Back to Journals » Clinical Ophthalmology » Volume 15
ETOILE: Real-World Evidence of 24 Months of Ranibizumab 0.5 mg in Patients with Visual Impairment Due to Diabetic Macular Edema
Authors Kodjikian L, Lecleire-Collet A, Dot C , Le Lez ML, Baillif S, Erginay A, Souied E, Fourmaux E, Gain P, Ponthieux A
Received 27 March 2021
Accepted for publication 18 May 2021
Published 3 June 2021 Volume 2021:15 Pages 2307—2315
DOI https://doi.org/10.2147/OPTH.S313081
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Laurent Kodjikian,1 Amélie Lecleire-Collet,2 Corinne Dot,3 Marie-Laure Le Lez,4 Stéphanie Baillif,5 Ali Erginay,6 Eric Souied,7 Eric Fourmaux,8 Philippe Gain,9 Anne Ponthieux10
1Department of Ophthalmology, Croix-Rousse University Hospital, Lyon, France; 2Mathilde Clinic, Rouen, France; 3Department of Ophthalmology, Desgenettes Military Hospital, Lyon, France and French Military Health Service Academy, Val-de-Grâce, Paris, France; 4Ophthalmology Department, Centre Hospitalier Regional Universitaire de Tours, Tours, France; 5Department of Ophthalmology, Centre Hospitalier Universitaire de Nice, Hôpital Pasteur-2, Nice, France; 6Department of Ophthalmology, Lariboisière Hospital, AP HP, University Paris-Diderot Paris-7, Paris, France; 7Department of Ophthalmology, Hospital Intercommunal de Creteil, University Paris Est Creteil, Creteil, France; 8Centre Gallien, Bordeaux, France; 9Ophthalmology Department, University Hospital, Saint-Etienne, France, Corneal Graft Biology, Engineering and Imaging Laboratory, BiiGC, EA2521, Federative Institute of Research in Sciences and Health Engineering, Faculty of Medicine, Jean Monnet University, Saint‐Etienne, France; 10Novartis Pharma SAS, Rueil-Malmaison, France
Correspondence: Laurent Kodjikian
Department of Ophthalmology, Croix-Rousse University Hospital, 103 Grande Rue de la Croix-Rousse, Lyon, 69004, France
Tel +33 4 26 10 94 29
Fax + 33 4 72 07 26 45
Email [email protected]
Purpose: To evaluate the real-world effectiveness of intravitreal ranibizumab 0.5 mg (Lucentis) in improving visual acuity (VA) in adults with decreased VA due to diabetic macular edema (DME).
Patients and Methods: Real-world prospective observational 24-month study. Ranibizumab-naïve patients (n=116) were enrolled, treated and followed up according to investigators’ usual procedures. Outcomes included change from baseline to month 24 in best-corrected VA (BCVA; primary outcome), central retinal thickness (CRT), treatment exposure and safety.
Results: Overall, 62.9% of patients completed the study per protocol, 68.6% completed the induction phase (first three injections one month apart). On average, patients had 12.5 ophthalmologist visits and 5.74 injections in year 1, decreasing to 4.6 visits and 1.94 injections in year 2. Mean baseline BCVA was 58.4 letters, mean gain at M24 was +6.08 letters (95% CI: 2.95, 9.21). Gains were higher for patients who completed induction, and for patients who did not switch treatment. Mean CRT improved by 149.17 μm at M24. There were no new safety signals. BCVA variation of ≥ 6 letters by M3 was predictive of BCVA gains at M24 (p=0.007), as was hypertension medication at baseline (p=0.022).
Conclusion: Real-world ranibizumab treatment improved VA in DME patients, despite fewer injections than recommended.
Keywords: real-world study, retinal thickness, visual acuity, switch, induction
Introduction
Diabetic retinopathy (DR) is a common complication of diabetes occurring in people with Type 1 and 2 diabetes aged over 20 years.1 Diabetic macular edema (DME), a complication of DR, is strongly associated with visual impairment.1,2 In diabetes, the prevalence of DR and DME is estimated to be 34.6% and 6.81%, respectively, yielding a global DME population of 21 million people in 2010.3 DME is multifactorial and the pathogenesis is not completely understood. Hyperglycemia initiates a cascade of events resulting in hypoxia, increased vascular permeability of the blood retinal barrier and retinal fluid accumulation (edema). Vascular endothelial growth factor (VEGF) is implicated in retinal vascular permeability and is regulated by hypoxia and hyperglycemia.
