Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 15
Estimates of Chronic Kidney Diseases Associated with Proton-Pump Inhibitors Using a Retrospective Hospital-Based Cohort in Thailand
Authors Pannoi T , Promchai C, Apiromruck P, Wongpraphairot S, Yang CC, Pan WC
Received 11 September 2022
Accepted for publication 1 December 2022
Published 10 December 2022 Volume 2022:15 Pages 371—381
DOI https://doi.org/10.2147/IJNRD.S389238
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pravin Singhal
Tanavij Pannoi,1,2 Chissanupong Promchai,3 Penjamaporn Apiromruck,3 Suwikran Wongpraphairot,4 Chen-Chang Yang,1,5,6 Wen-Chi Pan1,5
1International Health Program, Institute of Public Health, National Yang Ming Chiao Tung University, Taipei City, Taiwan; 2Department of Pharmaceutical Care, School of Pharmacy, Walailak University, Nakhon-Si-Thammarat, Thailand; 3Department of Pharmacy, Songklanagarind Hospital, Prince of Songkla University, Songkla, Thailand; 4Department of Nephrology Unit, Songklanagarind Hospital, Prince of Songkla University, Songkla, Thailand; 5Institute of Environmental and Occupational Health Sciences, National Yang Ming Chiao Tung University, Taipei City, Taiwan; 6Department of Occupational Medicine and Clinical Toxicology, Taipei Veteran General Hospital, Taipei City, Taiwan
Correspondence: Wen-Chi Pan, Institute of Environmental and Occupational Health Sciences, National Yang Ming Chiao Tung University, No. 155, Sec.2, Li-Nong St, Taipei City, 11221, Taiwan, Tel +886-228-267-000 #67057, Fax +886-2-28218165, Email [email protected]
Purpose: Potential adverse outcomes of Proton pump inhibitors (PPIs) have increasingly been reported. The potential risks to PPIs include hypomagnesemia and chronic kidney disease (CKD). Unlike a real-world electronic medical record (RW-EMR) with active-comparator design, claim databases and special population cohort with non-user design, using in previous studies, resulted in a wide range of strength of association with indication bias. This study aimed to measure the total effect of association between PPIs use and CKD incidence using Thai RW-EMR.
Patients and Methods: A retrospective hospital-based cohort was applied into this study. Electronic medical records and administrative data of out- and inpatient were retrieved from October 1st, 2010 to September 30th, 2017. On-treatment with grace period as well as propensity score matching was used in data analysis. Cox proportional hazard models were applied to evaluate the PPIs-CKD association.
Results: Of all 63,595 participants, a total of 59,477 new PPIs and 4118 Histamine 2-receptor antagonist (H2RA) users were eligible for follow-up. As compared with H2RA, the PPI users were non-elderly and more likely being female. The association of PPIs with CKD was statistically significant (adjusted hazard ratio [HR] = 3.753, 95% CI = 2.385– 5.905). The HR were not statistically different by concomitant use PPIs with NSAIDs and by medication possession ratio levels.
Conclusion: The association between PPIs and CKD incidence was statistically significant in this hospital-based cohort. However, self-treatment with over-the-counter PPIs, as well as, smoking, drinking alcohol and body mass index could not be fully retrieved, affecting the estimation of treatment effect.
Keywords: proton-pump inhibitors, chronic kidney disease, retrospective cohort, hospital-based medical database
Introduction
Proton-pump inhibitors (PPIs) is a class of medication, which decrease gastric acid by inhibiting the parietal cell H+/K+ ATP pump. Pharmacologically, PPIs demonstrate superior efficacy to histamine-2 receptor antagonists (H2RAs) in order to treat acid-related disorders. Accordingly, the United States Food and Drug Administration (US FDA) approved PPIs for the treatment of duodenal ulcers, gastric ulcers, erosive esophagitis, gastroesophageal reflux disorder (GERD), Helicobacter pylori eradication and pathological hypersecretory conditions, such as Zollinger–Ellison syndrome.1–4
Since the World Health Organization included PPIs in the list of essential medicines and health products, PPIs were globally given to millions of patients.5 In US, the prevalence of ambulatory care visits in which patients receiving PPIs increased by 5% between 2002 and 2009. In addition, 46.7% of those patients taking PPIs were 65 years and older, while, PPIs were prescribed in the ambulatory setting by a three-fold increase during the study period.6 In Thailand, PPIs were 1 out of 5 most prescriptions among 33 hospitals during the fiscal year of 2009, while, it was dispensed to patients with regardless of the diagnoses of gastrointestinal diseases or none of indication to use in medical records.7
Potential adverse outcomes due to PPIs use have been observed since the PPIs initiated to market. The US FDA announced safety warnings for potential risks to PPIs, including hypomagnesemia and kidney disease.1,8 The number of evidence of PPIs safety has alarmed public consumers, who have a likelihood of exposing PPIs as they are over-the-counter drugs in many countries, including Thailand.1,7 However, a couple of scientific gaps need to be further explored and clarified.
