Back to Journals » OncoTargets and Therapy » Volume 12
Ethyl acetate extracts of Nepenthes ventricosa x sibuyanensis leaves cause growth inhibition against oral cancer cells via oxidative stress
Authors Tang JY, Yu TJ, Lin LC, Peng SY, Wang CL, Ou-Yang F, Cheng YB , Chang HW
Received 11 October 2018
Accepted for publication 11 March 2019
Published 3 July 2019 Volume 2019:12 Pages 5227—5239
DOI https://doi.org/10.2147/OTT.S190460
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr William C. Cho
Jen-Yang Tang,1,2 Tzu-Jung Yu,3 Li-Ching Lin,4–6 Sheng-Yao Peng,3 Chun-Lin Wang,7 Fu Ou-Yang,8,9 Yuan-Bin Cheng,10 Hsueh-Wei Chang9,11–13
1Department of Radiation Oncology, Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung 80708, Taiwan; 2Department of Radiation Oncology, Kaohsiung Medical University Hospital, Kaohsiung 80708, Taiwan; 3Graduate Institute of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung 80708, Taiwan; 4Department of Radiation Oncology, Chi-Mei Foundation Medical Center, Tainan 71004, Taiwan; 5School of Medicine, Taipei Medical University, Taipei 11031, Taiwan; 6Department of Optometry, Chung Hwa University of Medical Technology, Tainan 71703, Taiwan; 7Bioresource Collection and Research Center, Food Industry Research and Development Institute, Hsinchu 30062, Taiwan; 8Division of Breast Surgery, Department of Surgery, Kaohsiung Medical University Hospital, Kaohsiung 80708, Taiwan; 9Cancer Center, Kaohsiung Medical University Hospital, Kaohsiung 80708, Taiwan; 10Graduate Institute of Natural Products, Kaohsiung Medical University, Kaohsiung 80708, Taiwan; 11Institute of Medical Science and Technology, National Sun Yat-sen University, Kaohsiung 80424, Taiwan; 12Department of Biomedical Science and Environmental Biology, Kaohsiung Medical University, Kaohsiung 80708, Taiwan; 13Drug Development and Value Creation Research Center, Kaohsiung Medical University, Kaohsiung 80708, Taiwan
Introduction: The genus Nepenthes of the pitcher plants contains several natural and hybrid species that are commonly used in herbal medicine in several countries, but its possible use in cancer applications remains unknown as yet.
Methods: In this study, we investigated the antioral cancer properties using ethyl acetate extracts of the Nepenthes hybrid (Nepenthes ventricosa x sibuyanensis), namely EANS. The bioactivity was detected by a MTS-based cell proliferation assay and flow cytometric or Western blot analysis for apoptosis, oxidative stress, and DNA damage.
Results: Treatment for 24 hrs of EANS inhibited all three types of oral cancer cells that were tested (Ca9-22, CAL 27, and SCC9), with just a small difference to normal oral cells (HGF-1). This antiproliferation was inhibited by pretreatments with the reactive oxygen species (ROS) scavenger N-acetylcysteine (NAC), and the apoptosis inhibitor (Z-VAD). EANS treatment increased the subG1 population and it also dose- and time-dependently induced annexin V- and pancaspase-detected apoptosis as well as cleaved caspases 3 and 9 overexpressions in the oral cancer cells (Ca9-22). After EANS treatment of Ca9-22 cells, intracellular ROS and mitochondrial superoxide (MitoSOX) were overexpressed and mitochondrial membrane potential (MMP) was disrupted. Moreover, DNA damages such as γH2AX and 8-oxo-2ʹ-deoxyguanosine (8-oxodG) were increased after EANS treatment to Ca9-22 cells. The EANS-induced effects (namely, oxidative stress, apoptosis, and DNA damage) were suppressed by ROS scavenger.
Conclusion: Our findings demonstrated that EANS inhibits ROS-mediated proliferation against oral cancer cells.
Keywords: Nepenthes, oral cancer, oxidative stress, apoptosis, DNA damage
Introduction
Oral cancer is one of the main threats to public health worldwide, especially for patients with the habits of alcohol drinking, betel quid-chewing, or smoking.1 In general, oral cancer patients have 50% of five-year survival.2 The traditional therapy for oral cancer is surgery with or without chemo- or radiotherapy,2 which occasionally associates with side effects.3 Therefore, there is a challenge to use alternative or supplementary therapy for cancer treatment without side effects.
Recently, chemoprevention effects of natural products against oral cancer cells have been emphasized.4–8 Many natural product-derived bioactive compounds9-12 have shown anticancer potential because of their high cytotoxicity to cancer cells and low toxicity to normal cells. Moreover, cancer progression is known to involve multiple signaling pathways and different drugs may have differential targets. Therefore, it is important and helpful to identify more natural product candidates for oral cancer therapy.