Ranibizumab (Lucentis, Novartis) is a humanized monoclonal antibody fragment administered by intravitreal injection that antagonizes VEGF with high affinity. The European Medicines Agency (EMA) approved ranibizumab for age-related macular degeneration (AMD) in 2007 and for DME in 2011. Ranibizumab’s approval for DME was based on significant visual acuity (VA) gains in the studies RESOLVE,4 RETAIN5 RESTORE,6 supported by an independent Phase 3 study.7 Interventional randomized controlled trials (RCTs) assess efficacy and safety data in narrow populations with strict inclusion/exclusion criteria. Observational real-world studies offer a complementary view of how medicines are prescribed and used, and how this affects outcomes. However, most observational trials are retrospective with lower levels of evidence.
Here, we report results from ETOILE, a prospective observational study evaluating the efficacy of ranibizumab in previously treatment-naïve patients with decreased VA due to DME over 24 months, under real-world conditions in France.
Patients and Methods
Study Design
This 24-month Phase 4 prospective multicenter observational study assessed real-world outcomes of ranibizumab in DME. We invited 38 ophthalmologists capable of administering ranibizumab injections and who had access to spectral domain optical coherence tomography (SD-OCT) to be study investigators. Each investigator had 6 months to include ≥8 consecutive patients.
Investigators informed patients about study objectives and data collection. Patients gave their “non-opposition” to participation, according to French regulations. The relevant French data protection committee [Comité Consultatif sur le Traitement de l’Information en matière de Recherche dans le domaine de la Santé (CCTIRS)] approved the study protocol. French ethics committee approval was not required, according to French law at the time of study conduct. Research was performed according to the Declaration of Helsinki.
Study Population
Eligible patients were aged ≥18 years, had decreased best corrected VA (BCVA) due to DME linked to type I or II diabetes in at least 1 eye that was previously untreated, and whose ophthalmologist recommended ranibizumab. If both eyes were eligible, the investigator designated the studied eye and injected this eye first. Exclusion criteria were hypersensitivity to any component of ranibizumab, ocular/periocular infection, severe intraocular inflammation, surgery in the studied eye within 3 months, and vitreomacular traction in the studied eye.
Treatment
Patients received ranibizumab 0.5 mg by intravitreal injection, according to the ophthalmologist’s usual practice. Ophthalmologists monitored patients for disease progression and treatment continued until maximum VA was achieved and/or disease absence, according to the ophthalmologist’s judgment.
Assessments
Demographics, medical history, diabetes disease characteristics, prior and concomitant medications were collected at baseline.
Data were collected ≥4 times: at baseline, first ranibizumab injection, Month (M) 12 (±2) and M24 (±2). If patients visited their ophthalmologists more frequently, data were also collected at M3 (±1), M6 (±1), M9 (±1) and M18 (±2).
Ophthalmologists assessed BCVA as per their usual practice. Data collected in Snellen or Monoyer scales were converted to Early Treatment Diabetic Retinopathy Study (ETDRS) letters. Central retinal thickness (CRT) was measured using SD-OCT. Number of visits and injections, concomitant DME treatments in studied eye, anatomical parameters, adverse events (AEs) were recorded.
Statistical Analysis
The primary outcome was the mean absolute change in BCVA at M24 in ETDRS letters. In RCTs, mean BCVA gains ranged from 7.9 to 14.9 after 24 months of ranibizumab.6,7 Assuming a mid-range gain of +11 letters and that 40% of patients would be unevaluable, a sample size of 150 patients was needed for an evaluable population of 90 patients, with a precision of +2.2273 letters.
We describe quantitative variables using mean, standard deviation (SD), median and extreme values, and 2-sided 95% confidence intervals (CIs). We describe qualitative variables as absolute frequency and percentage by modality. Sub-group analyses were performed by baseline disease characteristics and demographics.
Results are presented without imputation of missing data. A sensitivity analysis for robustness and reliability was performed for the primary outcome, replacing missing data with last observation carried forward (LOCF).
We identified predictive factors of the absolute mean change of BCVA at M12 using analysis of variance (ANOVA) of the absolute mean change of BCVA at M12 and at M24. Multivariate ANOVA allowed stepwise selection of significant factors identified in the univariate analysis.
We used SAS to analyze data.
