Back to Journals » International Journal of Nanomedicine » Volume 13
Establishment of a novel homogeneous nanoparticle-based assay for sensitive procalcitonin detection of ultra low-volume serum samples
Authors Li P, Chen Z, Liu B, Li K, Wang H, Lin L, He L, Wei J, Liu T
Received 17 May 2018
Accepted for publication 4 July 2018
Published 13 September 2018 Volume 2018:13 Pages 5395—5404
DOI https://doi.org/10.2147/IJN.S173776
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Peng Li,1 Zhenhua Chen,1 Bing Liu,1 Kun Li,1 Hao Wang,1 Li Lin,1 Ling He,1 Jie Wei,2 Tiancai Liu1
1State Key Laboratory of Organ Failure Research, Institute of Antibody Engineering, School of Laboratory Medicine and Biotechnology, Southern Medical University, Guangzhou, Guangdong, People’s Republic of China; 2Department of Clinical Laboratory, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, Guangdong, People’s Republic of China
Purpose: Sepsis is a potentially fatal systemic body infection with a significant mortality rate worldwide. Although C-reactive protein (CRP), interleukin-6 (IL-6), and procalcitonin (PCT) might be biomarkers for sepsis diagnosis, PCT is more sensitive and specific than CRP or IL-6. We aimed to establish an efficient immunoassay that precisely detects PCT in human serum for the early diagnosis of sepsis.
Materials and methods: We developed a novel amplified luminescent proximity homogeneous assay (AlphaLISA) for the quantitative detection of PCT in serum. In this assay, a pair of antibodies was used to capture PCT in serum and to form sandwich complexes after incubating for 15 minutes at 37°C.
Results: PCT concentrations were determined within a linear range of 0.016–100 ng/mL. The limit of detection was 18.6 pg/mL. The results demonstrate that the reproducibility, recovery, and specificity of this assay for PCT meet the requirements of clinical detection. The coefficient of determination (R2) between this method and commercially available enzyme-linked fluorescent assay (ELFA) kits was estimated to be 0.93045 in clinical serum testing.
Conclusion: The novel assay for PCT detection was robust with high sensitivity and a broad dynamic range. Compared with conventional heterogeneous detection methods such as ELISA, this assay measured the concentration of the homogeneous form of PCT and provided results that are more accurate within a shorter detection time. We expect that this novel method will be useful for the early screening and prognosis evaluation of patients with sepsis.
Keywords: sepsis, procalcitonin, nanoparticles, quantitative detection, homogeneous immunoassay
Introduction
Sepsis is a life-threatening infection caused by microbial pathogens including bacteria, viruses, and fungi.1 It is reported that an estimated 750,000 patients develop sepsis in the United States each year, with a mortality rate of up to 60% for severe sepsis.2,3 Thus, the early detection and diagnosis of severe sepsis have attracted continuous interest.
Procalcitonin (PCT) is a small protein composed of 116 amino acids with a molecular weight of 13 kDa,4 which is produced by C cells of the thyroid in healthy individuals. Normally, the PCT concentration in human serum from healthy individuals is very low (<0.01 ng/mL) and is undetectable via common methods.5 However, when having severe infectious disease and sepsis, serum PCT levels increase rapidly (>100 ng/mL) in response to pro-inflammatory stimulation.6,7 Compared with other biomarkers, such as C-reactive protein (CRP), white blood cell count (WBC), tumor necrosis factor-α (TNF-α), or interleukin-6 (IL-6), PCT is generally accepted as the most promising because of its better sensitivity and specificity than other known biomarkers for severe bacterial infection and sepsis.8–10 In addition, PCT concentration is used to evaluate sepsis severity and to assess the appropriateness of therapy (antibiotics or surgery), which leads to a reduction in antibiotic consumption.11 Therefore, a quantitative method with high degree of accuracy and sensitivity to detect PCT concentration in sera is needed.