Nepenthes plants are tropical plants with pitcher-shaped leaves that trap animal victims for the nutrient provision of these carnivorous plants. The genus contains a number of original species, and natural or manmade hybrids that increase its diversity to more than 170 species.13 Nepenthes plants have a wide range of habitats located in the tropical belt of Australia, Madagascar, Papua New Guinea, the Seychelles, and Southeast Asia. Several representatives have been used for herbal medicine in several countries. For example, N. mirabilis is famous for its treatments against cough, jaundice, fever, hypertension,14 and inflammation.15 Cell model studies showed that Nepenthes plant extracts based on several solvents suppressed the growth of certain bacteria16 and fungi.17 However, the anticancer effect of Nepenthes plants remains unclear.
Nepenthes plants are rich in antioxidant components. For example, N. mirabilis and N. gracilis were reported to contain flavonoids18 and phenolic compounds,19 respectively. Methanolic extracts of N. bicalcarata leaves also displayed high antioxidant properties.20 Antioxidants have a potential for oral cancer prevention.21 Hence, possible anticancer effects of Nepenthes plants warrant in-depth investigation. Moreover, the ethyl acetate extraction for Nepenthes plants is rarely investigated. Therefore, we focused on evaluating the antioral cancer effect of Nepenthes plants. Using ethyl acetate extract of N. ventricosa x sibuyanensis (EANS), the changes of cell viability, apoptosis, oxidative stress, and DNA damage were investigated using oral cancer cells.
Materials and methods
Plant materials, ethyl acetate extract, and drug inhibitors
Species identification and sample collection of Nepenthes species (N. ventricosa x sibuyanensis) was performed by Mr. Jui-Hsuan Kuo in the Dr. Celica Koo Botanic Conservation Center (KBCC), Taiwan, in October, 2014. The voucher sample (K45532) was air-dried and deposited in the Graduate Institute of Natural Products, Kaohsiung Medical University. N. ventricosa x sibuyanensis twigs and leaves (210 g) were soaked in methanol (1 L) to provide crude extract. Subsequently, this was partitioned between water and EtOAc. Finally, the EtOAc layer, namely EANS, was harvested (96 mg) and stored at 4°C. All treatments with or without EANS had the same concentration of dimethyl sulfoxide (DMSO) (Sigma-Aldrich; St. Louis, MO, USA) as a carrier of the active compounds.
In subsequent experiments with EANS, several kinds of inhibitors were pretreated as follows: Free radical scavenger N-acetylcysteine (NAC) (2 mM, 1 hr) (Sigma),22 pan-caspase inhibitor Z-VAD-FMK (Z-VAD) (20 μM, 2 hrs) (Selleckchem. com; Houston, TX, USA),11 and mitochondrial superoxide inhibitor Mito TEMPO (20 μM, 1 hr) (Cayman Chemical, Ann Arbor, Michigan, USA).23
Cell culture and cell viability
Oral cancer (Ca9-22, SCC9, and CAL 27) and normal oral (HGF-1) cells were used as described previously.24 All cell lines were purchased from American Type Culture Collection (ATCC) and the Japanese Collection of Research Bioresources (JCRB) Cell Bank. Cells were incubated (37°C, 5% CO2) in a humidified atmosphere and maintained with Dulbecco’s Modified Eagle Medium (DMEM)/Nutrient Mixture F-12 (Gibco, Grand Island, NY, USA) at 3:2 (oral cancer cells) and 4:1 (HGF-1 cells) ratio with 10% fetal bovine serum and common antibiotics (Gibco). Cell viability is measured by mitochondrial-activity-based cell proliferation MTS assay (Promega Corporation, Madison, WI) as described previously.25
Cell cycle phases
Cellular DNA content was measured by 7-aminoactinmycin D (7AAD) (Biotium, Inc., Hayward, CA, USA) staining and is commonly used for cell cycle phase analysis as previously described.26 After 75% ethanol fixation, cells were stained with 7AAD (1 μg/mL in phosphate-buffered saline (PBS), 30 mins) for Accuri™ C6 flow cytometry (Becton-Dickinson, Mansfield, MA, USA). The G1, S, and G2/M populations added up to 100% where the sub-G1 population was calculated separately as described before.27
Annexin V/7AAD apoptosis assay
Apoptosis was analyzed by the phosphatidylserine-based annexin V kit (Strong Biotech Corporation, Taipei, Taiwan) as described previously.28 Cells were double-stained with annexin V-labeled with FITC (10 μg/mL) and 7AAD (1 μg/mL) for 30 mins. Finally, cells were analyzed by Accuri™ C6 flow cytometry.