Results
Patients
Of 38 ophthalmologists solicited, 26 became ETOILE investigators, and 20 enrolled ≥1 patient. Six ophthalmologists were from public hospitals, six from private practice and eight had mixed public-private activity.
Between January 2014 and June 2015, 128 patients were enrolled and 116 received ≥1 ranibizumab injection. Safety analyses were based on these 116 patients (Figure 1). Eleven patients had inclusion criteria violations, missing baseline BCVA or missing follow-up visits. The remaining 105 patients comprised the population used to assess demographics, drug exposure and efficacy. Of this population, 89.5% had a surveillance visit at M3, 78.1% at M6, 66.7% at Month 9, 75.2% at M12, 68.6% at M18 and 62.9% at M24. Overall, 66 patients (62.9%) completed the study as planned. The remainder did not return for the M24 visit (n=35) or died (n=4).
 |
Figure 1 Flow chart of patients in study. |
Mean (SD) age was 65.5 (11.4) years. Mean visual acuity at baseline was 58.4 [55.7;61.0] ETDRS letters. See Table 1 for other demographics and disease characteristics.
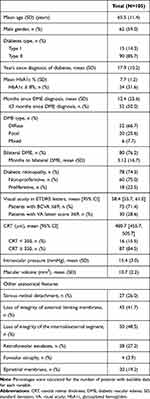 |
Table 1 Patient and Studied Eye Baseline Characteristics |
Treatment
Patients had a mean (SD) of 7.31 (4.18) intravitreal injections over 24 months. Mean injections decreased from 5.74 (2.5) in year 1 to 1.94 (2.38) in year 2. Patients had a mean (SD) of 12.5 (6.5) surveillance visits over the 24 months. Similarly, the mean (SD) number of visits decreased from 8.0 (3.3) in year 1 to 4.6 (3.6) in year 2. During induction, 68.6% patients had their first three ranibizumab injections one month apart (mean of 32.72 [13.09] days between injections). The percentage of patients receiving an injection decreased from 39.4% at M3 to 20.7% at M6, 20.0% at Month 9, 19% at M12, 16.7% at M18 and 7.6% at M24.
Non-injection was most common because the purpose of the visit was follow-up, or due to disease improvement or stabilization. Concomitant treatment was reported for 14 patients (13.3%), who received ≥1 additional laser treatment during the study, of whom 12 were treated during year 1.
Efficacy
Visual Acuity
Mean BCVA improvement from baseline to M24 (Figure 2A) was +6.08 letters (95% CI: 2.95, 9.21). In the sensitivity analysis with missing data imputed by LOCF, mean improvement was 5.51 (95% CI: 3.32, 7.71). Patients achieved a mean BCVA of 64.3 letters (95% CI: 60.5, 68.1). Improvements were evident from M3 (+5.5 letters [95% CI: 3.5, 7.4]). Gains of ≥10 and ≥15 letters from baseline to M24 were reported for 44.4% and 22.2% of patients, respectively. Loss of ≥15 letters was reported for 6.3% of patients.
 |
Figure 2 Change from baseline in (A) visual acuity and, (B) central retinal thickness. |
The proportion of patients with BCVA ≥70 letters increased from 26.7% at baseline to 45.2% at M3, 40% at M12 and 42.9% at M24.
Induction: Patients who completed induction (receiving three initial monthly injections) had higher mean (SD) BCVA change at M12 than patients who did not (+6.4 [10.7] letters vs +3.0 [17.2] letters). We observed similar trends at M24 (Figure 3A).
 |
Figure 3 Change from baseline in (A) visual acuity and (B) central retinal thickness, according to completion of induction phase. |
Switch: Overall, 74.3% patients were treated with ranibizumab only (non-switchers) and 25.7% switched from ranibizumab to another anti-VEGF or corticosteroid (switchers) after a mean (SD) of 14.2 (6.3) months. Similar proportions of patients switched to aflibercept (12.4%) and to dexamethasone implant (14.3%). Just one patient switched twice: to aflibercept then to dexamethasone implant. Baseline BCVA was similar in switchers and non-switchers. Non-switchers had higher mean (SD) BCVA gains than switchers at M12 (+7.4 [8.5] letters vs +1.7 [18.0] letters) and at M24 (+8.2 [10.1] letters vs +2.1 [15.4] letters) (Figure 4A and B).
