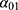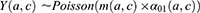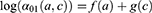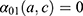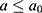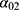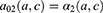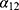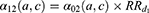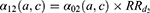Back to Journals » Clinical Epidemiology » Volume 14
Estimating the Future Burden of Myocardial Infarction in France Until 2035: An Illness-Death Model-Based Approach
Authors Kuhn J , Olié V, Grave C, Le Strat Y, Bonaldi C , Joly P
Received 1 October 2021
Accepted for publication 17 January 2022
Published 5 March 2022 Volume 2022:14 Pages 255—264
DOI https://doi.org/10.2147/CLEP.S340031
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Irene Petersen
Johann Kuhn,1 Valérie Olié,2 Clémence Grave,2 Yann Le Strat,1 Christophe Bonaldi,1 Pierre Joly3
1Department of Support, Data Processing and Analysis, French National Public Health Agency, Saint-Maurice, France; 2Department of Chronic Diseases and Injuries, French National Public Health Agency, Saint-Maurice, France; 3Centre Inserm U1219 – Bordeaux Population Health, Université de Bordeaux - ISPED, Bordeaux, France
Correspondence: Johann Kuhn, Department of Support, Data Processing and Analysis, French National Public Health Agency, 12 rue du Val d’Osne, Saint-Maurice, 94410, France, Tel/Fax +33 1 71 80 15 44, Email [email protected]
Purpose: In France, myocardial infarction (MI) was the second leading cause of years of life lost in 2019. Estimating the burden of MI in future years could help policymakers and other actors anticipate care and prevention needs and guide them in public health decision-making.
Materials and Methods: Using data from the French hospital discharge database from 2007 to 2015 (n = 519,400), demographic data, and an illness-death model, we projected incidence, prevalence, number of prevalent cases and mean age of incident MI cases in France. The methodology took into account the age-cohort effect on MI incidence, mortality of healthy and diseased subjects, and the time since disease onset.
Results: Projections highlighted an increase in MI prevalence in men between 2015 and 2035 from 2.52% (95% uncertainty interval (UI): [2.48– 2.56]) in 2015 to 4.02% ([3.92– 4.12]) in 2035, and from 0.85% ([0.83– 0.87]) to 1.44% ([1.38– 1.50]) in women. This corresponds to an increase of 365,000 cases between 2015 and 2035 (+81.1%) for men and 146,000 cases for women (+88.0%). The difference in the mean age of incident cases between men and women decreased from 9.52 in 2015 to 5.49 years in 2035.
Conclusion: Our projections forecast an increase in MI prevalence between 2015 and 2035 in men and women, especially in relatively younger women. Using statistical models such as ours can help assess the impact of prevention campaigns for the main cardiovascular disease risk factors on the future MI prevalence.
Keywords: myocardial infarction, projection, burden, prevalence, incidence, age-period-cohort model
Introduction
Cardiovascular disease is the main cause of death worldwide and in Europe. According to the 2019 Global Burden of Disease (GBD), global mortality from cardiovascular disease was estimated at 18.6 million deaths, or 32.8% of all deaths worldwide.1 Ischaemic heart disease, including myocardial infarction (MI), has been identified as the second leading cause of years of life lost (YLL) in France with approximately 840,000 YLL in 2019.1 The number of MI cases is expected to increase as a result of several phenomena: first, the French population is ageing.2 Second, MI hospitalization incidence in adults under 65 has increased since the early 2000s, with a decrease in mean age at MI onset in women.3 This increase is expected to continue, given the high proportion of the main cardiovascular risk factors (tobacco, cholesterol, hypertension, diabetes, obesity) in the French population.4–8 Finally, the decline in MI mortality over recent decades is contributing to increased MI prevalence in the population.