Caspase activity assays: pancaspase flow cytometry and Western blotting
Pancaspase activity (caspases-1, 3–9) was analyzed by a generic caspase activity assay kit (Abcam, Cambridge, UK) using flow cytometry as previously described.29 After treatment with 0.5X TF2-VAD-FMK for 2 hrs, cells were washed and resuspended for Accuri™ C6 flow cytometry.
Protein lysates (45 μg) were performed with 8% SDS-PAGE and transferred to a PVDF membrane for 5% nonfat milk blocking overnight. Apoptosis antibodies (diluted 1:1,000) were chosen for Western blotting, including: cleaved forms of caspase 3 (c-cas 3) and caspase 9 (c-cas 9) from the Apoptosis Antibody Sampler Kit and cleaved caspase 8 (c-cas 8) (1C12) (Cell Signaling Technology, Inc., Danvers, MA, USA). Loading control was mAb-β-actin (Sigma-Aldrich). WesternBright™ enhanced chemiluminescence (ECL) horseradish peroxidase (HRP) kit (Advansta, Menlo Park, CA, USA) was used for detecting HRP activity to secondary antibody.
Cellular ROS and mitochondrial superoxide (MitoSOX) assays
The reactive oxygen species (ROS)-reacting chemical 2ʹ,7ʹ-dichlorodihydrofluorescein diacetate (DCFH-DA) (Sigma-Aldrich, St. Louis, MO, USA) was oxidized by ROS and became fluorescent for analysis by flow cytometry.30,31 After treatment with DCFH-DA (10 μM, 37°C, 30 mins), cells were analyzed by flow cytometry (Accuri™ C6). Similarly, superoxide-reacting dye MitoSOX Red (Molecular Probes, Invitrogen, Eugene, OR, USA) was specifically oxidized with mitochondrial superoxide32 and became a fluorescent chemical for flow cytometry.33 After treatment with MitoSOX™ Red (5 μM, 37°C, 30 mins), cells were analyzed by flow cytometry (Accuri™ C6).
Mitochondrial membrane potential (MMP) assay
A membrane-potential-sensitive cyanine dye DiOC2(3) (Invitrogen, San Diego, CA, USA) was used for MMP analysis as previously described.34 After treatment with DiOC2 (3) (50 nM, 30 mins), cells were analyzed by flow cytometry (Accuri™ C6).
γH2AX DNA damage assay
Flow cytometry indications using the DNA double-strand break marker (γH2AX) was described in an earlier study.9 After treatment with primary antibody p-Histone H2A.X (Ser 139) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) (1:50 dilution, 4°C, 1 hr), the secondary antibody was further reacted for 30 mins for Accuri™ C6 flow cytometry.
8-Oxo-2ʹ-deoxyguanosine (8-oxodG) assay
For flow cytometric detection, the antibody from fluorometric OxyDNA kit (EMD Millipore, Darmstadt, Germany) was added to recognize 8-oxodG as described previously.35 After fixation and washing, cells were immersed in 10X diluted FITC-labeled antibody for 1 hr and were subsequently resuspended in PBS buffer for Accuri™ C6 flow cytometry.
Statistical analysis
Significant differences between multiple comparisons were determined by one-way analysis of variance (ANOVA) and the Tukey HSD test (JMP12; SAS Institute, Cary, NC, USA). Data analysis was performed in triplicate and presented as mean ± SD. Different groups without the same small letters indicate significant differences.
Results
Effect of EANS on cell viability
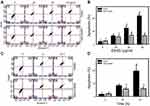
In this study, HGF-1 cells were used as a normal control compared to three oral cancer cell lines, ie, Ca9-22, CAL 27, and SCC9. In MTS assay, EANS dose-dependently reduced the viability (%) of these oral cancer cells (Figure 1A). However, EANS-treated normal oral cells (HGF-1) showed less cytotoxic effect (~80% viability) compared to oral cancer cells.
The EANS-inhibiting viabilities of those test cells were significantly suppressed by NAC pretreatment (p<0.05~0.0001) (Figure 1B). In the example of Ca9-22 cells, the EANS-inhibiting viabilities of oral cancer cells were suppressed by a pancaspase inhibitor Z-VAD pretreatment (Figure 1C).
Effect of EANS on cell cycle distributions of oral cancer cells
Proliferation, cell cycle progression, and apoptosis may interact between each other.36 Therefore, we investigated whether EANS may influence cell cycle progression. DNA histograms of different concentrations of EANS treatments in oral cancer cells were shown in Figure 2A. Incubation of Ca9-22 cells for 24 hrs with EANS dose-dependently increased the sub-G1 and G2/M populations, and decreased the G1 population, but it showed little change in the S population (Figure 2B). Furthermore, those EANS-affected cell cycle disturbances of Ca9-22 cells were suppressed by NAC.