In recent years, various immunoassays for PCT detection in clinical applications have been developed, including chemiluminescence immunoassay (CLIA), ELISA, electro-chemiluminescence immunofluorescence analysis (ECLIA), time-resolved fluoroimmunoassay (TRFIA), and point-of-care testing (POCT).12–15 All the above-mentioned methods are certified and are applied broadly in the clinic. However, they are also expensive because of equipment cost and the detection process. Moreover, most methods require multiple wash steps to remove non-specifically bound reactants; thus, they are also time-consuming.16
In this study, we developed a novel method for PCT detection using amplified luminescent proximity homogeneous assay (AlphaLISA) technology, which is a homogeneous nanobead-based non-radioactive immunoassay with obvious advantages.17,18 Two types of nanobeads, the “donor” beads and the “acceptor” beads, are used in this method. Upon illumination with laser light at 680 nm, the photosensitizer within the donor beads activates 60,000 singlet-state oxygen molecules per second. Because it possesses a high concentration of photosensitizers, donor beads convert ambient oxygen to singlet-state oxygen, greatly amplifying the signal.19 When acceptor beads and donor beads are brought into proximity during a specific biological interaction, the singlet-state oxygen molecules react with thioxene within the acceptor beads, resulting in chemiluminescence emission at 370 nm. This chemiluminescence further activates europium chelate doped in the acceptor beads and emits fluorescence at 615 nm. This emission has a high intensity, long lifetime, and sharp peak.20–22 Although this technology has existed for some years, further work is required prior to developing the PCT detection kits that could be applied to clinics.
Optical and physical characteristics of naked and conjugated acceptor beads were evaluated. Optimal concentration of conjugated acceptor beads and streptavidin–donor beads were determined for optimal signal in this study. The results indicated that this novel method for PCT detection has high sensitivity, precision, a wide linear range, and a simplified operation protocol. Therefore, this homogeneous nanoparticle-based assay is suitable for the early diagnosis of sepsis.
Materials and methods
Materials and reagents
Anti-PCT monoclonal antibody (MJG03) and antigen (JG01) were obtained from Hangzhou Kitgen Biotechnology Co., Ltd. (Hangzhou, Zhejiang, China). Anti-PCT monoclonal antibody (16B5) was purchased from HyTest Ltd. (Joukahaisenkatu, Turku, Finland). The acceptor beads, donor–streptavidin beads, 96-Optiplate™ microplates, and EnSpire™ Alpha Plate Reader were purchased from PerkinElmer Inc. (Waltham, MA, USA). Biotin N-hydroxysuccinimide ester, ProClin-300, and all other chemical reagents, unless stated otherwise, were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). The commercial enzyme-linked fluorescent assay kits (ELFA) for PCT detection were obtained from bioMerieux Inc (Boston, MA, USA). Clinical serum samples were made available by Sun Yat-sen Memorial Hospital, Sun Yat-sen University (Guangzhou, China).
Buffer solutions
The buffer solutions were as follows: binding buffer used for coupling acceptor beads to anti-PCT monoclonal antibody (16B5) was 0.13 M phosphate buffer solution (containing 25 mg/mL of NaBH3CN and 0.01% Tween 20, pH 8.0). Blocking buffer was 65 mg/mL of carboxymethoxylamine hemihydrochloride (CMO) dissolved in 0.8 M NaOH. Washing buffer was 25 mM Tris-HCl containing 0.2% Tween 20 and 0.05% ProClin-300, pH 7.8. Biotinylation buffer used for the biotinylation of anti-PCT monoclonal antibody (MJG03) was 0.01 M Na2CO3/NaHCO3 at pH 9.5. The composition of the analysis buffer was 25 mmol/L HEPES containing 2 mg/mL Dextran-500, 50 mmol/L NaCl, 0.5% bovine serum albumin, 0.02% cattle γ-globulin, and 0.5% Tween 20 at pH 7.4.
Biotinylation of anti-PCT monoclonal antibodies (Ab1)
The following protocol was used for the biotinylation of anti-PCT monoclonal antibodies (MJG03), denoted Ab1. Prior to biotinylation, 200 μg of Ab1 dissolved in 500 μL of biotinylation buffer was concentrated and purified using Sephadex G-50. According to the mole ratio of Ab/biotin =1/25, purified Ab1 was mixed with 3.3 μL of 10 mM biotin N-hydroxysuccinimide ester solution dissolved in dimethyl sulfoxide (DMSO), which was then incubated at room temperature for 1 hour or at 4°C overnight. Then, the biotinylated protein was purified by Sephadex G-50 to remove non-binding biotin. Bovine serum albumin and NaN3 were added into the biotinylated antibody solution, and the mixture solution was stored at 4°C.