Firstly, the previous observational studies related with PPIs and adverse effects were studied in US and European countries, as well as, different types of data set may lead to different outcomes due to its confounders. In addition, some cohort studies9,10 were to use no-PPIs as comparator that might lead to confounding by indication. Secondly, many studies could not fully generalize the association between PPIs use and CKD incidence among the general population due to selection bias. For instance, Lazarus et al,11 using population with registered atherosclerosis, studied the association between PPIs and CKD incident. Other than that, Xie et al,12 using US veteran hospital database, was limited in generalizability because there were more male (93.4%) than female (6.6%) participants. Thirdly, based on literature review,13–15 there were none of studies taking individual medication persistent use of PPIs into real-world data analysis and observed its effect to the strength of association between PPIs and CKD events.
To address these issues, the study aims to observe the effect of PPIs associated with CKD among Thai population using Thai real-world clinical data as compared with other observational studies.
Materials and Methods
Data Source
The health information of study patients was primarily retrieved from medical and administrative databases at Songklanagarind hospital. Databases consist of inpatient and outpatient information, medication data, laboratory, and administrative data. This study was reviewed by institutional review boards of National Yang Ming Chiao Tung University, as well as, by study site’s human research ethical committee.
Study Design
A retrospective cohort study was conducted (Figure 1). We retrieved data of PPIs and H2RAs users from out- and inpatient-departments from October 1, 2010 to September 30, 2012 followed through September 30, 2017, and the maximum follow-up duration was 7 years (Figure 1A in Supplementary Material).
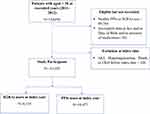 |
Figure 1 Flowchart of Participant’s Selection. |
Study Sample and Disease Diagnosis
For inclusion criteria, all participants, who were over 20 years old and given either PPIs or H2RAs between Thai fiscal year 2011 (from October 1, 2010 to September 30, 2011) and 2012 (from October 1, 2011 to September 30, 2012), were recruited. At least 6 months prior to each fiscal year, PPIs, H2RAs prescription, acute kidney injury (AKI), hypomagnesemia and CKD were not recorded among these participants.
Exposure and Comparator Definitions
New PPIs or H2RAs (as an active comparator) user was defined as any participants who were given the first PPIs prescription or the first H2RAs prescription since the entry periods. Details of drug’s codes were informed in Supplementary Data 1. “As treated” or “on treatment” scheme was applied in the main analysis, while, we only follow-up exposure-comparator during treatment episode. Discontinuation of medication date is unknown in observational database. Therefore, we defined discontinuation date of medication regarding the last prescription dispensing date plus the days’ supply and grace period (Figure 2). A person who did not refill a prescription of PPIs or H2RAs before discontinuation date was censored. We pre-defined 30, 90, 180, and 365 days of grace period. Eventually, the main analyses relied on a 90-day grace period because a steady increase in 3-month prescriptions dispensed were observed, whereas, this dispensing plan has evidently improved medication persistence as compared to a 30-day refill plan.16
 |
Figure 2 On-treatment scheme with grace period = 90 days. |
Covariates Assessment and Adjustment
Individual characteristics including hospital service uses, medications, and diagnoses were retrieved and presented as baseline characteristics. Individual serum creatinine and serum magnesium levels before the index time (T0) were collected based on the availability of data.
At baseline covariates with their absolute standardized difference (Table 1), we eventually adjusted for sex, age, Charlson comorbidity index (CCI), number of hospitalizations, hypertension (I10-I16), nephrotoxic drugs (Clopidogrel, Non-steroidal Anti-inflammatory drugs [NSAIDs], Steroids), and drugs-induced hypomagnesemia (Statin, Diuretics, Insulin, Digoxin, Aminoglycosides, Polyenes antifungals). We included baseline covariates leading to hypomagnesemia due to the potentially hypomagnesemia patients linking with renal impairment.17,18 Estimated glomerular filtration rate (eGFR) was calculated from the observed serum creatinine (Scr) with CKD-Epi formula. Due to missing baseline serum creatinine in some participants, we, therefore, imputed the rest of participants’ eGFR whose serum creatinine were unobserved in accordance with sex and age. Details of ICD-10 for covariates and outcomes were informed in Supplementary Data 1.
