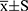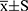Back to Journals » Cancer Management and Research » Volume 11
Estimation of radiotherapy modalities for patients with stage I-II nasal natural killer T-Cell lymphoma
Authors Liu X, Wu F, Guo Q, Wang Y, He Y, Luo H, Li Q, Zhong M, Li C, Yang H, Zhou J, Jin F
Received 14 January 2019
Accepted for publication 29 June 2019
Published 30 July 2019 Volume 2019:11 Pages 7219—7229
DOI https://doi.org/10.2147/CMAR.S201514
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Xianfeng Liu,1 Furong Wu,1 Qishuai Guo,1 Ying Wang,1 Yanan He,1 Huanli Luo,1 Qicheng Li,1 Mingsong Zhong,1 Chao Li,1 Han Yang,1 Juan Zhou,2 Fu Jin1
1Department of Radiation Oncology, Chongqing University Cancer Hospital, Chongqing Cancer Institute, Chongqing Cancer Hospital, Chongqing, People’s Republic of China; 2Forensic Identification Center, College of Criminal Investigation, Southwest University of Political Science and Law, Chongqing, People’s Republic of China
Purpose: The objective of this study is to estimate radiotherapy (RT) modalities for patients with stage I-II nasal natural killer T-Cell lymphoma (NNKTCL), including plan quality, radiation delivery efficiency, cost of RT and excess absolute risk (EAR).
Materials and methods: Twenty-four representative patients with stage I-II NNKTCL treated with fix-field intensity-modulated radiotherapy (FF-IMRT) were re-planned for volumetric modulated arc therapy (VMAT), TomoDirect (TD) and TomoHelical (TH) on the TomoHDA system, respectively. Plan characteristics, cost of RT and EAR were compared.
Results: Compared with IMRT, TD and TH showed significant improvement in terms of D98%, D2%, cold spot volume and homogeneity index (HI) of planning target volume (PTV), while achieving worse Dmean and conformity index (CI). The mean dose of oropharynx, thyroid and left salivary, and the maximum dose of right salivary by TD (249.20%, p=0.000; 52.94%, p=0.000; 160.23%, p=0.022; 122.67%, p=0.027), VMAT (15.76%, p=0.000; 23.53%, p=0.000; 34.09%, p=0.000; 31.33%, p=0.000) and TH (250.32%, p=0.000; 58.82%, p=0.000; 120.45%, p=0.020; 117.33%, p=0.032) increased significantly compared to IMRT. VMAT reduced treatment time (p=0.000; 0.000; 0.000) and monitor units (MUs) (p=0.000; 0.000; 0.000) obviously compared with IMRT, TD and TH. The cost of RT for TD and TH increased 150% compared with IMRT and VMAT. IMRT obtained the lowest EAR to oropharynx, thyroid, left and right salivary gland in the four treatment modalities.
Conclusion: The results show that both TD and TH can achieve higher conformal target quality while getting worse organs at risk (OARs) sparing and EAR to some organs than IMRT for patients with stage I-II NNKTCL. IMRT delivers the lowest dose to most OARs, VMAT requires the lower cost of RT and shortest delivery time, and TH obtained the optimal target coverage. The results could provide direction for selecting proper RT modalities for different cases.
Keywords: nasal natural killer T-Cell lymphoma, excess absolute risk, IMRT, VMAT, TomoHelical
Background
Nasal natural killer T-Cell lymphoma (NNKTCL) usually occurs in Eastern Asia, Mexicans and South Americans, and accounts for <10% of primary non-Hodgkin lymphoma disease.1–3 Radiotherapy (RT) is recommended as the main therapy method for stage I-II NNKTCL, and previous research have shown an overall survival benefit in favor of RT for patients with the NNKTCL.2,4,5
To achieve better target coverage and organs at risk (OARs) sparing, and improve the control rates and reduce the treatment morbidity subsequently, various RT methods such as medical linear accelerator-based fix-field intensity-modulated radiotherapy (FF-IMRT) and volumetric modulated arc therapy (VMAT), TomoHDA system-based TomoHelical (TH) are all used to treat NNKTCL, showing that each method has both advantage and disadvantage at target dose coverage, OARs sparing, and therapeutic efficiency, respectively, of which the out-of-field dose also differs significantly.3,6,7
Exposure to therapeutic doses of RT unavoidably leads to radiation damage and toxicity for normal tissue, and previous literature have shown therapy-related second cancer risk (SCR) as a late complication of treatment becomes obvious for patients who received RT previously, although the precise-related data remain unknown.8–10 Model excess absolute risk (EAR) developed by Schneider can be utilized to estimate SCR for normal tissues, which are usually unavoidably irradiated during a radiation treatment.11,12 Currently, advances in RT have resulted in large cases of long-term cancer survivors, and late sequelae of RT are becoming the next core concern. Hence, SCR remains studying further, especially involving younger patients who potentially have a longer life expectancy.13,14
Clinically, selecting the optimal treatment modality for patients with stage I-II NNKTCL has crucial importance, previous studies showed that each modality has its own characteristic in plan quality.3,6,7 However, the SCR and cost of RT triggered by various RT modalities have not studied. The purpose of this study is to compare the plan quality, radiation delivery efficiency, cost of RT and SCR among four treatment modalities (FF-IMRT, VMAT, TH and TomoDirect[TD]) for patients with stage I-II NNKTCL, and provide guide for choosing reasonable treatment modalities.