The financial burden of MI on public health is growing, with significant costs linked to hospitalization and treatment during the acute phase, as well as cardiac rehabilitation and secondary and tertiary prevention. There is also a social cost; patients who have a MI have a poorer quality of life and an increased risk of functional limitation and dependence.9 In this context, estimating the future burden of MI could help actors anticipate care and prevention needs, and adapt to public health policies. Estimating future prevalences of chronic diseases requires a methodology which takes into account the mortality trend for both diseased and healthy subjects, in particular for diseases which are more prevalent in the elderly.10 Multi-state models and especially “illness-death” models are particularly suitable for prevalence projection and represent a good alternative to classic survival models.11 Using “illness-death” methodology, we aimed to provide a French nationwide estimation of MI prevalence in 2035 and other epidemiological indicators.
Materials and Methods
Identification of Incident MI Cases
The French National Health Data System (SNDS) contains individual and anonymized demographic data (age, sex) and covers almost the entire French population.12 It includes a hospital discharge database (“Programme de médicalisation des systèmes d’information – Médecine, chirurgie, obstétrique”, PMSI-MCO) from which patients for the present study were identified.13 We defined MI incidence as being equal to MI hospitalization incidence because all-living MI cases are systematically hospitalized in France. Between 2007 and 2015 in metropolitan France, 519,400 patients (67.4% for men, 32.6% for women) aged 35 to 95 years old were hospitalized for MI. Those hospitalized with a principal diagnosis of MI (codes I21-I23 in the 10th revision of International Classification of Diseases (ICD-10)) and no medical history of MI in the two years preceding inclusion, who were in-patients (ie, at least one night in hospital) or who died on the day of hospital admission, were included. The annual crude incidence rate of hospital admissions by age and sex was calculated using annual estimates of the national average population from the National Institute of Statistics and Economic Studies (INSEE).
Demographic Data
Estimates of the French population size on 1st January and age-specific mortality rates (from 35 to 95 years old) by sex for each year from 1955 to 2070 were obtained from INSEE. Data for the years before 2013 came from censuses, while post-2013 data were based on INSEE projections. We used three INSEE-proposed projected scenarios for population size and age-specific mortality rates from 2013 to 2070, based on different assumptions on life expectancy, fecundity and net migration (Supplementary Table S1).14
Illness-Death Model
The projection method used was based on a three-state Markov model, called “illness-death” (Figure 1), which describes the possible transitions between three states: state 0 (alive, no MI), state 1 (alive, with MI), state 2 (dead). Subjects in state 0 are designated as “healthy”. The model is said to be irreversible because an individual cannot recover from the disease (ie, cannot return to state 0). The model is fully characterized by its transition intensities. Transition  represents the incidence rate of MI,
represents the incidence rate of MI,  the mortality rate among people without MI, and
the mortality rate among people without MI, and 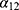 the mortality rate among people with MI. Transition intensities are usually dependent on age or calendar time depending on the application.
the mortality rate among people with MI. Transition intensities are usually dependent on age or calendar time depending on the application.
 |
Figure 1 Illness-death model. |
The incidence rate of individuals hospitalized for MI denoted 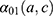 for an age
for an age  and a cohort
and a cohort  , was estimated from the following age-cohort model:
, was estimated from the following age-cohort model:
where 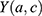 is the number of MI incident cases,
is the number of MI incident cases, 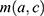 the number of person-years,
the number of person-years, 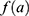 the age effect and
the age effect and 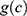 the cohort effect.15 This model was compared with other models from the age-period-cohort family, including the age-period model. The parameters for this model were estimated using a generalized linear model (GLM) and the effects of age and the cohort were modelled with natural splines. For
the cohort effect.15 This model was compared with other models from the age-period-cohort family, including the age-period model. The parameters for this model were estimated using a generalized linear model (GLM) and the effects of age and the cohort were modelled with natural splines. For 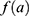 11 knots were placed between 35 and 95 (approximately one knot every five years) for both sexes, and one knot every 11 and 10 years for men and women, respectively, for
11 knots were placed between 35 and 95 (approximately one knot every five years) for both sexes, and one knot every 11 and 10 years for men and women, respectively, for 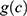 . Details of the knot placements and goodness-of-fit graphics are provided in the appendix (Supplementary Table S2). The choice of the model was based on the Akaike information criterion (AIC), on the goodness of fit of the predicted data to the observed data, and on the shape of the projections calculated by the model (Supplementary Figures S1–S6). A suitable compromise between goodness of fit and projections is necessary to validate the model. Accordingly, we assessed whether the projections were plausible from an epidemiological point of view (ie, no sudden increases or decreases in the incidence rate). In addition, we assumed that