Coupling of anti-PCT monoclonal antibodies (Ab2) to acceptor beads
Anti-PCT monoclonal antibody (16B5), denoted as Ab2, was directly coupled to acceptor beads via aldehyde reactive groups. The following reagents were added to a 1.5 mL Eppendorf tube, and the reaction was incubated for 48 hours at 37°C: 200 μg of Ab2, 1 mg of unconjugated acceptor beads, and 10 μL of binding buffer. Next, 10 μL of blocking buffer was added for 1 hour at 37°C to block unreacted aldehyde sites, washed three times with washing buffer by centrifugation at 20,000× g for 10 minutes, and resuspended in phosphate buffer solution containing 0.05% Proclin-300.23 The functional acceptor beads were stored at 4°C.
PCT detection protocol
One-step no wash bioassays were used. As shown in Figure 1, the order of reagent addition and reaction conditions was as follows: 5 μL of samples or PCT calibration solution was added to 96-well plates, and 70 μL of antibody–acceptor beads (0.01 mg/mL) and biotinylated antibody (1.79 ng/mL) mixture solution in assay buffer was added to each well. The plates were covered with a lid and incubated at 37°C for 15 minutes to form a sandwich complex. Subsequently, 175 μL of streptavidin–donor beads (0.1 mg/mL) in assay buffer was added. The plates were covered with a lid and incubated at 37°C for 15 minutes in the dark to complete the interaction between biotin and streptavidin. Finally, the plates were measured on an EnSpire Alpha Plate Reader to obtain signal. All the steps in this assay could be completed within approximately 30 minutes.
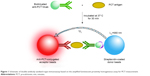  | Figure 1 Schematic of double-antibody sandwich-type immunoassay based on the amplified luminescent proximity homogeneous assay for PCT measurement. |
Results
During the development of the quantitative determination for PCT antigen based on AlphaLISA technology, we explored a large series of different assay component concentrations, optimized reaction conditions and their combinations, which included the dosage of biotinylated antibody, and matrix titration between functionalized acceptor beads and streptavidin–donor beads. Several assay parameters including repeatability, recovery, linearity, and feasibility were also explored.
Evaluation of functionalized acceptor beads versus naked acceptor beads
Fluorescent spectra of various concentrations (0, 2, 200, and 20,000 ng/mL in PBS) of the acceptor beads after conjugation are shown in Figure 2A, and the maximum emission peak at a wavelength of 614 nm when excited at 340 nm was determined. Fluorescence intensity increased with increasing concentration of the conjugated acceptor beads. Upon comparing naked acceptor beads with conjugated beads, a slight redshift was observed (Figure 2B).
Dynamic light scattering (DLS) analysis was performed to evaluate the physical characterization of acceptor beads. As shown in Figure 3A, the Mcab-labeled acceptor beads possessed acceptable narrow size distribution and monodispersion. Compared with naked acceptor beads, the conjugated beads had a broader range of size distribution by light intensity, but the symmetrical distribution curve indicated that functionalized acceptor beads were relatively uniform. As shown in Figure 3B, the mean of the diameter calculated by intensity distribution, volume distribution, number distribution, and Z-average suggested that the mean diameter of conjugated acceptor beads was approximately 250 nm. A change in diameter and zeta potential indicated that the anti-PCT Mcab was conjugated successfully onto the acceptor beads. The mean zeta potential and polydispersity index also suggested that colloidal stabilization was achieved during the conjugation process. The following results indicated that the physical properties of conjugated acceptor beads were good for the assays of PCT.