Materials and methods
Patients and materials
Planning computer tomography (CT) datasets of 24 different patients with stage I-II NNKTCL who were recently treated at our Institution were used for this study. This study was approved by the Chongqing Cancer Hospital’s ethics committee, and written informed consent was obtained from each patient. Diversity in shape and stage of the treated NNKTCL in the selected patients were ascertained, with 22 stage I and 2 stage II cases. The 24 patients were without distant metastases and had not received prior RT.
The CT scans were acquired on a Philips Brilliance Big Bore CT (Philips, Holland) simulation in 3 mm slice thickness, in the supine position with the scan scope from the inferior margin of the clavicular heads to the vertex of the skull. Image registration and delineation of the target and OARs were performed using the Eclipse treatment planning system (TPS, Varian Medical Systems, Version 11.0, Inc.). Gross tumor volume (GTV) contained primary tumor and regional metastatic lymph nodes, diagnosed by magnetic resonance imaging or CT, endoscopic examination and physical examination. Clinical target volume (CTV) included GTV and adjacent tissues at risk for contiguous spread. Planning target volume (PTV) was obtained from the respective CTV expanding symmetrically by 3 mm in all dimensions to offset setup errors. The mean volume of PTV was 248.8 cm3, ranging from 60.7 to 471.3 cm3. Surrounding OARs of lenses, eyes, optic nerves, optic chiasm, brainstem, spinal cord, parotid glands, salivary gland, oropharynx and thyroid gland were contoured. All CT images and delineation of the target and OARs in the Eclipse TPS were conveyed to the tomotherapy TPS (Tomotherapy, Madison, WI, USA).
RT plans
For each of the 24 cases, four different planning modalities were adopted: FF-IMRT, VMAT, TH and TD. FF-IMRT and VMAT plans were designed on the Eclipse TPS. For all Eclipse plans, dose calculations were done with 6 MV photon beams generated by Varian IX linear accelerator, Progressive Resolution Optimizer and Dose–Volume Optimizer algorithms were utilized for VMAT and FF-IMRT optimizations, respectively.15 Anisotropic analytical algorithm was used for final dose calculations.16
TH and TD plans were designed on tomotherapy TPS, utilizing 6 MV photon beams, a fixed dose rate (DR) of 877 monitor units (MUs)/min and least squares optimization method.17,18 A 0.5 cm bolus was added for all plans optimizations, the inner edges of the paropias on both sides were the right and left bounds of the bolus, while the lower edge of the palate and the upper edge of the frontal sinus were the lower and upper bounds of the bolus, respectively.
FF-IMRT
All FF-IMRT plans owned 10 coplanar fields, where the gantry angles were 200°, 240°, 280°, 320°, 0°, 0°, 40°, 80°, 120° and 160°, respectively. The angles of collimator and couch rotation for all fields were set to 0°. To minimize the dose to the lenses and eyes, the position of jaws in the two fields of 0° should be adjusted. After fitting the jaws to the structure of PTV with a margin of 6 mm, the position of the upper jaw of one field with gantry angle 0° was adjusted to 3 mm below the lower edge of the lenses, and the position of the left and right jaws of the other field with gantry angle 0° was adjusted to keep the lenses out of radiation field. And dynamic sliding-window IMRT delivery technique and a fixed DR of 400 MUs/min were adopted to deliver the dose.
VMAT
All VMAT plans were set as two coplanar arcs of 360°, with the collimator rotation of 45° and 315°, respectively, and the couch rotation of 0°. The maximum DR of all plans was set to 600 MUs/min.
TH
All the TH plans were optimized with a field width of 2.5 cm, pitch values of 0.287 and modulation factor of 3, and dose calculated with fine dose calculation grid. In addition, the function of complete block was adopted to protect the lenses.