. Details of the knot placements and goodness-of-fit graphics are provided in the appendix (Supplementary Table S2). The choice of the model was based on the Akaike information criterion (AIC), on the goodness of fit of the predicted data to the observed data, and on the shape of the projections calculated by the model (Supplementary Figures S1–S6). A suitable compromise between goodness of fit and projections is necessary to validate the model. Accordingly, we assessed whether the projections were plausible from an epidemiological point of view (ie, no sudden increases or decreases in the incidence rate). In addition, we assumed that 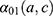 was null before a given age
was null before a given age 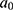 (equal to 35 years old for this application) because MI are rare in younger adults:
(equal to 35 years old for this application) because MI are rare in younger adults:
We assumed that the mortality of healthy subjects was equivalent to the mortality in the general population, denoted by 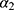 . This assumption is plausible because both MI prevalence and mortality from MI are low (approximately 5% and 3%, respectively). Since age, calendar time (
. This assumption is plausible because both MI prevalence and mortality from MI are low (approximately 5% and 3%, respectively). Since age, calendar time ( ) and cohort are linked by the following relation:
) and cohort are linked by the following relation: 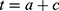 , it is possible to estimate the age-cohort-specific mortality rate of healthy individuals from the age-calendar time-specific mortality rate. The age-specific mortality rate for healthy subjects in a cohort
, it is possible to estimate the age-cohort-specific mortality rate of healthy individuals from the age-calendar time-specific mortality rate. The age-specific mortality rate for healthy subjects in a cohort  can therefore be written as:
can therefore be written as:
We then fitted a Gompertz-Makeham model to the INSEE mortality rates to obtain a continuous function of age for each cohort and for the three projection scenarios.16,17
Mortality in diseased individuals was considered proportional to that in healthy individuals with a relative risk depending on time since disease onset, as the mortality risk after a MI is very high during the first year and then decreases over time. Let us denote 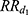 the relative risk associated with the first year following the MI occurrence and
the relative risk associated with the first year following the MI occurrence and 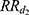 the relative risk thereafter. Mortality in the first year after a MI was:
the relative risk thereafter. Mortality in the first year after a MI was:
while mortality in subsequent years was:
We estimated 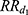 and
and 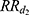 using a Cox regression with age as the time scale and a time dependent covariate which is the time after MI occurrence (
using a Cox regression with age as the time scale and a time dependent covariate which is the time after MI occurrence (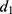 and
and 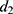 ) in men and women. Estimations were made with a sample of the French population from SNDS (n = 996,391) in which patients were followed from the 1st January 2008 to the 1st October 2021. Patients were at least 35 years old in 2008 without medical history of MI in 2006 and 2007. A description of the sample and values of the estimations are given in the Supplementary Material (Supplementary Table S3).
) in men and women. Estimations were made with a sample of the French population from SNDS (n = 996,391) in which patients were followed from the 1st January 2008 to the 1st October 2021. Patients were at least 35 years old in 2008 without medical history of MI in 2006 and 2007. A description of the sample and values of the estimations are given in the Supplementary Material (Supplementary Table S3).
We ran 1000 iterations of Monte-Carlo method to compute 95% uncertainty intervals (UIs) for the number of prevalent cases, the prevalence, and the mean age of incident cases.10 Details of the formula and distributions for each parameter used to compute the epidemiological indicators and their uncertainty interval are presented in the appendix (Supplementary Methods and Table S4).
Results
Estimations and projections of the epidemiological indicators were computed separately for men and women. Between 2007 and 2015, 519,400 patients were hospitalized for an MI in France with a higher proportion of men than women. Mean age was 65.0 years in men and 74.9 years in women (64.0 and 79.0, respectively, for median age) (Table 1). Almost half of the men hospitalized for a MI (45.8%) belonged to the age group 55–74 years old. For women, MI was more common in those over 75 years (61.6%).
 |
Table 1 Demographic Characteristics of Patients Hospitalized for a Myocardial Infarction Between 2007 and 2015 (PMSI Database) |
Figure 2 represents the predicted incidence rate of MI hospitalizations used to estimate the epidemiological indicators by age, according to gender and cohorts between 1935 and 1985 as obtained by the age-cohort model. For all cohorts of both sexes, the incidence rate increased with age. Furthermore, in those aged 35 to 45 years old, incidence across cohorts was effectively the same and remained constant at 90 years of age. For each age group, trends increased over successive cohorts for both sexes except for cohorts 1935 and 1945 in women.