Optimal concentration of conjugated acceptor beads and streptavidin–donor beads
To achieve optimal signal-to-background ratio (S/B), matrix titrations were conducted between conjugated acceptor beads and streptavidin–donor beads. As shown in Figure 4, when the concentration of conjugated acceptor beads was 0.01 mg/mL, the S/B ratio approached a plateau in a range of streptavidin–donor bead concentrations from 0.001 to 0.1 mg/mL. For conjugated acceptor beads at concentrations from 0.0001 to 0.01 mg/mL, the optimal S/B ratio was reached at 0.1 mg/mL of streptavidin–donor beads. Based on the sensitivity and expense of the assay, optimal concentrations of 0.01 mg/mL of conjugated acceptor beads and 0.1 mg/mL of streptavidin–donor beads were selected for this study.
  | Figure 4 Matrix titration of conjugated acceptor beads and streptavidin–donor beads. |
Optimization of the concentration of biotinylated antibody
To minimize assay costs, the optimal concentration of biotinylated antibody required to retain assay sensitivity was investigated. In this study, a fixed concentration of PCT antigen (100 ng/mL) was mixed with 0.45, 0.89, 1.79, 3.57, and 7.14 ng/mL of biotinylated antibody and incubated at 37°C for 15 minutes. As shown in Figure 5, the fluorescence intensity increased gradually with an increase in the concentration of biotinylated antibody. However, fluorescence intensity declined sharply when the biotinylated antibody concentration was greater than 1.79 ng/mL. The curve exhibited a typical bell shape in the assays as the ligand concentration exceeded the capacity of the nanobeads, which is called the “hook” effect. Therefore, 1.79 ng/mL of biotinylated antibody was selected as the optimal concentration for all subsequent experiments.
Analytical sensitivity and linear range
We determined the sensitivity of this assay as well as the dynamic range for the quantification of PCT antigen in human serum. The analytical sensitivity (detection limit) for the current assay corresponded to the lowest concentration of detectable PCT which was 18.6 pg/mL, defined as the mean plus two SD (n=20) of the 0 calibrator. As shown in Figure 6A, a best-fit standard curve was obtained based on the measurement of eight serial reference standards (0, 0.016, 0.032, 0.16, 0.8, 4, 20, and 100 ng/mL of PCT antigen) under optimal conditions. Standard curve determinations were carried out using log–log regression and were represented by the equation: lg(y)=3.3097+1.0078×lg(x) (R2=0.9909). As shown in Figure 6B, the linear range of this assay was determined to be 0.02–100 ng/mL. Hook effects were observed when the range exceeded 100 ng/mL of PCT antigen. These results demonstrate the excellent performance of this PCT detection assay.
Assay precision (intra- and inter-assay variation)
The assay variation was studied using three serum samples. Intra-assay precision was determined by measuring the reference serum samples in five replicates at 3-hour intervals within 1 day, and the inter-assay precision was determined for 5 days continuously. As shown in Table 1, the intra-assay precision coefficients of variation (CV) ranged from 2.12% to 3.28% and the inter-assay precision CV ranged from 4.46% to 8.56%, which indicated the excellent reproducibility of this assay.
  | Table 1 Intra- and inter-assay precision of the PCT detection |
Analytical recovery and specificity
The analytical recovery was studied by adding purified recombinant PCT antigen to patient serum samples. The results are shown in Table 2. The recovery was in the range of 97.10%–106.55%, suggesting that recovery of the different serum samples was quantitative and that PCT was accurately measured in the real samples. The results demonstrated that the recovery of this method is acceptable. To evaluate the specificity of the detection kit for PCT determination, a range of possible interferent was evaluated by the proposed method, including CRP, IL-2, and IL-6. Table 3 illustrates that no effective cross-reactivity was observed for each interferent.
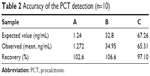  | Table 2 Accuracy of the PCT detection (n=10) |
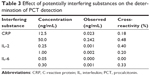  | Table 3 Effect of potentially interfering substances on the determination of PCT detection |
Comparison with ELFA
Overall, 116 serum samples were analyzed by commercial ELFA kits and the novel method. The comparisons of concentration values obtained by different methods are shown in Figure 7. The regression curve suggested a good correlation between the two methods (coefficient of determination R2=0.93045) and the equation of the regression curve was y=1.5042 x - 2.7156 (P<0.0001).