 |
Table 1 Baseline Demographic and Health Characteristics of Overall Cohort Between Proton-Pump Inhibitors (PPIs) and Histamine-2-Receptor Antagonist (H2RAs) Users, at Index Time |
Statistical Analysis
All individuals who developed either CKD events, hypomagnesemia, or death before their index date were excluded, while, those who were given by PPIs or H2RAs, considering exposed and comparator under the allowable gap. Switching between exposure and comparator, as well as, death during the follow-up period will be right-censored (Supplementary Figure 1A).
Baseline characteristics of cohort patients for the PPI and H2RAs users were reported as frequency, percentage, mean and standard deviation, or median and interquartile range, as appropriate. In addition, baseline characteristics of the exposure group and active comparator were compared using absolute standardized difference19 between 2 groups for continuous and categorical data. The strength of association between PPIs and CKD was assessed with stratified Cox-proportional hazard (CPH) because the CPH assumption complied with Global Schoenfeld residuals test (p = 0.1407), including a separate test for each covariate (Supplementary Figure 2A). Collinearity assessment with variance inflation factor (VIF) was less than 4, assumingly none of multicollinearity among covariates using in CPH model (Supplementary Figure 3A).
In addition, we used propensity score matching to estimate the treatment effect of PPIs on those who received it accounting for confounding by the included covariates. The propensity score was estimated using 1:1 nearest neighbor matching without replacement20 of PPIs on the baseline covariates-- sex, age category (<=60;>60), baseline eGFR, Steroids, Clopidogrel, NSAIDs, Charlson comorbidity index (CCI), hypertension, index year, and hospital visits (Supplementary Data 1, Figures 1B and 2B). To balance covariates between PPIs and H2RAs user, propensity score matching (PSM) was applied and modeled with time-to-event analysis.
Subgroup and Sensitivity Analysis
To assess if the HR would be differed by some covariates, subgroup analyses were conducted with sub-categories--baseline age (elderly or not), concomitant use of NSAID and PPIs and individual medication persistent use. On the basis of medication persistent use, we adopted “Medication Possession Ratio (MPR)”, defined as ratio between the days of medication supply of all prescriptions fills within a time interval.21 AdhereR package in R was used to calculate MPR, which takes the first medical event and accounts for carry over within observational window and excluding the supply left22 (Figure 4A in Supplementary Material). R software (version 4.0.5) was used for data management and analysis. A p-value of <0.05 was considered as statistical significance.
Results
During the 2-year entry periods, 154,056 participants age 20 years old or more were included in the study cohort. There were 90,285 participants who were excluded with reasons, such as incomplete of socio-demographic data and people who were diagnosed either AKI, hypomagnesemia, or CKD events before their index dates. A total of 59,477 new PPIs and 4118 H2RAs users were eligible for follow-up (Figure 1).
Baseline health characteristics and socio-demographics are described in Table 1. As compared with H2RAs, the number of PPI users who aged under or equal 60 years was higher and more often in women. Of all eligible participants, there were 22,015 (34.62%) users, whose baseline serum creatinine (Scr.) were provided in the hospital database. The individual measures of Scr were converted to eGFR, while, eGFR were imputed by sex and age for any participants whose baseline eGFRs were not measured. In this study, among of H2RAs users had higher CCI score at baseline than PPIs users. A small proportion of participants (4.70%) were diagnosed with diseases of esophagus, stomach and duodenum for PPIs or H2RAs use with regard to ICD codes. After matching on propensity score, there were 8174 matched-pairs, as well as, standard mean difference of age, hypertension, clopidogrel, NSAIDs, steroids, eGFR and number of hospital visits was balanced by SMD less than 10%.