TD
The TD plans had nine equally distributed beam, with the angel of 200°, 240°, 280°, 320°, 0°, 40°, 80°, 120° and 160°, respectively. The optimization and calculation settings of the TD plans were the same as the TH plans. Similarly, the function of complete block was used to minimize the dose to the lenses.
For the PTV of all plans, the prescribed dose was 50 Gy in 25 fractions, the prescribed 95% isodose covered no <95% of the PTV, and the percentage volume of PTV receiving over 107.5% of the prescription dose was no more than 2%.7,19 Based on the Radiation Therapy Oncology Group 0615 Protocol,20 dose constraints for the four types of plans were slightly modified. Dose constraint for OARs was defined as follows: maximum dose (Dmax) of eyes, optic nerves, optic chiasm and brainstem were limited to <50 Gy; Dmax of spinal cord and lenses were limited to 45 and 15 Gy, respectively; mean dose (Dmean) of parotid glands was limited to <26 Gy; Dmean of salivary gland, oropharynx and thyroid gland were limited to <40 Gy.
Treatment plan evaluation
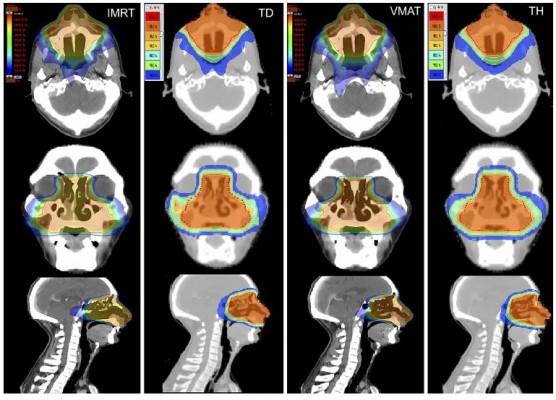
All the data acquired from Dose–Volume Histogram (DVH) of the 96 plans were analyzed. Figures 1 and 2 show representative dose distribution and DVHs for the four types of modalities, respectively. The plan evaluation focused on the items as in the following:
PTV
The dose received by 2%, and 98% (D2% and D98%) of the PTV volume were defined as the maximum and minimum dose of PTV, respectively. The Dmean of PTV was also used. The percentage volumes of PTV receiving <93% of the prescribed dose were defined as the cold spot volume of PTV, which should be <1%. The homogeneity index (HI) and conformity index (CI) of the PTV were compared by treatment modalities. CI was evaluated using the Paddick conformity index, defined as CI=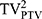 /(TV×PIV), where TV was the PTV volume, PIV was the body volume receiving 95% of the prescription dose and
/(TV×PIV), where TV was the PTV volume, PIV was the body volume receiving 95% of the prescription dose and 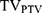 was the PTV volume covered by 95% of the prescribed dose. Ranging from 0 to 1, the value of CI approaching 1 meant fine conformity of PTV. HI represented dose uniformity, calculated with D5% minus D95%, divided by Dmean, where D5% and D95% was the minimum dose delivered to 5% and 95% of the PTV. Lower HI indicated better uniformity irradiation of the target volume.
was the PTV volume covered by 95% of the prescribed dose. Ranging from 0 to 1, the value of CI approaching 1 meant fine conformity of PTV. HI represented dose uniformity, calculated with D5% minus D95%, divided by Dmean, where D5% and D95% was the minimum dose delivered to 5% and 95% of the PTV. Lower HI indicated better uniformity irradiation of the target volume.
Organs at risk
For all the plans, dosimetric analysis was executed for the Dmax and Dmean of OARs, including left and right lens, left and right eye, left and right optic nerve, left and right parotid gland, left and right salivary gland, thyroid gland, optic chiasm, oropharynx, spinal cord and brainstem.
MUs and treatment time
The total MUs and treatment time were compared for the four kinds of modalities. Treatment time was calculated only from beam-on to the end of total MUs delivery, including the time of gantry rotation and dose delivery.
Cost of RT
The cost of RT through the whole radiation treatment process includes the expense of simulation, delineation, dose calculation, treatment delivery and quality assurance. In People's Republic of China, different provinces have different RT costs. In our western region, under the policies regulation of the Chongqing price bureau, the four treatment modalities only have different cost of treatment delivery in our institute, so we only compare the cost of treatment delivery among four treatment modalities in this study.