Figure 3 displays the distributions of MI incident cases. According to the central INSEE projected scenario in men, the number of incident cases increases by 10,929 (+32.5%) between 2015 and 2025 and specifically by 11,352 (+25.5%) between 2025 and 2035 in persons over 50 years old. The number of incident cases among women increases by 4161 (+60.3%) between 2015 and 2025 for those aged 50 to 75, then decreases by 218 (+2.5%) for persons 75 to 90 years old, and then remains almost constant up to the age of 95. Comparing 2025 and 2035, it increases by 9005 (+55.1%) between those aged 60 and 90 years old. No difference in incident cases is observed between 2015 and 2035 for persons of either sex aged under 50 years old.
In 2015, the difference between the mean age of male and female incident cases is approximatively 10 years (Table 2). In the central INSEE scenario, this gap tends to decrease over the years, reaching +5.5 years in 2035. Similar trends are observed for the other two INSEE scenarios (Supplementary Table S5).
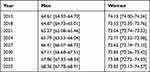 |
Table 2 Estimated Mean Age of Incident Cases for Myocardial Infarction (with 95% Uncertainty Intervals) in France by Gender According to the Central INSEE Scenario |
Figure 4 displays the prevalence for both sexes from 2015 to 2035 according to the central scenario. Prevalences were calculated using the annual size of the French population, aged between 35 and 95 years old from 2015 to 2035 as a denominator. Prevalence increases from 2.52% (95% UI: [2.48–2.56]) in 2015 to 4.02% ([3.92–4.12]) in 2035 in men and from 0.85% ([0.83–0.87]) to 1.44% ([1.38–1.50]) in women (Table 3). This corresponds to an increase of 365,000 (+81.1%) and 146,000 cases (+88.0%), respectively (Table 4). The two other INSEE scenarios show a similar increase in terms of prevalence and number of prevalent cases (Supplementary Tables S6 and S7). Comparing our projections with another which uses an incidence that is homogeneous over time (ie, it depends on age only), the difference in the number of prevalent cases of these two projections amounts to −23,000 cases in men and −12,000 cases in women in 2015, and +154,000 cases, and +61,000 cases, respectively, in 2035 (Supplementary Figure S7). Finally, in order to extract the share of the increase in prevalence linked to aging only (ie, demographical effect), we computed a projection of the number of prevalent cases in 2035 by applying the estimated prevalences in 2015 to the population structure of 2035. The difference between our projections and the latter one, which may be approximately attributed to an epidemiological effect, amounted to +206,000 cases for men and +90,000 cases for women (Supplementary Figure S8).
 |
Table 3 Estimated Prevalence (in Percentage) of Myocardial Infarction (with 95% Uncertainty Interval) in France for 2015, 2025 and 2035 Based on the Central INSEE Scenario |
 |
Table 4 Estimated Number of Prevalent Cases (N) for Myocardial Infarction (with 95% Uncertainty Interval) in France for 2015 and 2035 Based on the Central INSEE Scenario |
 |
Figure 4 Estimated prevalence of myocardial infarction (with 95% uncertainty interval) from 2015 to 2035 in men (left) and in women (right) according to the central INSEE scenario. |
Discussion
Our MI prevalence projections indicated an increase in the coming years irrespective of the INSEE demographic scenario used. The central scenario showed an increase for men from 2.52% to 4.02%, representing an 81.1% rise in the number of prevalent cases, while for women the increase was from 0.85% to 1.44%, representing an 88.0% rise. The projections showed a decrease of approximately four years in the difference in the mean age of incident cases between men and women between 2015 and 2035, with an increase in MI cases among younger women (ie, younger than the mean age in women for a MI).