The Strength of Association Between PPIs and CKD Events
Table 2 describes the number of events--AKI, Hypomagnesemia, and CKD, crude incidence rates of each study event. The median follow-up time was 0.25 years for before and after PSM. As many as 745 participants were diagnosed as CKD, as well as, 326 individuals were diagnosed as hypomagnesemia. The crude incidence rates and the proportion of participants developing CKD and hypomagnesemia events were higher among PPI users than among H2RAs users.
 |
Table 2 Number of Events, Follow-Up and Incidence Rate for the Study Mediators and Outcomes Between PPI and H2RA Users (Grace Period = 90 Days) |
To comply with Cox proportional hazard (Cox-PH) regression’s assumption, different Cox-PH regressions were modeled (Supplementary Table 1A). As shown in Table 3, a stratified Cox-PH adjusted for baseline characteristics was proposed, and the hazard ratio for PPI users, as compared with H2RA users was 3.753 (95% CI:2.385–5.905) for the CKD events in accordance with 90 days of grace period, whereas, HR on the basis of 1:1 PSM was 5.164 (95% CI:3.110–8.574). The association was consistent in both stratified Cox-PH and propensity score matching models with different grace periods (Supplementary Table 2A).
 |
Table 3 Multivariable Cox Proportional Hazards: Overall Survival Following CKD for PPIs and H2RAs Users by Null, and Stratified Cox-PH, with Grace Periods = 90 Days |
Sub-Group and Sensitivity Analysis
To measure confounding effect of concomitant use of NSAID to PPI users at baseline, in Table 4, their HRs were not statistically different. In addition, the HR was significant with both MPR 50% or less and with MPR over 50% (HR = 3.880; 95% CI = 2.336–6.445 vs HR = 4.401; 95% CI = 1.184–16.356, respectively). Over 95% of Thai patients were given by Omeprazole, which showed a strong significant association to CKD incidence, while, the strength of association between non-Omeprazole and CKD was observed only before PSM (HR 3.612; 95% CI: 1.263–10.326) with a small number of sample size.
 |
Table 4 Subgroup Analysis Depicting the HR with 95% CI for CKD Events (Before and After Propensity Score Matching (PSM)) |
Discussion
In this large observational cohort study of using electronic medical record in Thailand, PPIs use associated, as compared with H2RAs, with diagnosed CKD outcome, was focused. Regarding the systematic reviews across countries from the United States, Brazil, Taiwan, Sweden, and Denmark, effect estimates for the association between PPI treatment (compared with non-PPI users or H2 blocker users) and CKD ranged from an adjusted HR of 1.12 (95% CI 1.08–1.17) to 7.34 (95% CI 3.94–13.71).23
As compared with this study, Guedes et al10 resulted the HR was 7.34 (95% CI: 3.94–13.71) (Supplementary Table 3A), indicating a higher risk of worse stages of CKD in omeprazole users than in non-users. This is relevant to our sub-group analysis (Table 4) that HR among omeprazole as compared to H2RAs was 3.783 (95% CI: 2.404–5.952). It is noticeable that omeprazole was most likely to be prescribed to the study participants in Thailand, which accounted over 90% of all gastric acid suppressants.
Most large observational databases were used in the study of PPIs associated with renal impaired outcomes that led to different incidence rate of CKD events, whereas, heterogeneity of data sources and baseline characteristics affected the magnitude of the CKD risk. For instance, Xie et al12 observed that a higher risk of CKD and CKD progression was dependent on healthcare-related eGFR measurement. Likewise, Lazarus et al11 studied among PPI users in the Atherosclerosis Risk in Community cohort, where the higher odds of CKD relied on the ascertainment of ICD codes.
Having said that, our results were consistent as compared to those previous publications, while, we carefully control measurable confounders, such as a number of hospital visits among participants leading to differently ascertain eGFR measures, CKD incidence based on ICD coding by physician, co-prescription of nephrotoxic and hypomagnesemia-related medications were considered. However, baseline serum creatinine was largely missing due to unmeasured eGFR conditioning by clinical practice. Multiple imputation of eGFR based on sex and age was adopted to mitigate the bias estimation. Nevertheless, any other confounders, such as, personal health risk behaviors (smoking, drinking, sodium intakes, etc.) or time-varying confounders (BMI, eGFR changes along with the survival period) were not completely observed throughout our study database. This may cause underestimation of HR due to the modification effects between these unmeasured confounders and PPIs use to CKD.
