.jbi.2008.08.010
15. Pasternak AL, Kidwell KM, Dempsey JM, et al. Impact of CYP3A5 phenotype on tacrolimus concentrations after sublingual and oral administration in lung transplant. Pharmacogenomics. 2019;20(6):421–432. doi:10.2217/pgs-2019-0002
16. Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transpl. 2008;8(4):753–760. doi:10.1111/j.1600-6143.2008.02159.x
17. Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Hear Lung Transpl. 2005;24(11):1710–1720. doi:10.1016/j.healun.2005.03.019
18. R Core Team. R: a language and environment for statistical computing; 2020. R Foundation for Statistical Computing Austria. URL V, Available from: https://www.R-project.org/.
19. Renders L, Frisman M, Ufer M, et al. CYP3A5 genotype markedly influences the pharmacokinetics of tacrolimus and sirolimus in kidney transplant recipients. Clin Pharmacol Ther. 2007;81(2):228–234. doi:10.1038/sj.clpt.6100039
20. Strohbehn GW, Pan WW, Petrilli CM, et al. Large-scale variability of inpatient tacrolimus therapeutic drug monitoring at an academic transplant center: a retrospective study. Ther Drug Monit. 2018;40(4):394–400. doi:10.1097/FTD.0000000000000526
21. Min SI, Kim SY, Ahn SH, et al. CYP3A5 *1 allele: impacts on early acute rejection and graft function in tacrolimus-based renal transplant recipients. Transplantation. 2010;90(12):1394–1400. doi:10.1097/TP.0b013e3181fa93a4
22. Quteineh L, Verstuyft C, Furlan V, et al. Influence of CYP3A5 genetic polymorphism on tacrolimus daily dose requirements and acute rejection in renal graft recipients. Basic Clin Pharmacol Toxicol. 2008;103(6):546–552. doi:10.1111/j.1742-7843.2008.00327.x
23. Singh R, Srivastava A, Kapoor R, Sharma RK, Mittal RD. Impact of CYP3A5 and CYP3A4 gene polymorphisms on dose requirement of calcineurin inhibitors, cyclosporine and tacrolimus, in renal allograft recipients of North India. Naunyn Schmiedebergs Arch Pharmacol. 2009;380(2):169–177. doi:10.1007/s00210-009-0415-y
24. Ferraresso M, Tirelli A, Ghio L, et al. Influence of the CYP3A5 genotype on tacrolimus pharmacokinetics and pharmacodynamics in young kidney transplant recipients. Pediatr Transpl. 2007;11(3):296–300. doi:10.1111/j.1399-3046.2006.00662.x
25. Tirelli S, Ferraresso M, Ghio L, et al. The effect of CYP3A5 polymorphisms on the pharmacokinetics of tacrolimus in adolescent kidney transplant recipients. Med Sci Monit. 2008;14(5):CR251–CR254.
26. Rojas L, Neumann I, Herrero MJ, et al. Effect of CYP3A5*3 on kidney transplant recipients treated with tacrolimus: a systematic review and meta-analysis of observational studies. Pharmacogenomics J. 2015;15(1):38–48. doi:10.1038/tpj.2014.38
27. Seibert SR, Schladt DP, Wu B, et al. Tacrolimus trough and dose intra-patient variability and CYP3A5 genotype: effects on acute rejection and graft failure in European American and African American kidney transplant recipients. Clin Transpl. 2018;32:e13424. doi:10.1111/ctr.13424
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.