EAR calculations
For a person exposed to a radiation dose, the SCR of an organ can be obtained from Equation (1) utilizing the EAR model.11,21
(1)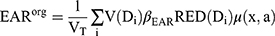
where VT represented the total organ volume, (2)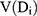 was the organ volume to receive a dose Di, and
was the organ volume to receive a dose Di, and 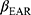 represented the slope of the dose–response curve at low dose.
represented the slope of the dose–response curve at low dose. 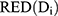 was the dose–response mechanistic model which accounts for cell killing and fractionation effects described as Equation (2),
was the dose–response mechanistic model which accounts for cell killing and fractionation effects described as Equation (2), 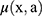 was the modifying function given by Equation (3).
was the modifying function given by Equation (3).
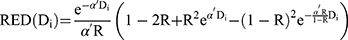
where R was the repopulation/repair parameter, which characterized the repopulation/repairability of the tissue between two dose fractions, and (3)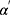 was given by Equation (4).
was given by Equation (4).
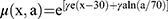
where (4)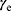 and
and 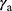 were the age modifying parameters.
were the age modifying parameters.
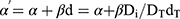
where DT and dT represented the prescribed dose to the target volume with the corresponding fractionation dose, respectively.
The EAR has been investigated to the organs of oropharynx, thyroid, left and right salivary gland. The reported values of α and α/β22–25 were applied in this study. As far as we know, there is no published value of R for thyroid, therefore, we set R=0.5 which represented intermediate repopulation just like the previous literature did.26 All other parameters utilized in EAR calculations in this study were selected from published research.21 All the parameters are shown in Table 1.
 |
Table 1 All the parameters utilized in EAR calculations in this study |
Statistical analysis
Utilizing the SPSS statistical software (SPSS, Chicago, IL, USA), paired t-test was carried out for determining if there was a significant difference in each of the pair parameters. A p<0.05 was considered statistically significant.
Results
PTV coverage
D98%, D2%, Dmean, cold spot volume, CI and HI were compared for assessing the quality of PTV coverage. TD and TH could provide better D98% and D2% than IMRT and VMAT (p<0.05), while achieving worse Dmean than IMRT and VMAT (p<0.05). Compared with IMRT, cold spot volume (%) of PTV with TD, VMAT and TH decreased by 68.45% (p=0.000), 53.57% (p=0.000) and 76.79% (p=0.000). The CI was worse with TD (0.830±0.007) and TH (0.850±0.008) than with IMRT (0.905±0.004) and VMAT (0.920±0.004), while achieving better HI than IMRT and VMAT (p<0.05). The findings on PTV are listed in Table 2.
 |
Table 2 Results of dosimetric comparison for PTV from DVH ( |
OARs
The radiation dose to the OARs is listed in Table 3. In comparison with TD and TH, IMRT and VMAT reduced the maximum and the mean dose of optic chiasm (p<0.05), left and right optic nerve (p<0.05) and spinal cord (p<0.05). Similarly, compared with TD, VMAT and TH, the maximum to right eye with IMRT was decreased by 5.04%, 4.35% and 6.28% (p=0.028; 0.019; 0.014). Compared with TH, the maximum dose to left lens with IMRT, TD and VMAT were significantly decreased by 11.65%, 28.19% and 9.13% (p=0.021; 0.000; 0.000), the mean dose to left lens with TD and VMAT were decreased by 26.62% and 15.82% (p=0.000; 0.001), and the maximum dose to right lens with TD and VMAT were decreased by 28.15% and 13.96% (p=0.000; 0.035). In comparison with IMRT, the mean dose to oropharynx with TD, VMAT and TH were significantly increased by 249.20%, 15.76% and 250.32% (p=0.000; 0.000; 0.000), the mean dose to thyroid with TD, VMAT and TH were significantly increased by 52.94%, 23.53% and 58.82% (p=0.000; 0.000; 0.000), the mean dose to left salivary with TD, VMAT and TH were significantly increased by 160.23%, 34.09% and 120.45% (p=0.022; 0.000; 0.020), and the maximum dose to right salivary with TD, VMAT and TH were significantly increased by 122.67%, 31.33% and 117.33% (p=0.027; 0.000; 0.032).
 |
Table 3 Results of dosimetric comparisons for OARs from DVH ( |
MUs and treatment time
The MUs and treatment time of the four treatment modalities are shown in Table 2. The mean MUs of IMRT, TD, VMAT and TH were 1191.08, 3415.33, 451.42 and 3441.42. Compared with VMAT, the mean MUs of IMRT, TD and TH were significantly increased by 163.85%, 656.57% and 662.35% (p=0.000; 0.000; 0.000). The mean treatment time of IMRT, TD, VMAT and TH were 421.66, 327.10, 189.27 and 245.46 s. In comparison with VMAT, the mean treatment time of IMRT, TD and TH were significantly increased by 122.78%, 72,82% and 29.69% (p=0.000; 0.000; 0.000).