Preceding articles have adopted many renowned study designs and advanced statistical analysis. In our study, we used retrospective with on-treatment analysis to determine whether being on either PPIs or H2RAs is associated with CKD event, assumingly the outcome was assessed on the basis of what treatments each participant actually prescribed, irrespective of the randomize of treatment.24 “On-treatment” with grace period approach measures CKD event that occurred while patients are taking PPIs or H2RAs with subsequent days after discontinuation of medication; for each patient who had no CKD event, time in the study is censored on 90 days after discontinuation of PPIs or H2RAs. Our result showed that the HR was persistent across the different grace periods (Supplementary Table 2A). However, our study approach excluded different periods of time for either PPIs or H2RAs, causing selection bias into the evaluation of risk, even if the direction of that bias remained unclear.24
Having reported from some previous studies9,10 with indication bias, H2RA as an active comparator (AC) and new user (NU) study design were also applied in our study. The aim of selecting AC is to mitigate confounding by indication and other unmeasured participant’s characteristics (eg, baseline health status, frailty, and assignment mechanism to treatment), while, the potential for immortal time bias is reduced as the start of follow-up time can be defined as the switch date for either PPIs or H2RAs.25,26 Although, H2RAs is not fully substitutable for PPIs indication, based on choices of treatment available on Thai health service during the study period, this is the best active comparator we had. For a new user approach, the time-varying hazard and temporality of covariates assessment are preserved.25 We fixed a wash-out window prior to the index date that individuals did not use any of either PPIs or H2RAs at least 6 months before the entry years.
The study’s result should be deliberately interpreted with its pros-and cons. Largely electronic hospital database is comprehensive and powerful in order to assess the safety of medication use in regular practice. As compared to previous cohort studies, our population is not predominant in some certain characteristics. Therefore, the study result could be more generalizable, particularly general population. However, our participants were patients accessing hospital, while reasons to measure serum creatinine could be confounders leading to selection bias.
In addition, dispensing data are more valid reflection of medication intake than claim data, particularly in terms of drug use continuation. However, dispensing data does not necessarily guarantee the adherence of medication. Therefore, a grace period approach was applied in data analysis with the concern of drug discontinuation causing the bias result, while, medication possession ratio (MPR) was also used in sub-group analysis to demonstrate that medication persistence did not affect the strength of association in this study (Table 4).
Nevertheless, there are some limitations in this study. On-treatment with exclusion of immortal time period (time from entry date to the index date) prone to selection bias. Potential self-treatment with over-the-counter PPIs could not be retrieved from our study database, which is a crucial confounder leading to misclassification of exposures. In addition, unmeasured confounders, particularly, smoking, drinking alcohol, and individual BMI may affect the estimation of HR. For further study, the additive effect of concomitant use between PPIs and other nephrotoxic drugs, particularly NSAID, while, potential mechanism or mediation effect between PPIs and CKD should be explored.
Conclusion
To conclude, the effect of PPIs associated with CKD was statistically significant in this hospital-based cohort. Although causality could not be assumed with a single study, the result is still relevant to previous publications, while, our study’s outcome was more generalizable to general population using active comparator new user design. PPIs should be prescribed under the approval indication, while, it should be considered to remove from over-the-counter drug list in order to avoid long-term adverse events among general population.
Informed Consent Statement
Patient’s informed consents according to the Declaration of Helsinki and the International Conference on Harmonization in Good Clinical Practice were waived by institutional review boards of National Yang Ming Chiao Tung University (YM108156E), as well as, by study site’s human research ethical committee (REC.63-096-19-9). All retrieved data was anonymized and maintained with confidentiality under the study’s site regulations. Due to Thai and Taiwan restrictions apply to the availability of these data, raw datasets with personal identifications will not publicly shared.
Acknowledgments
We thank Professor Dong Yaa-Hui at the Department of pharmacy, College of Pharmaceutical Sciences, National Yang Ming Chiao Tung University (Taiwan), who guided all the statistical methods applied in this manuscript. We also thank Hsin-Jin (Jack) Huang for his assistance and advices to wrangle data with R program.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received no external funding.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Makunts T, Cohen IV, Awdishu L, Abagyan R. Analysis of postmarketing safety data for proton-pump inhibitors reveals increased propensity for renal injury, electrolyte abnormalities, and nephrolithiasis. Sci Rep. 2019;9(1). doi:10.1038/s41598-019-39335-7
2. Maes ML, Fixen DR, Linnebur SA. Adverse effects of proton-pump inhibitor use in older adults: a review of the evidence. Ther Adv Drug Saf. 2017;8(9):273–297. doi:10.1177/2042098617715381
3. Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z. Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney Int. 2017;91(6):1482–1494. doi:10.1016/j.kint.2016.12.021
4. Olbe L, Carlsson E, Lindberg P. A proton-pump inhibitor expedition: the case histories of omeprazole and esomeprazole. Nat Rev Drug Discov. 2003;2(2):132–139. doi:10.1038/nrd1010
5. World Health Organization. 21st WHO Model List of Essential Medicines. World Health Organization; 2019.
6. Rotman SR, Bishop TF. Proton pump inhibitor use in the U.S. ambulatory setting, 2002–2009. PLoS One. 2013;8(2):e56060. doi:10.1371/journal.pone.0056060
7. Chariyawet P, Dilokthornsakul P. Omeprazole overuse and its financial loss in a community hospital. J Pharm Pract. 2018;10(2):12.