Discussion
In this study, we developed a novel homogeneous immunoassay for PCT detection, which was based on luminescent oxygen channeling assay technology. Although several PCT detection methods have been developed for clinical diagnosis, most of them are heterogeneous methods that have reactants in two or more phases. Physical separation of bound and free reporters is essential in these methods; however, such washing processes are time-consuming and may reduce the specificity and sensitivity of the immunoassay. The current homogeneous method reported here provides a lower limit of detection and a wider measuring range compared with common methods (reference limit of detection [LOD] of 31.2 pg/mL and assay range of 31.2–2,000 pg/mL for ELISA kits from R&D Systems, Inc., Minneapolis, MN, USA).
A 30-minute incubation time was chosen as the optimal time for this novel method based on our previous study.24 Such a short incubation time is an advantage over traditional ELISA that generally takes longer than 60 minutes. DLS data in Figures 2 and 3 suggested that the size of acceptor beads was not significantly increased and the beads were well dispersed after conjugation. The emission spectrum of conjugated acceptor beads was sharp with full width at a half maximum of 8 nm. The slight redshift suggested that the covalent attachment process had no impact on the optical properties of the acceptor beads. To construct an appropriate reaction system, we determined the optimal concentrations of the acceptor and donor beads (Figure 4; 0.01 mg/mL of conjugated acceptor beads and 0.1 mg/mL of streptavidin–donor beads to achieve the optimal S/B and to minimize the assay cost). In addition, the biotinylated antibody had a high binding affinity to recombinant PCT antigen, and we optimized the biotinylated antibody concentration with consideration for the sensitivity and specificity of the assay. Based on the data from Figure 5, an optimal concentration of 1.79 ng/mL of biotinylated antibody was selected in this study. Furthermore, we investigated the assay sensitivity and accuracy (recovery) and demonstrated that the developed PCT detection method has a high degree of sensitivity (LOD of 18.6 pg/mL) and accuracy (range of 97.1%–106.6%), indicating that the assay precision of this method is excellent.
A further evaluation of clinical serum detection was carried out to compare the novel developed method and ELFA. Linear regression analyses revealed good correlations between the novel developed method and the approved commercial kit. This indicated no significant statistical difference between the two methods and thus the newly established assay might be used for the clinical determination of PCT in human serum. Furthermore, the comparison of assay characteristics with the reported methods is shown in Table S1.
This method is based on AlphaLISA technology, which is simple, accurate, sensitive, and easy to operate.17,25 To the best of our knowledge, this study is the first report of a luminescent oxygen channeling-based homogeneous assay for the accurate and reliable quantitative detection of PCT for clinical diagnosis. In this particle-based method, the nanoparticle beads suspended in the assay solution provide a relatively large surface area. This enabled more antibody molecules to be attached to the surface of the acceptor beads, thereby reducing the consumption of reagents and improving the immobilization of more antibodies. The fluorescent signal depends on antibody–antigen reactions, which form a bridge linking the donor and acceptor beads. The chemiluminescent reaction was confined to a pair of adjacent nanobeads that permitted the detection of immunocomplex formation without interference from excess particles. In this method, the coupled donor–acceptor bead pairs were irradiated by a 680 nm solid-state laser, which minimized autofluorescence from the serum matrix. This characteristic led to an appreciable improvement of the sensitivity and precision of detection. The flexibility and efficiency of this method, by changing antibodies, extend its applicability to the detection of other biomarkers. However, one limitation is that the composition of the assay reagents requires further optimization because nonspecific binding was occasionally observed in this study. Moreover, more samples should be collected in order to further improve the accuracy and precision of this assay.
Conclusion
We have developed a novel homogeneous nanoparticle-based immunoassay, which was designed specifically as a hypersensitive, precise, and rapid measurement method for the quantitative determination of PCT in human serum. This novel method demonstrated excellent analytical performance and convenience. Additionally, the method established here had an excellent correlation with conventional ELFA when applied to the determination of PCT in clinical serum samples. Based on this investigation, we have established a good foundation for the further development of kits to detect other biomarkers, such as IL-6, using the same platform. It also has great value in clinical settings for determining the severity and prognosis of bacterial infection and for providing guidance when choosing to administer antibiotics.