Cost of RT
The cost of per treatment delivery for IMRT and VMAT are ~1200 RMB, and for TH and TD are ~3000 RMB, respectively. For all RT plans, the prescribed dose was 50 Gy in 25 fractions, so the total cost of treatment delivery for IMRT and VMAT are ~3000 RMB, while TD and TH are ~75,000 RMB, enhancing 150% compared to IMRT and VMAT.
EAR calculations
The EAR to the organs of oropharynx, thyroid, left and right salivary gland with four treatment modalities are shown in Table 4. Compared with IMRT, the EAR to the organs of oropharynx with TD, VMAT and TH were increased by 83.67%, 14.97% and 82.31% (p=0.000; 0.000; 0.000), the EAR to the organ of thyroid with TD, VMAT and TH were increased by 36.36%, 18.18% and 36.36% (p=0.002; 0.000; 0.001), the EAR to the organ of left salivary gland with TD, VMAT and TH were increased by 38.71%, 29.03% and 41.94% (p=0.000; 0.000; 0.000) and the EAR to the organ of right salivary gland with TD, VMAT and TH were increased by 35.48%, 29.03% and 35.48% (p=0.000; 0.000; 0.000).
 |
Table 4 The EAR of oropharynx, thyroid, left and right salivary gland with four treatment modalities |
Discussion
The study concerning estimation of various RT modalities for stage I-II NNKTCL is rare, hence, this study reporting a comparison about plan quality, radiation delivery efficiency, cost of RT and SCR among IMRT, TD, VMAT and TH for treating stage I-II NNKTCL is significant. The purpose of the study is to estimate the properties of four types of RT modalities, and further provide guidance for selecting the optimal RT modality in clinical practice for stage I-II NNKTCL.
The reported researches confirmed that IMRT therapy has demonstrated excellent target dose distribution and OARs sparing in stage I-II NNKTCL.3,6,7 Compared with IMRT, TD, VMAT and TH were the hotter issues in recent years. VMAT therapy in stage I-II NNKTCL has demonstrated excellent target coverage quality, improvements in OARs sparing and delivery time.3,7 TH therapy in stage I-II NNKTCL also has demonstrated its advantages in target coverage quality.3,17 However, only this study utilized TD for stage I-II NNKTCL up to present. In the study, we generally found that TD, VMAT and TH offered better D98%, cold spot volume and HI than IMRT, and TH plan had the optimal cold spot volume and HI, while CI with IMRT was slightly superior to with TD and TH. VMAT provided the optimal MUs and radiation delivery time, which has confirmed in previous studies.3,7
However, the unexpected results showed that IMRT obtained the optimal sparing of OARs in current study, and TD, VMAT and HD had higher maximum and mean radiation dose in most OARs. But previous researches confirmed that VMAT and HT offered improved OARs sparing compared with IMRT, and especially reduced side effects of parotid glands.27,28 The opposite findings about OARs sparing could be caused by the different spatial relationship of OARs and PTV. In general, the target volume of NNKTCL is not just close to lenses, optic nerves, optic chiasm and eyes, but also surrounded by them in space. Once TD, VMAT and TH pursue deceased cold spot volume or improved HI in target volume, the radiation dose to OARs would unavoidably increase. This implies that a good treatment plan should achieve a balance between target coverage and critical organ protection.
With the rising cost of RT, the cost-effectiveness of the treatment modality must be concerned.29 In this study, we only compare the cost of treatment delivery among the four treatment modalities, and find that the total cost of TD and TH dramatically increased 150% compared with IMRT and VMAT. Andrea Peeters et al30 evaluated the cost of external beam RT with carbon-ions, protons and photons and illuminated the higher cost of particle therapy, which might reduce by fewer fractions. However, in our study, the four treatment modalities had the same prescribed dose of 50 Gy in 25 fractions, the difference of RT cost for the four treatment modalities was determined by the cost of per treatment delivery.