Anatomical features at baseline: Baseline BCVA was worse in patients with loss of integrity of internal segment-outer segment (53.5 letters) and external limiting membrane (52.2 letters). These patients had mean (SD) BCVA gains from baseline to M24 of +6.7 (12.6) and +8.8 (11.9) letters, respectively.
Central Retinal Thickening
CRT improved from baseline to M12 (Figure 2B) by an absolute mean (SD) decrease of −111.4 μm (95% CI: −148.5, −74.2) and to M24 by −149.2 μm (95% CI: −187.3, −111.0). CRT improvement was evident from M3 onwards.
Baseline mean (SD) CRT was 487.0 μm (124.2) for patients with full induction phase (3 initial monthly injections) and 467.2 μm (135.8) for patients without. Similarly to BCVA, CRT improvement (Figure 3B) appeared better in patients who completed induction than those who did not at M12 (−133 µm vs −51 µm) and M24 (−171 µm vs −80 µm). Final CRT was 321.9 μm (76.9) in patients with full induction and 376.7 μm (137.8) without.
Baseline mean (SD) CRT was higher in switchers (518.0 μm [141.2]) than non-switchers (who stayed on ranibizumab) (468.1 μm [121.3]). Switchers had a bigger decrease than non-switchers at M12 (−149 µm vs −95 µm) and M24 (−174 µm vs −137 µm) (Figure 4C and D). However, final CRT was similar in both sub-groups at 12 and 24 months: 364 µm and 328 µm in non-switchers versus 359 µm and 348 µm in switchers.
Predictive Factors
According to multivariate ANOVA, BCVA variation of ≥6 letters in the first 3 months of follow-up (p= 0.007) and ≥1 hypertension medication at baseline (p=0.022) predicted BCVA gains at M24. Baseline CRT ≥ 300 μm was predictive of BCVA loss at M24.
Safety
Overall, 75.9% of patients had ≥1 AE: 10.3% had AEs in both eyes, 51.7% in the studied eye only and 19% in the contralateral eye only. Ocular AEs occurring in >5% of patients were cataract (9.5%), diabetic retinal edema (7.8%), ocular hypertension (5.2%), vitreous hemorrhage (5.2%), drug ineffective (24.1%) and inappropriate schedule of drug administration (24.1%). 36.2% of patients had non-ocular AEs.
Seventeen patients had AEs considered related to ranibizumab. The only related AE reported by >1 patient was “drug ineffective” (reported by 15/17 patients).
Serious AEs (SAEs) were reported by 26.7% of patients. The only ocular SAE reported by >2% of patients was cataract (2.6%). No patient had an SAE considered related to ranibizumab; 3 (2.6%) had SAEs that the investigator considered related to the intravitreal injection (cataract, retinal detachment and vitreous hemorrhage).
Four patients (3.4%) died following cardiac disorder, SAEs that were not considered related to ranibizumab or its administration.
Discussion
In this prospective real-life study of French DME patients, we observed visual gains with ranibizumab from M3 (+5.5 letters) that were maintained up to M24 (+6.1 letters). This effect was maintained in a sensitivity analysis imputing missing data with LOCF (+5.5 letters). Gains of ≥15 letters were achieved by 22.2% of patients, and 42.9% had final BCVA ≥70 letters. VA gain appeared higher when patients completed induction (+6.4 and +7.1 letters at M12 and M24, respectively). Patients who stayed on ranibizumab with no switch appeared to have better VA results, although these patients may represent “good responders” to ranibizumab. Further work and statistical comparisons are needed to confirm these findings. Mean CRT also improved from baseline to M3 (−125.8 μm) and to M24 (−149.1 μm). Safety was similar to the established safety profile of ranibizumab.
RCTs of ranibizumab in DME reported BCVA gains of +7.24 letters after 6 months,9 +10.3 letters after 12 months (with mean [SD] numbers of injections: 10.2 [2.5]),4 +7.7 letters after 24 months (with 3 monthly injections initially, and a mean of 5.3 injections during the 18 months follow-up)10 and +7–9 letters after 24 months (with a median number of 8–9 and 2–3 injections during years 1 and 2).11
RESTORE used a less intensive regimen, comprising a 3-month induction followed by pro re nata (PRN) “as needed” injections. BCVA gain was +6.8 letters after 12 months of ranibizumab alone6 and +8.0 letters after the 2-year extension study.12 We observed similar gains in ETOILE.