Effect of EANS on annexin V-based apoptosis of oral cancer cells
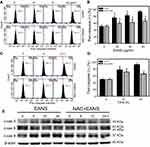
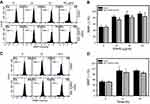
To confirm whether the subG1 accumulation of EANS on Ca9-22 cells was related to apoptosis, we detected apoptosis using phosphatidylserine exposure-dependent annexin V/7AAD flow cytometry. Annexin V/7AAD dot-plot graphs of different concentrations and time course of EANS treatments in Ca9-22 cells were shown in Figure 3A and C. EANS increased apoptosis in terms of annexin V (+) % in Ca9-22 cells in a dose- and time-dependent manner (Figure 3B and D). Furthermore, those EANS-induced apoptosis expressions of Ca9-22 cells were suppressed by NAC.
Effect of EANS on caspases-based apoptosis of oral cancer cells
To further confirm apoptosis-inducing ability of EANS on Ca9-22 cells, we detected caspase activation using flow cytometry. Pancaspase graphs of different concentrations and time course of EANS treatments in Ca9-22 cells were shown in Figure 4A and C. EANS increased apoptosis in terms of pancaspase (+) % in Ca9-22 cells in a dose- and time-dependent manner (Figure 4B and D). Furthermore, those EANS-induced pancaspase (apoptosis) activations of Ca9-22 cells were suppressed by NAC.
Since the applied pancaspase kit was developed to generically detect the pancaspase activity (caspases (cas)-1, 3, 4, 5, 6, 7, 8, 9),29 it was necessary to further examine the involvement of caspase signaling for apoptosis. Therefore, we detected several members of caspase signaling by Western blotting. After EANS treatment to Ca9-22 cells, the cleaved forms of caspases such as c-cas 3 and c-cas 9 expressions were increased but c-cas 8 was undetectable (Figure 4E), suggesting that EANS mainly induced intrinsic apoptosis in Ca9-22 cells. Furthermore, those EANS-induced expressions of apoptosis signaling proteins of Ca9-22 cells were suppressed by NAC.
Effect of EANS on ROS generation of oral cancer cells
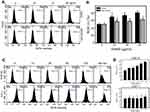
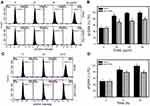
Excess ROS may induce DNA damage and apoptosis.37 Therefore, we investigated whether EANS may influence intracellular ROS generation. ROS graphs of different concentrations of EANS treatments in Ca9-22 cells were shown in Figure 5A. EANS treatment to Ca9-22 cells enhanced the ROS generation in a dose-dependent manner (Figure 5B). Furthermore, those EANS-induced ROS generation of Ca9-22 cells were suppressed by NAC.
A time course of ROS development after EANS treatments in oral cancer cells (Ca9-22) and normal oral cells (HGF-1) is shown in Figure 5C. As shown in Figure 5D, EANS dramatically induces ROS generation of Ca9-22 cells in a time-dependent manner within 3 hrs. In contrast, HGF-1 cells maintain basal level of ROS after the same treatment by EANS.
Effect of EANS on the MitoSOX generation of oral cancer cells
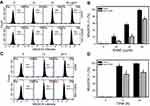
Oxidative stress is mainly produced in mitochondria,38 especially for mitochondrial superoxide. Therefore, we examined MitoSOX upon EANS treatment. MitoSOX graphs of different concentrations and time course of EANS treatments in Ca9-22 cells are shown in Figure 6A and C. EANS increases MitoSOX (+) % in Ca9-22 cells in a dose- and time-dependent manner (Figure 6B and D). Furthermore, those EANS-induced MitoSOX generations of Ca9-22 cells were suppressed by NAC.
Effect of EANS on MMP of oral cancer cells
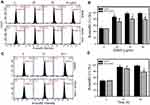
Oxidative stress generated from mitochondria may regulate apoptotic signaling.39 Therefore, we examined MMP upon EANS treatment. MMP graphs of different concentrations and time course of EANS-treated Ca9-22 cells are shown in Figure 7A and C. EANS increased MMP (–) % in Ca9-22 cells in a dose- and time-dependent manner (Figure 7B and D). Furthermore, those EANS-induced MMP destructions of Ca9-22 cells are suppressed by the pretreatment of MitoSOX-specific inhibitor Mito TEMPO.