For decades, improving curative effects in RT results in longer survival time for cancer patients, the assessment of SCR caused by RT has become increasingly important.14 The second cancer exists highly for patients who received RT, which have demonstrated by numerous clinical studies, and usually occur in volumes receiving total dose irradiation or nearby areas radiated with the doses from 2 to 50 Gy.31,32 Thyroid is known to have a low dose threshold for radiation-induced cancer, which is as low as 0.05 Gy in children and young adults.33,34 Hence, in RT treatment, second cancer for thyroid or other organs may be affected by out-of-field dose, which is likely to be highly associated with treatment facility due to leakage and scattering from head and accessories.35 Featured with more field and MUs, intensity-modulated techniques will increase leakage and scattering radiation unavoidably.36 Recently, particle therapy is introduced into clinical routine, which can often obtain excellent target converge while delivering minimal dose to normal tissue nearby tumor targets, and significantly decrease the risk of second cancer.37 However, due to the high cost of the particle therapy confirmed by Andrea Peeters et al,30 the total benefit still needs to be discussed.
For predicting cancer induction, based on the DVH data of the RT plan and some parameters, model EAR and model excess relative risk or a mixture of the two models were developed to calculate SCR.11,38,39 Previous literature26,40 compared SCR of RT modalities in whole-breast irradiation and rectal cancer and showed that RT modalities had obvious difference in SCR to some organs, but similar study was rare in head and neck cancer. In this study, owing to the insufficient data of survival rate for NNKTCL, we used EAR to determine SCR. Our study showed that IMRT obtained the lowest EAR to the organs of oropharynx, thyroid, the left and right salivary gland, hence, IMRT was suitable for the young patients who probably have a longer life expectancy. However, uncertainties inevitably existed in the accuracy of the commercially TPS models and the risk models, the results about EAR evaluating carried with inherent limitation.
In general, each RT modality had its own characteristics, adapting to individualized patient treatment. The optimal treatment modality for every individual patient depended on the balance between all relevant factors, such as target converge, normal tissue damage, economic aspects and long-term risk effects.
Conclusion
Both TD and TH showed slight improvements in target quality compared with IMRT for stage I-II NNKTCL. However, IMRT could offer lower cost of RT, lowest EAR in some organs and lowest dose to most OARs, and VMAT featured with lower cost of RT, lowest MUs and shortest delivery time. Each treatment modality has its own advantage, so selecting appropriate RT modality for individualized patient is necessary.
Abbreviation list
RT, radiotherapy; NNKTCL, nasal natural killer T-Cell lymphoma; EAR, excess absolute risk; FF-IMRT, fix-field intensity-modulated radiotherapy; VMAT, volumetric-modulated arc therapy; TD, TomoDirect; TH, TomoHelical; HI, homogeneity index; PTV, planning target volume; CI, conformity index; MUs, monitor units; OARs, organs at risk; SCR, second cancer risk; TPS, treatment planning system; CT, computer tomography; GTV, gross tumor volume; CTV, clinical target volume; DR, dose rate; DVH, Dose–Volume Histogram.
Acknowledgments
This work was supported by the National Natural Science Foundation of China under Grant Nos. 11575038 and 11805025, the Special Funds for Central Guiding Local Scientific and Technological Development, the Innovation Project of Social Undertakings and Livelihood Security Technology of Chongqing Science & Technology Commission (cstc2016shms-ztzx10002), and the Technical Innovation Project on Social Services & Livelihood Security of Science and Technology Commission, Chongqing Municipality, People's Republic of China (Grant No. cstc2015shmszx10013).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wu X, Li P, Zhao J, et al. A clinical study of 115 patients with extranodal natural killer/T-cell lymphoma, nasal type. Clin Oncol. 2008;20:619–625. doi:10.1016/j.clon.2008.05.011
2. Au WY, Weisenburger DD, Intragumtornchai T, et al. Clinical differences between nasal and extranasal NK/T-cell lymphoma: a study of 136 cases from the international peripheral T-cell lymphoma project. Blood. 2009;113:3931–3937. doi:10.1182/blood-2008-03-146472
3. Liu X, Huang E, Wang Y, et al. Dosimetric comparison of helical tomotherapy, VMAT, fixed-field IMRT and 3D-conformal radiotherapy for stage I-II nasal natural killer T-cell lymphoma. Radiat Oncol. 2017;12:76. doi:10.1186/s13014-017-0812-1
4. Huang MJ, Jiang Y, Liu WP, et al. Early or up-front radiotherapy improved survival of localized extra nodal NK/T-cell lymphoma, nasal-type in the upper aerodigestive tract. Int J Radiat Oncol Biol Phys. 2008;70:166–174. doi:10.1016/j.ijrobp.2007.05.073
5. Yang Y, Zhang YJ, Lin XB, et al. Role of radiotherapy in the combined treatment of patients with early-stage extra nodal nasal-type NK/T-cell lymphoma and analysis of prognostic factors. Chin J RadiatOncol. 2009;18:285–289.