These projections allowed us to quantify the increase in MI prevalence in order to anticipate the patient management and needs, as MI systematically leads to hospitalization and often cardiac rehabilitation. Patients’ quality of life may deteriorate, leading to a state of dependency in the most severe cases. Our projections highlight different dynamics in the distribution of incident cases between 2015 and 2035 for men and women. In men, the increase in incident cases is particularly pronounced in those over 65 years of age. In women, incident cases were higher in those aged between 60 and 75 years old. These two dynamics explain the decline in the mean age for MI in men, and the relative stability in women. The projected increase in the number of prevalent MI cases over time would therefore increase the financial burden of associated patient management. In addition, our projections forecast an increase in the number of relatively younger women who survive MI (particularly those aged 60 to 75 years of age), leading to an increased financial burden due to a longer period of patient management. Several studies projecting the financial and social burdens of coronary heart disease (CHD) – which includes MI – in different countries (China, Germany, Sweden, Australia, U.S.) also forecast an increase in both incidence and the number of events until at least 2030, and consequent greater financial burden.18–25 Our MI-specific forecasts are consistent with CHD forecasts by the US and German studies. In the US, projections highlighted an increase in prevalence from 8.6% in 2021 to 9.3% in 2030, while in Germany the increase was from 5.9% in 2018 to 9.5% in 2060.
The illness-death model is a multi-state model widely used in the medical literature, especially for chronic diseases.26–28 To estimate MI incidence, we used a model from the age-period-cohort models as we had little information on the temporal dynamics of the incidence (effect of calendar time, cohort or both). In the literature, age-period-cohort models are frequently used to model incidence and mortality rates,15,29 but we restricted ourselves to simple models such as the age-cohort model as it allowed an appropriate compromise between the goodness of fit of the predicted data to the observed data and the pertinence of the incidence projection from an epidemiological point of view. We also tested more complex models, but the gain in goodness of fit was not noticeable, and the projections were not epidemiologically plausible. Initially, our projected incidence depended only on age; however, as we wanted to have more accurate projections, we modelled incidence as a function of both age and cohort. The projected incidence for these two models was much closer for 2015 than those for 2035, as the data used were close to 2015 (2007 to 2015) (Supplementary Figure S7). It should also be noted that our incidence estimates were computed using hospitalization data. However, this may have led to an underestimation of the burden of MI because some cases may have died before hospitalization. We also estimated mortality in healthy subjects with INSEE to test the impact of demography on our projections. Results showed very little impact on the projections. Furthermore, trends were similar for all three INSEE scenarios tested (Supplementary Tables S6 and S7). For mortality in persons having an MI, we took into account the time since disease onset, making the hypothesis that the excess risk was higher in the year following the MI than in subsequent years.
The projected increase in MI prevalence, which we observed, was related to demographical and epidemiological conditions during the study period (2015–2035). However, the fact that our model took into account the mortality of adults without MI made it difficult to provide an accurate estimate of the respective impact of each dimension on the observed increase in prevalence. Nevertheless, we tried to extract the role played by risk factors from our projections by subtracting our projection of the number of prevalent cases in 2035 from a projection calculated by applying the estimated prevalence in 2015 to the INSEE-projected population for 2035, assuming that living and healthcare conditions in 2015 are identical to those in 2035, which is equivalent to erasing the effect of ageing (Supplementary Figure S8). The observed difference, attributable to the epidemiological impact, corresponded to approximately 45–55% of the observed increase. Note that this estimated observed difference is probably overestimated because general mortality and MI share the same risk factors.
Conclusion
We computed projections of MI prevalence until 2035 for both sexes by modelling incidence as a function of age and cohort, according to three different INSEE-proposed demographic scenarios. Our estimates projected that prevalence will increase between 2015 and 2035 with an almost doubling of cases, and that cases will increase in relatively younger women (ie, younger than the mean age of women with MI) between 2015 and 2035. Prevention measures for the main vascular risk factors could help contain this projected increase. The use of these statistical models could allow us to calculate the impact of prevention campaigns on the future prevalence of MI.