8. Brisebois S, Merati A, Giliberto JP. Proton pump inhibitors: review of reported risks and controversies. Laryngoscope Investig Otolaryngol. 2018;3(6):457–462. doi:10.1002/lio2.187
9. Aguilera AI, Rodríguez-Poncelas A, Barceló MA, Saez M, Coll-de-Tuero G. Duration and dosing of Proton Pump Inhibitors associated with high incidence of chronic kidney disease in population-based cohort. PLoS One. 2018;13(10):e0204231. doi:10.1371/journal.pone.0204231
10. Guedes JVM, Aquino JA, Castro TLB, et al. Omeprazole use and risk of chronic kidney disease evolution. PLoS One. 2020;15(3):e0229344.
11. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176(2):238–246. doi:10.1001/jamainternmed.2015.7193
12. Xie Y, Bowe B, Li T, Xian H, Balasubramanian S, Al-Aly Z. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J Am Soc Nephrol. 2016;27(10):3153–3163. doi:10.1681/ASN.2015121377
13. Hung SC, Liao KF, Hung HC, et al. Using proton pump inhibitors correlates with an increased risk of chronic kidney disease: a nationwide database-derived case-controlled study. Fam Pract. 2018;35(2):166–171. doi:10.1093/fampra/cmx102
14. Cholin L, Ashour T, Mehdi A, et al. Proton-pump inhibitor vs. H2-receptor blocker use and overall risk of CKD progression. BMC Nephrol. 2021;22(1):264. doi:10.1186/s12882-021-02449-0
15. Nochaiwong S, Ruengorn C, Awiphan R, et al. The association between proton pump inhibitor use and the risk of adverse kidney outcomes: a systematic review and meta-analysis. Nephrol Dial Transplant. 2018;33(2):331–342. doi:10.1093/ndt/gfw470
16. Parker MM, Moffet HH, Adams A, Karter AJ. An algorithm to identify medication nonpersistence using electronic pharmacy databases. JAMIA. 2015;22(5):957–961. doi:10.1093/jamia/ocv054
17. Sakaguchi Y, Tsubakihara Y, Rakugi H, Isaka Y. Does hypomagnesemia predict faster progression of nondiabetic chronic kidney disease? Am J Med. 2014;127(2):e13. doi:10.1016/j.amjmed.2013.08.020
18. Van Laecke S, Nagler EV, Verbeke F, Van Biesen W, Vanholder R. Hypomagnesemia and the risk of death and GFR decline in chronic kidney disease. Am J Med. 2013;126(9):825–831. doi:10.1016/j.amjmed.2013.02.036
19. Yang D, Dalton JE A unified approach to measuring the effect size between two groups using SAS; 2012.
20. Zhao QY, Luo JC, Su Y, Zhang YJ, Tu GW, Luo Z. Propensity score matching with R: conventional methods and new features. Ann Transl Med. 2021;9(9):812. doi:10.21037/atm-20-3998
21. Sperber CM, Samarasinghe SR, Lomax GP. An upper and lower bound of the medication possession ratio. Patient Prefer Adherence. 2017;11:1469–1478. doi:10.2147/PPA.S136890
22. Dima AL, Dediu D. Computation of adherence to medication and visualization of medication histories in R with AdhereR: towards transparent and reproducible use of electronic healthcare data. PLoS One. 2017;12(4):e0174426. doi:10.1371/journal.pone.0174426
23. Rajan P, Iglay K, Rhodes T, Girman CJ, Bennett D, Kalantar-Zadeh K. Risk of bias in non-randomized observational studies assessing the relationship between proton-pump inhibitors and adverse kidney outcomes: a systematic review. Therap Adv Gastroenterol. 2022;15:17562848221074183. doi:10.1177/17562848221074183
24. Yang F, Wittes J, Pitt B. Beware of on-treatment safety analyses. Clin Trials. 2019;16(1):63–70. doi:10.1177/1740774518812774
25. Lund JL, Richardson DB, Sturmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep. 2015;2(4):221–228. doi:10.1007/s40471-015-0053-5
26. Yoshida K, Solomon DH, Kim SC. Active-comparator design and new-user design in observational studies. Nat Rev Rheumatol. 2015;11(7):437–441. doi:10.1038/nrrheum.2015.30
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