6. Shen Q, Ma X, Hu W, Chen L, Huang J, Guo Y. Intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy for stage I-II natural killer/Tcell lymphoma nasal type: dosimetric and clinical results. Radiat Oncol. 2013;8:152. doi:10.1186/1748-717X-8-152
7. Liu X, Yong Y, Fu J, et al. A comparison of volumetric modulated arc therapy and sliding-window intensity-modulated radiotherapy in the treatment of stage I-II nasal natural killer/T-cell lymphoma. Med Dosim. 2016;4(1):42–46. doi:10.1016/j.meddos.2015.07.003
8. Deasy JO, Moiseenko V, Marks L, et al. radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys. 2010;76(3):58–63. doi:10.1016/j.ijrobp.2009.06.090
9. Tward JD, Wendland MM, Shrleve DC, Szabo A, Gaffney DK. The risk of secondary malignancies over 30 years after the treatment of non-Hodgkin’s lymphoma. Cancer. 2006;107:108–115. doi:10.1002/cncr.21971
10. Toda K, Shibuya H, Hayashi K, Ayukawa F. radiation-induced cancer after radiotherapy for non-hodgkin’s lymphoma of the head and neck: a retrospective study. Radiat Oncol. 2009;4:21. doi:10.1186/1748-717X-4-21
11. Schneider U. Modeling the Risk of Secondary Malignancies after Radiotherapy. Genes. 2011;2:1033–1049. doi:10.3390/genes2041033
12. Schneider U. Mechanistic model of radiation-induced cancer after fractionated radiotherapy using the linear-quadratic formula. Med Phys. 2009;36:1138–1143. doi:10.1118/1.3089792
13. Grantzau T, Mellemkjaer L, Overgaard J. Second primary cancers after adjuvant radiotherapy in early breast cancer patients: a national population based study under the Danish Breast Cancer Cooperative Group (DBCG). Radiother Oncol. 2013;106:42–49. doi:10.1016/j.radonc.2013.01.002
14. Bartkowiak D, Humble N, Suhr P, et al. Second cancer after radiotherapy,1981–2007. Radiother Oncol. 2012;105:122–126. doi:10.1016/j.radonc.2011.09.013
15. Mingzan Z, Tuodan Z, Zhijian C, et al. Advanced nasopharyngeal carcinoma radiotherapy with volumetric modulated arcs and the potential role of flattening filter-free beams. Radiat Oncol. 2013;8:120. doi:10.1186/1748-717X-8-120
16. Bragg CM, Wingate K, Conway J. Clinical implications of the anisotropic analytical algorithm for IMRT treatment planning and verification. Radiother Oncol. 2008;86:276–284. doi:10.1016/j.radonc.2008.01.011
17. Tomita N, Kodaira T, Tachibana H, et al. A comparison of radiation treatment plans using IMRT with helical tomotherapy and 3Dconformal radiotherapy for nasal natural killer/T-cell lymphoma. Br J Radiol. 2009;82:756–763. doi:10.1259/bjr/83758373
18. Shepard DM, Olivera GH, Reckwerdt PJ, Mackie TR. Iterative approaches to dose optimization in tomotherapy. Phys Med Biol. 2000;45:69–90. doi:10.1088/0031-9155/45/1/306
19. Peters S, Schiefer H, Plasswilm L. A treatment planning study comparing Elekta VMAT and fixed field IMRT using the varian treatment planning system eclipse. Radiat Oncol. 2014;9:153. doi:10.1186/1748-717X-9-153
20. Radiation Therapy Oncology Group (RTOG) 0615. Available from: http://irochouston.mdanderson.org/RPC/CREDENTIALING/files/0615-Master-2-16-11.pdf.