Data Sharing Statement
The data underlying this article cannot be shared publicly due to privacy reasons. Access to data is regulated by French legislation (request for access to data to the National Institute of Health Data (INDS) and authorization by the French Data Protection Authority (CNIL)). INSEE data are available at: https://www.insee.fr/fr/statistiques/2496228.
Ethics Approval and Informed Consent
In order to carry out its public interest missions in terms of health monitoring (article L. 1413-1 code de la santé publique*), the French National Public Health Agency has been granted by French law (code de la santé publique: articles L. 1461-3 I 2° and R. 1461-12 and following) permanent access to the SNDS. The examination of the study protocol by the National Ethics Committee is not, in this case, required by French law. The French National Public Health Agency is only required to keep an internal record of the studies it conducts on the basis of these data, as part of its public interest missions (article R 1461-17). Besides, analyses of SNDS data by French National Public Health Agency were performed with the permission of the CNIL (French data protection authority) and by decree (regulatory decision DE-2011-078).
*The code de la santé publique (public health code) contains laws (articles beginning with L) and decrees (articles beginning with à D or R) taken by French authorities in the field of public health.
Acknowledgments
We thank Zoé Uhry for her explications and advices for the modelling with age-period-cohort models.
Author Contributions
VO and YLS designed the study. VO, CG and CB performed the data acquisition and helped for the literature review. JK performed the literature review, performed the analysis of data with the help of PJ and drafted the manuscript. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The Authors declare that there is no funding.
Disclosure
The Authors declare that there are no conflicts of interest.
References
1. Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi:10.1016/S0140-6736(20)30925-9
2. Institut national de la statistique et de études économiques.[Population projections up to 2070]. Insee Première – 1619; 2016. Available from: https://www.insee.fr/fr/statistiques/2496228.
3. Gabet A, Danchin N, Juillière Y, Olié V. Acute coronary syndrome in women: rising hospitalizations in middle-aged French women, 2004–14. Eur Heart J. 2017;38(14):1060–1065. doi:10.1093/eurheartj/ehx097
4. Blacher J, Gabet A, Vallée A, et al. Prevalence and management of hypercholesterolemia in France, the Esteban observational study. Medicine. 2020;99(50):e23445. doi:10.1097/MD.0000000000023445
5. Lailler G, Piffaretti C, Fuentes S, et al. Prevalence of prediabetes and undiagnosed type 2 diabetes in France: results from the national survey ESTEBAN, 2014–2016. Diabetes Res Clin Pract. 2020;165:108252. doi:10.1016/j.diabres.2020.108252
6. Pasquereau A, Andler R, Arwidson P, Nguyen-Thanh V. [Tobacco consumption among adults: five years of the national program against smoking], 2014–2019. Bulletin Epidémiologique Hebdomadaire. 2020;14:273–281. French.
7. Vallée A, Gabet A, Grave C, Sorbets E, Blacher J, Olié V. Patterns of hypertension management in France in 2015: the ESTEBAN survey. J Clin Hypertens. 2020;22(4):663–672. doi:10.1111/jch.13834
8. Verdot C, Torres M, Salanave B, Deschamps V. [Corpulence of children and adults in metropolitan France in 2015. Results of the Esteban study and evolution since 2006]. Bulletin Epidémiologique Hebdomadaire. 2017;13(1):234–241. French.
9. De Peretti C, Danchin N, Danet S, Olie V, Gabet A. [Prevalence and functional status of ischaemic heart disease and heart failure in the adult population in France: contributions of the Handicap-Santé declarative surveys]. Bulletin Epidémiologique Hebdomadaire. 2014;9(10):172–181. French.