Effect of EANS on γH2AX-based DNA damage of oral cancer cells
To investigate the effects of EANS on inducting apoptosis in Ca9-22 cells, DNA damage in terms of γH2AX were evaluated. γH2AX graphs of different concentrations and time course of EANS treatments in Ca9-22 cells were shown in Figure 8A and C. EANS increased γH2AX (+) % in Ca9-22 cells in a dose- and time-dependent manner (Figure 8B and D). Furthermore, those EANS-induced γH2AX expressions of Ca9-22 cells were suppressed by NAC.
Effect of EANS on 8-oxodG-based DNA damage of oral cancer cells
To investigate the effects of EANS on inducting apoptosis in Ca9-22 cells, oxidative DNA damage in terms of 8-oxodG were evaluated. 8-OxodG graphs of different concentrations and time course of EANS treatments in Ca9-22 cells were shown in Figure 9A and C. EANS increased 8-oxodG (+) % in Ca9-22 cells in a dose- and time-dependent manner (Figure 9B and D). Furthermore, those EANS-induced 8-oxodG expressions were suppressed by NAC.
Discussion
Nepenthes plants are used for herbal medicine14,15 and display diverse biological effects against bacteria16 and fungi.17 However, an anticancer effect of Nepenthes plants remains unclear. Moreover, different solvents were used to extract Nepenthes plant in earlier studies20,40,41 with the exception of ethyl acetate which was used here for the first time.
In this study, we used ethyl acetate extract of N. ventricosa x sibuyanensis (EANS) to evaluate the antiproliferative effect for oral cancer cells. Incubation of oral cancer cells (Ca9-22, CAL 27, and SCC9) for 24 hrs with EANS show IC50 values with 25, 20, and 32 μg/mL. Incubation of normal oral cells (HGF-1) for 24 hrs with the highest test concentration of EANS (40 μg/mL) shows about 80% viability. Therefore, EANS has higher cytotoxicity against oral cancer cells than normal oral cells. The antiproliferative effect of EANS treatment against oral cancer cells was suppressed by the ROS scavenger NAC and the pancaspase inhibitor Z-VAD (Figure 1), suggesting that oxidative stress and apoptosis play vital roles in the antioral cancer effect of EANS. It should be emphasized that the concentration of EANS used in this study was based on a cell line model and may not be suitable to apply to blood or tissue levels.
Although antioxidants at normal concentration may reduce the ROS generation, antioxidants at high concentration may induce intracellular ROS generation.42 Many antioxidant components were reported from Nepenthes plants.18–20 In our test concentrations (10–40 μg/mL), 3 hrs incubation of EANS enhanced ROS generation in oral cancer cells (Ca9-22) ranging from 70% to 80% ROS (+) (Figure 5B). Moreover, 2 hrs incubation of EANS (40 μg/mL) induced 80% ROS (+) of ROS generation in Ca9-22 cells (Figure 5C). Accordingly, Nepenthes plants containing antioxidant constitutes may have concentration effects on ROS induction in Ca9-22 cells. In contrast, 3 hrs incubation of EANS (40 μg/mL) maintained basal level of ROS generation in normal oral cells (HGF-1) (Figure 5C), suggesting on differential ROS induction between oral cancer and normal oral cells. Since ROS may induce apoptosis43 and DNA damage,44 this differential ROS induction may partly explain the differential killing against oral cancer cells but less cytotoxic effects on normal oral cells.
In addition to ROS generation, EANS also induce MitoSOX generation and MMP destruction in oral cancer cells. Therefore, EANS induces oxidative stress against oral cancer cells. These inductions were inhibited by NAC or Mito TEMPO pretreatments, which further supported that oxidative stress was involved in antioral cancer effect of EANS. Furthermore, EANS-induced subG1 accumulation (Figure 2), annexin V-detected apoptosis (Figure 3), pancaspase-detected apoptosis (Figure 4B and 4D), and caspase signaling activation (Figure 4E) was also inhibited by NAC pretreatment. Accordingly, the role of oxidative stress in these EANS-induced apoptosis-related effects was validated.
Excessive oxidative stress frequently induces several types of DNA damages such as DNA double-strand breaks (γH2AX)44 and oxidative DNA damage (8-oxodG).45 Consistently, we found that EANS-induced oxidative stress, in turn, induced γH2AX and 8-oxodG expressions, which were inhibited by NAC pretreatment (Figures 8 and 9). Again, the function of oxidative stress in EANS-induced ROS-mediated DNA damage was validated. However, the signaling transduction for EANS-induced oxidative stress was not examined in the present study. It was reported that oxidative stress may activate mitogen-activated protein kinase (MAPK) signaling46 and in turn regulate apoptosis47 and DNA damage.48,49 A detailed investigation of the effect of MAPK of antioral cancer effects caused by EANS is warranted in the future.