In RETAIN, mean BCVA change at M24 was similar between the treat and extend (T&E) and laser (+8.3 letters), T&E (+6.5) and PRN (+8.1) groups. Mean injections over the 24 months numbered 12.4 and 12.8 in the T&E+laser and T&E groups, and 10.7 in the PRN group.5
Taken together, these RCTs show that BCVA and central retinal thickness improvements observed after one year are maintained during the second and third years, while the number of injections required declined progressively.
A 2018 meta-analysis of observational studies investigating pharmacological interventions in DME included 32 studies of anti-VEGF treatments, totaling 6842 studied eyes. After a 15.6-month mean follow-up, mean BCVA gain was +4.7 letters, with a final BCVA of 62 letters. Mean injections numbered 5.8 over the 15.6-month period, notably fewer than in RCTs.13 Anti-VEGF treatment response therefore appears to depend on the number of injections.
In ETOILE, only 68.8% of patients completed the 3-month induction. These patients appeared to have better outcomes, a key message for daily practice. French guidelines recommend intensive treatment during the first year of treatment, with 7–9 injections.14 In ETOILE, patients had a mean (SD) of 7.31 (4.18) injections over 24 months. More injections were administered in year 1 (mean 5.74) compared to year 2 (1.94) and patients were not injected at each visit, a well-known bias of the PRN regimen. On average patients had 12.5 visits and 7.31 injections. Patients are therefore under-treated compared to the French recommendations; this could be partially due to the PRN regimen. Under-treatment was also reported in another three-year French prospective real-world study of ranibizumab in DME that showed fewer visits (mean 13.4) and injections (mean 5.1) than recommended in year 1, decreasing further in subsequent two years.15
One third of patients in ETOILE were lost to follow-up by M24, for any reason. This rate was similar to previous real-world studies by Best et al (25.45% after one year of follow-up)16 and Massin et al (35.5% after three years).15 In ETOILE, 25.8% of patients had switched to other intravitreal therapy by M24. A similar rate of switching was observed after 4 years (25.4%) in a Danish retrospective cohort of 566 DME patients.17 In this study, as observed in ETOILE, there was no difference in baseline VA between the switchers and non-switchers, but switchers had higher baseline CRT. This phenomenon of switching treatments is not well documented.
ETOILE shows that ranibizumab achieves good visual results in real life. BCVA gains were lower than those observed in RCTs but were achieved with fewer injections. Moreover, the ETOILE cohort had a bad prognosis at baseline; with nearly half of patients showing loss of integrity of external limiting membrane and the internal/external segment. On average, patients in ETOILE had a one-year average delay between DME diagnosis and treatment initiation.
Our results support other real-world prospective studies in DME. A prospective study with 1226 participants (of whom 738 completed the 2-year follow-up) reported a mean baseline VA of 60.9 letters [95% CI: 59.7, 61.5].18 VA improved by +4.0 and +5.2 letters at M12 and M24, respectively. Patients received a mean of 4.4 injections in year 1 and 5.5 injections over the 2 years. Massin reported mean BCVA gains of +4.1 letters after 36 months.15 However, in this cohort, only half of the patients who completed the 36-month follow-up had been treated with ranibizumab only, the remaining patients switched to other intravitreal drugs. The ETOILE cohort had similar baseline BCVA and gains at M24, and similar numbers of injections.
Most of the published real-world studies are retrospective. In a study of ranibizumab in 78 patients (106 eyes),19 baseline BCVA was 48.3 letters (notably lower than ETOILE’s 58.4 letters). Mean BCVA gain was +10.7 letters at M12, with significant CRT reductions. On average, patients had 5.4 injections. This study demonstrated better BCVA gains than ETOILE, although the 38% of patients with BCVA >70 letters at M12 was similar to ETOILE (40%).
In a retrospective study in 80 patients (102 eyes), baseline mean BCVA was 60.8 letters.20 BCVA gain was +6.2 letters after the 3 initial induction doses and +7.0 letters at M24. Gains were sustained over the 4-year follow-up (+6.6 letters). Average injections per year was 4.7 in year 1, decreasing to 1.4, 0.7 and 0.9 during years 2, 3 and 4. These results are similar to ETOILE.