10. Joly P, Touraine C, Georget A, Dartigues JF, Commenges D, Jacqmin-Gadda H. Prevalence projections of chronic diseases and impact of public health intervention. Biometrics. 2013;69(1):109–117. doi:10.1111/j.1541-0420.2012.01827.x
11. Keiding N. Age-specific incidence and prevalence: a statistical perspective. J Royal Statis so Series A. 1991;154(3):371. doi:10.2307/2983150
12. Tuppin P, Rudant J, Constantinou P, et al. Value of a national administrative database to guide public decisions: from the système national d’information interrégimes de l’Assurance Maladie (SNIIRAM) to the système national des données de santé (SNDS) in France. Revue d’Épidémiologie et de Santé Publique. 2017;65:S149–S167. doi:10.1016/j.respe.2017.05.004
13. Boudemaghe T, Belhadj I. Data resource profile: the French national uniform hospital discharge data set database (PMSI). Int J Epidemiol. 2017;46(2):392–392d. doi:10.1093/ije/dyw359
14. [Population projections 2013-2070 for France: method and main results]. Documents de travail - F1606. Insee. Available from: https://www.insee.fr/fr/statistiques/2400057.
15. Clayton D, Schifflers E. Models for temporal variation in cancer rates. I: age–period and age–cohort models. Stat Med. 1987;6(4):449–467. doi:10.1002/sim.4780060405
16. Olshansky SJ, Carnes BA. Ever since Gompertz. Demography. 1997;34(1):1–15. doi:10.2307/2061656
17. Gompertz B. On the nature of the function expressive of the law of human mortality, and on a new mode of determining the value of life contingencies. In a letter to Francis Baily, Esq. F. R. S. &c. By Benjamin Gompertz, Esq. F. R. S. Philos Transact Royal Soc London. 1833;2:252–253. doi:10.1098/rspl.1815.0271
18. Marquina C, Talic S, Vargas-Torres S, et al. Future burden of cardiovascular disease in Australia: impact on health and economic outcomes between 2020 and 2029. Eur J Prev Cardiol. 2021;zwab001. doi:10.1093/eurjpc/zwab001
19. Bibbins-Domingo K, Coxson P, Pletcher MJ, Lightwood J, Goldman L. Adolescent overweight and future adult coronary heart disease. N Engl J Med. 2007;357(23):2371–2379. doi:10.1056/NEJMsa073166
20. Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–944. doi:10.1161/CIR.0b013e31820a55f5
21. Milan V, Fetzer S, Hagist C. Healing, surviving, or dying? – Projecting the German future disease burden using a Markov illness-death model. BMC Public Health. 2021;21(1):123. doi:10.1186/s12889-020-09941-6
22. Moran A, Gu D, Zhao D, et al. Future cardiovascular disease in China: Markov model and risk factor scenario projections from the coronary heart disease policy model–China. Cardiovasc Qual Outcomes. 2010;3(3):243–252. doi:10.1161/CIRCOUTCOMES.109.910711
23. Ben Ayed H, Ben Jemaa M, Trigui M, et al. Cardiovascular diseases in Southern Tunisia: current trends and future projections. Tunisie Medicale. 2019;97(5):659–666.
24. Modig K, Drefahl S, Andersson T, Ahlbom A. The aging population in Sweden: can declining incidence rates in MI, stroke and cancer counterbalance the future demographic challenges? Eur J Epidemiol. 2012;27(2):139–145. doi:10.1007/s10654-012-9653-2
25. Moran A, Zhao D, Gu D, et al. The future impact of population growth and aging on coronary heart disease in China: projections from the coronary heart disease policy model-China. BMC Public Health. 2008;8(1):394. doi:10.1186/1471-2458-8-394
26. Harezlak J, Gao S, Hui SL. An illness-death stochastic model in the analysis of longitudinal dementia data. Stat Med. 2003;22(9):1465–1475. doi:10.1002/sim.1506
27. Commenges D, Joly P, Letenneur L, Dartigues J. Incidence and mortality of Alzheimer’s disease or dementia using an illness-death model. Stat Med. 2004;23(2):199–210. doi:10.1002/sim.1709
28. Frydman H. Nonparametric estimation of a Markov ‘illness-death’ process from interval-censored observations, with application to diabetes survival data. Biometrika. 1995;82(4):773–789. doi:10.1093/biomet/82.4.773
29. Carstensen B. Age–period–cohort models for the Lexis diagram. Stat Med. 2007;26(15):3018–3045. doi:10.1002/sim.2764
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.