Conclusion
The antioral cancer effect of Nepenthes plants is reported here for the first time. In this study, we demonstrated that ethyl acetate extraction for N. ventricosa x sibuyanensis (EANS) preferentially inhibited the proliferation of oral cancer cells but showed little effect on normal oral cells. Oxidative stress detected by intracellular ROS and mitochondrial superoxide were also induced by EANS treatment for oral cancer cells. Oxidative stress-induced apoptosis and DNA damage appeared in EANS-treated oral cancer cells. Using NAC pretreatment to oral cancer cells, we further demonstrated that the EANS-induced antiproliferation, oxidative stress-associated changes, apoptosis, and DNA damages were mediated by ROS.
Acknowledgments
This work was partly supported by funds from the Ministry of Science and Technology (MOST 107-2320-B-037-016; MOST 107-2628-B-037-001; MOST 107-2314-B-037-048; and MOST 107-2314-B-037-057), the National Sun Yat-sen University-Kaohsiung Medical University Joint Research Project (#NSYSUKMU 108-P001), the Kaohsiung Medical University Hospital (KMUH105-5M24 and KMUH107-7R74), the Ministry of Economic Affairs (107-EC-17-A-22-0643 and 107-EC-17-A-22-0525), the Kaohsiung Medical University Research Center (KMU-TC108A03), the Chimei-KMU Jointed Project (108CM-KMU-11), and the Health and Welfare Surcharge of Tobacco Products, the Ministry of Health and Welfare, Taiwan, Republic of China (MOHW108-TDU-B-212-124016). The authors thank our colleague Dr. Hans-Uwe Dahms for editing the English of this manuscript.
Disclosure
The authors declare that they have no conflicts of interest in regard to this work.
References
1. Ko YC, Huang YL, Lee CH, Chen MJ, Lin LM, Tsai CC. Betel quid chewing, cigarette smoking and alcohol consumption related to oral cancer in Taiwan. J Oral Pathol Med. 1995;24(10):450–453.
2. Danaraddi S, Koneru A, Hunasgi S, Ramalu S, Vanishree M. Natural ways to prevent and treat oral cancer. J Oral Res Rev. 2014;6(1):34–39. doi:10.4103/2249-4987.140213
3. Silverman S
4. Singh A, Tripathi P. Potential of natural products for the prevention of oral cancer. In: Akhtar MS, Swamy MK, editors. Anticancer Plants: Natural Products and Biotechnological Implements. Vol. 2. Singapore: Springer Singapore; 2018:41–66.
5. Lee JC, Hou MF, Huang HW, et al. Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell Int. 2013;13(1):55. doi:10.1186/1475-2867-13-55
6. Pereira LO, Bicalho LS, Campos-da-Paz Lopes M, et al. DNA damage and apoptosis induced by Pteridium aquilinum aqueous extract in the oral cell lines HSG and OSCC-3. J Oral Pathol Med. 2009;38(5):441–447. doi:10.1111/j.1600-0714.2008.00705.x
7. Lin MH, Liu YC, Liu SY, et al. Clathrin-mediated endocytosis is required for ANE 30-100K-induced autophagy. J Oral Pathol Med. 2018;47(1):25–31. doi:10.1111/jop.12593
8. Shin JA, Ryu MH, Kwon KH, Choi B, Cho SD. Down-regulation of Akt by methanol extracts of Impatiens balsamina L. promotes apoptosis in human oral squamous cell carcinoma cell lines. J Oral Pathol Med. 2015;44(6):420–428. doi:10.1111/jop.12248
9. Chiu CC, Haung JW, Chang FR, et al. Golden berry-derived 4beta-hydroxywithanolide E for selectively killing oral cancer cells by generating ROS, DNA damage, and apoptotic pathways. PLoS One. 2013;8(5):e64739. doi:10.1371/journal.pone.0064739
10. Yen CY, Hou MF, Yang ZW, et al. Concentration effects of grape seed extracts in anti-oral cancer cells involving differential apoptosis, oxidative stress, and DNA damage. BMC Complement Altern Med. 2015;15(1):94. doi:10.1186/s12906-015-0621-8
11. Chen CY, Yen CY, Wang HR, et al. Tenuifolide B from Cinnamomum tenuifolium stem selectively inhibits proliferation of oral cancer cells via apoptosis, ROS generation, mitochondrial depolarization, and DNA damage. Toxins (Basel). 2016;8(11):319. doi:10.3390/toxins8110319