A real-world observational study using UK electronic medical records evaluated visual outcomes for DME patients (3103 eyes) treated with ranibizumab.21 Mean baseline BCVA for patients followed ≥2 years was 51.1 letters. Mean BCVA gain at M12 was +5 letters and 33% of patients had mean VA ≥70 letters compared to 25% at baseline. Despite a lower mean baseline VA, these BCVA gains are lower than in ETOILE. However, eyes followed for ≥6 months received only a mean of 3.3 injections over a mean of 6.9 surveillance visits in 1 year. Other ranibizumab real-world studies also reported low numbers of ranibizumab injections.17,22
ETOILE used multivariate ANOVA to identify two factors predictive of BCVA gains at M24. These were early BCVA gain of ≥6 letters in the first 3 months of follow-up and at least 1 hypertension medication at baseline. Early BCVA response and higher baseline BCVA were previously identified as predictors of better BCVA outcomes with ranibizumab.15,19,23 In post-hoc analyses of the RISE and RIDE studies, good baseline BCVA was a predictor of a good visual outcome of 20/40 or better. However, poor baseline BCVA was associated with an improvement in BCVA of ≥15 letters.24 Since better baseline BCVA and CRT values predicted better BCVA and CRT after 12 months, it has been suggested that ranibizumab should be initiated before BCVA and CRT deteriorate.
ETOILE gives insight into the real-life use of ranibizumab in France. The focus on France could limit the applicability of results to other countries. Bias could exist because ETOILE pre-selected investigators capable of administering ranibizumab by intravitreal injection. However, including inexperienced ophthalmologists would have been unethical. As an observational study, the data are inherently variable; however, data underwent strict validation and quality control steps. Another limitation was the variation in scales used to assess BCVA. However, this variation is a general limitation of real-world study designs. Conversion of results to ETDRS letters has been used in other studies of anti-VEGF in DME.15,18,19
Conclusion
In conclusion, this 24-month prospective observational study showed that ranibizumab treatment under normal clinical conditions improved VA in DME patients, confirming previous clinical studies with restricted populations. Induction with 3 injections one month apart is not yet performed systematically. VA gain at the end of the 3-month induction seems to predict the VA outcomes after 24 months of treatment.
Abbreviations
AE, adverse event; AMD, age-related macular degeneration; ANOVA, analysis of variance; BCVA, best corrected visual acuity; CCTIRS, Comité Consultatif sur le Traitement de l’Information en matière de Recherche dans le domaine de la Santé; CI, confidence interval; CRT, central retinal thickness; DME, diabetic macular edema; DR, diabetic retinopathy; EMA, European Medicines Agency; ETDRS, early treatment diabetic retinopathy study; HbA1c, glycosylated hemoglobin; LOCF, last observation carried forward; M, month; PRN, pro re nata; RCT, randomized controlled trial; SAE, serious adverse event; SD, standard deviation; SD-OCT, spectral domain optical coherence tomography; T&E, treat and extend; VA, visual acuity; VEGF, vascular endothelial growth factor.
Data Sharing Statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
Acknowledgments
Medical writing assistance was funded by Novartis and was provided by Dr. Fiona Dunlevy and Matrix Consultants, who prepared a manuscript draft for the named authors to edit.
Funding
This study was funded by Novartis Pharma SAS, Rueil-Malmaison, France, who participated in the design of the study, conduct, data collection, data management, data analysis, interpretation of the data, preparation, review and approval of the manuscript.