21. Schneider U, Sumila M, Robotka J. Site-specific dose-response relationships for cancer induction from the combined Japanese A-bomb and Hodgkin cohorts for doses relevant to radiotherapy. Theor Biol Med Modell. 2011;8:27. doi:10.1186/1742-4682-8-27
22. Schneider U, Zwahlen D, Ross D, Kaser-Hotz B. Estimation of radiation-induced cancer from three-dimensional dose distributions: concept of organ equivalent dose. Int J Radiat Oncol Biol Phys. 2005;61(5):1510–1515. doi:10.1016/j.ijrobp.2004.12.040
23. Pedicini P, Nappi A, Strigari L, et al. Correlation between egfr expression and accelerated proliferation during radiotherapy of head and neck squamous cell carcinoma. Radiat Oncol. 2012;7:143. doi:10.1186/1748-717X-7-143
24. Bakhshandeh M, Hashemi B, Mahdavi SRM, et al. Normal Tissue Complication Probability Modeling of Radiation-Induced Hypothyroidism After Head-and-Neck Radiation Therapy. Int J Radiation Oncol Biol Phys. 2013;85(2). doi:10.1016/j.ijrobp.2012.03.034
25. Murdoch-Kinch C-A, Kim HM, Vineberg KA, et al. Dose-Effect Relationships for the Submandibular Salivary Glands and Implications for Their Sparing by Intensity Modulated Radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(2):373–382. doi:10.1016/j.ijrobp.2007.12.033
26. Zwahlen DR, Bischoff LI, Gruber G, Sumila M, Schneider U. Estimation of second cancer risk after radiotherapy for rectal cancer: comparison of 3D conformal radiotherapy and volumetric modulated arc therapy using different high dose fractionation schemes. Radiat Oncol. 2016;11:149. doi:10.1186/s13014-016-0723-6
27. Bertelsen A, Hansen CR, Johansen J, Brink C. Single Arc volumetric modulated Arc therapy of head and neck cancer. Radiother Oncol. 2010;95:142–148. doi:10.1016/j.radonc.2010.01.011
28. Sheng K, Molloy JA, Read PW. Intensity-modulated radiation therapy (IMRT) dosimetry of the head and neck: a comparison of treatment plans using linear accelerator- based IMRT and helical tomotherapy. Int J Radiat Oncol Biol Phys. 2006;V65N3:917–923. doi:10.1016/j.ijrobp.2006.02.038
29. Ploquin NP, Dunscombe PB. The cost of radiation therapy. Radiother Oncol. 2008;86:217–223. doi:10.1016/j.radonc.2008.01.005
30. Peeters A, Grutters JPC, Pijls-Johannesma M, et al. How costly is particle therapy? Cost analysis of external beam radiotherapy with carbon-ions, protons and photons. Radiother Oncol. 2010;95:45–53. doi:10.1016/j.radonc.2009.12.002
31. Suit H, Goldberg S, Niemierko A, et al. Secondary carcinogenesis in patients treated with radiation: a review of data on radiation-induced cancers in human, non-human primate, canine and rodent subjects. Radiat Res. 2007;167(1):12–42. doi:10.1667/RR0527.1
32. Berrington de Gonzalez A, Gilbert E, Curtis R, et al. Second solid cancers after radiation therapy: a systematic review of the epidemiologic studies of the radiation dose-response relationship. Int J Radiat Oncol Biol Phys. 2013;86(2):224–233. doi:10.1016/j.ijrobp.2012.09.001
33. Cardis E, Howe G, Ron E, et al. Cancer consequences of the Chernobyl accident: 20 years on. J Radiol Prot. 2006;26(2):127–140. doi:10.1088/0952-4746/26/2/001
34. Jin F, Luo H-L, Zhou J, et al. Cancer risk assessment in modern radiotherapy workflow with medical big data. Cancer Manag Res. 2018;10:1665–1675. doi:10.2147/CMAR.S164980
35. Joosten A, Bochud F, Baechler S, Levi F, Mirimanoff RO, Moeckli R. Variability of a peripheral dose among various linac geometries for second cancer risk assessment. Phys Med Biol. 2011;56(16):5131–5151. doi:10.1088/0031-9155/56/16/004
36. Kim DW, Chung WK, Shin D, et al. Risk of second cancer from scattered radiation of intensity-modulated radiotherapies with lung cancer. Radiat Oncol. 2013;8:47. doi:10.1186/1748-717X-8-47
37. Eekers DB, Roelofs E, Jelen U, et al. Benefit of particle therapy in re-irradiation of head and neck patients. Results of a multicentric in silico ROCOCO trial. Radiother Oncol. 2016;121(3):387–394. doi:10.1016/j.radonc.2016.08.020
38. Sachs RK, Brenner DJ. Solid tumor risks after high doses of ionizing radiation. Proc Natl Acad Sci USA. 2005;102:13040–13045. doi:10.1073/pnas.0506648102
39. LindaWalsh U. A method for determining weights for excess relative risk and excess absolute risk when applied in the calculation of lifetime risk of cancer from radiation exposure. Radiat Environ Biophys. 2013;52:135–145. doi:10.1007/s00411-012-0441-x
40. Han EY, Paudel N, Sung J, Yoon M, Chung WK, Kim DW. Estimation of the risk of secondary malignancy arising from whole-breast irradiation: comparison of five radiotherapy modalities, including TomoHDA. Oncotarget. 2016;7(16):22960–22969. doi:10.18632/oncotarget.8392
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.