12. Chang YT, Wu CY, Tang JY, et al. Sinularin induces oxidative stress-mediated G2/M arrest and apoptosis in oral cancer cells. Environ Toxicol. 2017;32(9):2124–2132. doi:10.1002/tox.22425
13. Christenhusz MJM, Byng JW. The number of known plants species in the world and its annual increase. Phytotaxa. 2016;261(3):201–217. doi:10.11646/phytotaxa.261.3.1
14. Thanh NV, Thao NP, Dat le D, et al. Two new naphthalene glucosides and other bioactive compounds from the carnivorous plant Nepenthes mirabilis. Arch Pharm Res. 2015;38(10):1774–1782. doi:10.1007/s12272-015-0576-9
15. Thao NP, Luyen BT, Koo JE, et al. In vitro anti-inflammatory components isolated from the carnivorous plant Nepenthes mirabilis (Lour.) Rafarin. Pharm Biol. 2016;54(4):588–594. doi:10.3109/13880209.2015.1067234
16. Buch F, Pauchet Y, Rott M, Mithofer A. Characterization and heterologous expression of a PR-1 protein from traps of the carnivorous plant Nepenthes mirabilis.. Phytochemistry. 2014;100:43–50. doi:10.1016/j.phytochem.2014.01.014
17. Gwee PS, Khoo KS, Ong HC, Sit NW. Bioactivity-guided isolation and structural characterization of the antifungal compound, plumbagin, from Nepenthes gracilis.. Pharm Biol. 2014;52(12):1526–1531. doi:10.3109/13880209.2014.902083
18. Thanh NV, Thao NP, Huong PTT, et al. Naphthoquinone and flavonoid constituents from the carnivorous plant Nepenthes mirabilis and their anti-osteoporotic and antioxidant activities. Phytochem Lett. 2015;11:254–259. doi:10.1016/j.phytol.2015.01.009
19. Aung HH, Chia LS, Goh NK, et al. Phenolic constituents from the leaves of the carnivorous plant Nepenthes gracilis.. Fitoterapia. 2002;73(5):445–447.
20. Ismail NA, Kamariah AS, Lim LBL, Ahmad N. Phytochemical and pharmacological evaluation of methanolic extracts of the leaves of Nepenthes bicalcarata.. Int J Pharmacogn Phytochem Res. 2015;7(6):1127–1138.
21. Iqubal MA, Khan M, Kumar P, Kumar A, Ajai K. Role of vitamin E in prevention of oral cancer:-a review. J Clin Diagn Res. 2014;8(10):ZE05–07. doi:10.7860/JCDR/2014/9166.4958
22. Huang CH, Yeh JM, Chan WH. Hazardous impacts of silver nanoparticles on mouse oocyte maturation and fertilization and fetal development through induction of apoptotic processes. Environ Toxicol. 2018;33(10):1039–1049. doi:10.1002/tox.22590
23. Wang TS, Lin CP, Chen YP, Chao MR, Li CC, Liu KL. CYP450-mediated mitochondrial ROS production involved in arecoline N-oxide-induced oxidative damage in liver cell lines. Environ Toxicol. 2018;33(10):1029–1038. doi:10.1002/tox.22588
24. Chang YT, Huang CY, Li KT, et al. Sinuleptolide inhibits proliferation of oral cancer Ca9-22 cells involving apoptosis, oxidative stress, and DNA damage. Arch Oral Biol. 2016;66:147–154. doi:10.1016/j.archoralbio.2016.02.019
25. Yeh CC, Tseng CN, Yang JI, et al. Antiproliferation and induction of apoptosis in Ca9-22 oral cancer cells by ethanolic extract of Gracilaria tenuistipitata.. Molecules. 2012;17(9):10916–10927. doi:10.3390/molecules170910916
26. Vignon C, Debeissat C, Georget MT, et al. Flow cytometric quantification of all phases of the cell cycle and apoptosis in a two-color fluorescence plot. PLoS One. 2013;8(7):e68425. doi:10.1371/journal.pone.0068425
27. Chang HW, Tang JY, Yen CY, et al. Synergistic anti-oral cancer effects of UVC and methanolic extracts of Cryptocarya concinna roots via apoptosis, oxidative stress and DNA damage. Int J Radiat Biol. 2016;92(5):263–272. doi:10.3109/09553002.2016.1145753
28. Huang HW, Tang JY, Ou-Yang F, et al. Sinularin selectively kills breast cancer cells showing G2/M arrest, apoptosis, and oxidative DNA damage. Molecules. 2018;23(4):849. doi:10.3390/molecules23040849
29. Chang HW, Li RN, Wang HR, et al. Withaferin A induces oxidative stress-mediated apoptosis and DNA damage in oral cancer cells. Front Physiol. 2017;8:634. doi:10.3389/fphys.2017.00634