Disclosure
AP is an employee of Novartis Pharma SAS, France. LK received consulting fees from Novartis related to the current paper and reports personal fees from Allergan, AbbVie, Alimera, Horus, Bayer, Novartis, Roche, and Thea, during the conduct of the study. ALC reports payment for enrollment of patients and data from Novartis, during the conduct of the study; consultant for Novartis, Bayer, and Allergan, outside the submitted work. CD reports personal fees for consulting from AbbVie, Bayer, Novartis, and Horus, during the conduct of the study. SB is a board member for Allergan, Novartis, Bayer and Horus Pharma, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Klein R, Lee KE, Gangnon RE, Klein BE. The 25-year incidence of visual impairment in type 1 diabetes mellitus the Wisconsin epidemiologic study of diabetic retinopathy. Ophthalmology. 2010;117:63–70. doi:10.1016/j.ophtha.2009.06.051
2. Moss SE, Klein R, Klein BE. Ten-year incidence of visual loss in a diabetic population. Ophthalmology. 1994;101:1061–1070. doi:10.1016/S0161-6420(94)31217-6
3. Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564. doi:10.2337/dc11-1909
4. Massin P, Bandello F, Garweg JG, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter Phase II study. Diabetes Care. 2010;33:2399–2405. doi:10.2337/dc10-0493
5. Prünte C, Fajnkuchen F, Mahmood S, et al. Ranibizumab 0.5 mg treat-and-extend regimen for diabetic macular oedema: the RETAIN study. Br J Ophthalmol. 2016;100::787–795. doi:10.1136/bjophthalmol-2015-307249
6. Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–625. doi:10.1016/j.ophtha.2011.01.031
7. Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 Phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi:10.1016/j.ophtha.2011.12.039
8. Diabetic Retinopathy Clinical Research Network; Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–1077 e1035. doi:10.1016/j.ophtha.2010.02.031
9. Nguyen QD, Shah SM, Heier JS, et al. Primary end point (six months) results of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2009;116:2175–2181 e2171. doi:10.1016/j.ophtha.2009.04.023
10. Nguyen QD, Shah SM, Khwaja AA, et al. Two-year outcomes of the ranibizumab for edema of the macula in diabetes (READ-2) study. Ophthalmology. 2010;117:2146–2151. doi:10.1016/j.ophtha.2010.08.016
11. Elman MJ, Bressler NM, Qin H, et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118:609–614. doi:10.1016/j.ophtha.2010.12.033
12. Schmidt-Erfurth U, Lang GE, Holz FG, et al. Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension study. Ophthalmology. 2014;121:1045–1053. doi:10.1016/j.ophtha.2013.11.041
13. Kodjikian L, Bellocq D, Mathis T. Pharmacological management of diabetic macular edema in real-life observational studies. Biomed Res Int. 2018;2018:8289253. doi:10.1155/2018/8289253
14. Creuzot-Garcher C, Massin P. Œdèmes maculaires (Société Française d’Ophtalmologie). 2016.
15. Massin P, Creuzot-Garcher C, Kodjikian L, et al. Real-world outcomes after 36 months treatment with ranibizumab 0.5 mg in patients with visual impairment due to diabetic macular edema (BOREAL-DME). Ophthalmic Res. 2020. doi:10.1159/000511591
16. Best AL, Fajnkuchen F, Nghiem-Buffet S, et al. Treatment efficacy and compliance in patients with diabetic macular edema treated with ranibizumab in a real-life setting. J Ophthalmol. 2018;2018:4610129. doi:10.1155/2018/4610129
17. Hodzic-Hadzibegovic D, Sander BA, Monberg TJ, et al. Diabetic macular oedema treated with intravitreal anti-vascular endothelial growth factor – 2–4 years follow-up of visual acuity and retinal thickness in 566 patients following Danish national guidelines. Acta Ophthalmol. 2018;96:267–278. doi:10.1111/aos.13638
18. Ziemssen F, Wachtlin J, Kuehlewein L, et al. Intravitreal ranibizumab therapy for diabetic macular edema in routine practice: two-year real-life data from a non-interventional, multicenter study in Germany. Diabetes Ther. 2018;9:2271–2289. doi:10.1007/s13300-018-0513-2
19. Hrarat L, Fajnkuchen F, Boubaya M, et al. Outcomes after a 1-year treatment with ranibizumab for diabetic macular edema in a clinical setting. Ophthalmologica. 2016;236:207–214. doi:10.1159/000453006
20. Epstein D, Amren U. Long-time outcome in patients treated with ranibizumab for diabetic macular edema: a 4-year study. Retina. 2018;38:183–186. doi:10.1097/IAE.0000000000001501
21. Egan C, Zhu H, Lee A, et al. The United Kingdom Diabetic Retinopathy Electronic Medical Record Users Group, Report 1: baseline characteristics and visual acuity outcomes in eyes treated with intravitreal injections of ranibizumab for diabetic macular oedema. Br J Ophthalmol. 2017;101:75–80. doi:10.1136/bjophthalmol-2016-309313
22. Adelman R, Parnes A, Michalewska Z, et al. Strategy for the management of diabetic macular edema: the European vitreo-retinal society macular edema study. BioMed Res Int. 2015;2015:352487.
23. Wells JA, Glassman AR, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193–1203.
24. Sophie R, Lu N, Campochiaro PA. Predictors of functional and anatomic outcomes in patients with diabetic macular edema treated with ranibizumab. Ophthalmology. 2015;122:1395–1401. doi:10.1016/j.ophtha.2015.02.036
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