30. Yeh CC, Yang JI, Lee JC, et al. Anti-proliferative effect of methanolic extract of Gracilaria tenuistipitata on oral cancer cells involves apoptosis, DNA damage, and oxidative stress. BMC Complement Altern Med. 2012;12(1):142. doi:10.1186/1472-6882-12-142
31. Lu MC, Li TY, Hsieh YC, Hsieh PC, Chu YL. Chemical evaluation and cytotoxic mechanism investigation of Clinacanthus nutans extract in lymphoma SUP-T1 cells. Environ Toxicol. 2018:(In press). doi:10.1002/tox.22629
32. Mukhopadhyay P, Rajesh M, Yoshihiro K, Hasko G, Pacher P. Simple quantitative detection of mitochondrial superoxide production in live cells. Biochem Biophys Res Commun. 2007;358(1):203–208. doi:10.1016/j.bbrc.2007.04.106
33. Chang YT, Huang CY, Tang JY, et al. Reactive oxygen species mediate soft corals-derived sinuleptolide-induced antiproliferation and DNA damage in oral cancer cells. Onco Targets Ther. 2017;10:3289–3297. doi:10.2147/OTT.S138123
34. Chang HS, Tang JY, Yen CY, et al. Antiproliferation of Cryptocarya concinna-derived cryptocaryone against oral cancer cells involving apoptosis, oxidative stress, and DNA damage. BMC Complement Altern Med. 2016;16(1):94. doi:10.1186/s12906-016-1073-5
35. Tang JY, Huang HW, Wang HR, et al. 4beta-Hydroxywithanolide E selectively induces oxidative DNA damage for selective killing of oral cancer cells. Environ Toxicol. 2018;33(3):295–304. doi:10.1002/tox.22516
36. Alenzi FQ. Links between apoptosis, proliferation and the cell cycle. Brit J Biomed Sci. 2004;61(2):99–102. doi:10.1080/09674845.2004.11732652
37. Jena NR. DNA damage by reactive species: mechanisms, mutation and repair. J Biosci. 2012;37(3):503–517.
38. Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel?. Nat Rev Cancer. 2014;14(11):709–721. doi:10.1038/nrc3803
39. Fulda S, Debatin K-M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25(34):4798. doi:10.1038/sj.onc.1209608
40. Rodzali NN, Mydin MM. Antibacterial activity of leaves and pitchers extract of Nepenthes gracilis against Bacillus subtilis and Escherichia coli. J Fundam Appl Sci. 2017;9(6S):81–88. doi:10.4314/jfas.v9i6s.7
41. Shin KS, Lee S, Cha BJ. Suppression of phytopathogenic fungi by hexane extract of Nepenthes ventricosa x maxima leaf. Fitoterapia. 2007;78(7–8):585–586. doi:10.1016/j.fitote.2007.03.020
42. Bouayed J, Bohn T. Exogenous antioxidants–double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev. 2010;3(4):228–237. doi:10.4161/oxim.3.4.12858
43. Chang YC, Fong Y, Tsai EM, et al. Exogenous C(8)-ceramide induces apoptosis by overproduction of ROS and the switch of superoxide dismutases SOD1 to SOD2 in human lung cancer cells. Int J Mol Sci. 2018;19(10):3010. doi:10.3390/ijms19103010
44. Li Z, Yang J, Huang H. Oxidative stress induces H2AX phosphorylation in human spermatozoa. FEBS Lett. 2006;580(26):6161–6168. doi:10.1016/j.febslet.2006.10.016
45. Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2ʹ-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27(2):120–139. doi:10.1080/10590500902885684
46. Son Y, Kim S, Chung HT, Pae HO. Reactive oxygen species in the activation of MAP kinases. Methods Enzymol. 2013;528:27–48. doi:10.1016/B978-0-12-405881-1.00002-1
47. Kashyap D, Sharma A, Garg V, et al. Reactive oxygen species (ROS): an activator of apoptosis and autophagy in cancer. J Biol Chem Sci. 2016;3(2):256–264.
48. Lee ER, Kim JY, Kang YJ, et al. Interplay between PI3K/Akt and MAPK signaling pathways in DNA-damaging drug-induced apoptosis. Biochim Biophys Acta. 2006;1763(9):958–968. doi:10.1016/j.bbamcr.2006.06.006
49. Reinhardt HC, Aslanian AS, Lees JA, Yaffe MB. p53-deficient cells rely on ATM- and ATR-mediated checkpoint signaling through the p38MAPK/MK2 pathway for survival after DNA damage. Cancer Cell. 2007;11(2):175–189. doi:10.1016/j.ccr.2006.11.024
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.