Acknowledgments
Many thanks to Prof Ying-Song Wu, the director of Institute of Antibody Engineering, for his great help. The work was supported by the National Natural Science Foundation of China (grant number 21575058) and the National High Technology Research and Development Program 863 (No 2014AA020904).
Disclosure
The authors report no conflicts of interest in this work.
References
Liao T, Yuan F, Yu H, Li Z. An ultrasensitive ELISA method for the detection of procalcitonin based on magnetic beads and enzyme-antibody labeled gold nanoparticles. Anal Methods. 2016;8(7):1577–1585. | ||
Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. | ||
Engel C, Brunkhorst FM, Bone HG, et al. Epidemiology of sepsis in Germany: results from a national prospective multicenter study. Intens Care Med. 2007;33(4):606–618. | ||
Wang H, Wang H, Chen S, et al. Development of a fluorescent immnunochromatographic assay for the procalcitonin detection of clinical patients in China. Clin Chim Acta. 2015;444:37–42. | ||
Li P, Zhang W, Zhou X, Zhang L. C60 carboxyfullerene-based functionalised nanohybrids as signal-amplifying tags for the ultrasensitive electrochemical detection of procalcitonin. Clin Biochem. 2015;48(3):156–161. | ||
Meisner M, Tschaikowsky K, Palmaers T, Schmidt J. Comparison of procalcitonin (PCT) and C-reactive protein (CRP) plasma concentrations at different SOFA scores during the course of sepsis and MODS. Critical Care. 1999;3(1):45–46. | ||
Balci C, Sungurtekin H, Gürses E, Sungurtekin U, Kaptanoglu B. Usefulness of procalcitonin for diagnosis of sepsis in the intensive care unit. Crit Care. 2003;7(1):85. | ||
Sener G, Ozgur E, Rad AY, Uzun L, Say R, Denizli A. Rapid real-time detection of procalcitonin using a microcontact imprinted surface plasmon resonance biosensor. Analyst. 2013;138(21):6422–6428. | ||
Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care. 2010;14(1):R15. | ||
Carrigan SD, Scott G, Tabrizian M. Toward resolving the challenges of sepsis diagnosis. Clin Chem. 2004;50(8):1301–1314. | ||
Wolff M, Bouadma L. What procalcitonin brings to management of sepsis in the ICU. Crit Care. 2010;14(6):1007. | ||
Morgenthaler NG, Struck J, Fischer-Schulz C, Bergmann A. Sensitive immunoluminometric assay for the detection of procalcitonin. Clin Chem. 2002;48(5):788–790. | ||
Köszegi T. Immunoluminometric detection of human procalcitonin. J Biochem Biophys Methods. 2002;53(1–3):157–164. | ||
Li H, Sun Y, Elseviers J, Muyldermans S, Liu S, Wan Y. A nanobody-based electrochemiluminescent immunosensor for sensitive detection of human procalcitonin. Analyst. 2014;139(15):3718–3721. | ||
Krämer PM, Gouzy MF, Kess M, Kleinschmidt U, Kremmer E. Development and characterization of new rat monoclonal antibodies for procalcitonin. Anal Bioanal Chem. 2008;392(4):727–736. | ||
Qi X, Huang Y, Lin Z, Xu L, Yu H. Dual-Quantum-Dots-Labeled Lateral Flow Strip Rapidly Quantifies Procalcitonin and C-reactive Protein. Nanoscale Res Lett. 2016;11(1):167. | ||
Poulsen F, Jensen KB. A luminescent oxygen channeling immunoassay for the determination of insulin in human plasma. J Biomol Screen. 2007;12(2):240–247. | ||
Szekeres PG, Leong K, Day TA, Kingston AE, Karran EH. Development of homogeneous 384-well high-throughput screening assays for Abeta1-40 and Abeta1-42 using AlphaScreen technology. J Biomol Screen. 2008;13(2):101–111. | ||
Peppard J, Glickman F, He Y, Hu SI, Doughty J, Goldberg R. Development of a high-throughput screening assay for inhibitors of aggrecan cleavage using luminescent oxygen channeling (AlphaScreen). J Biomol Screen. 2003;8(2):149–156. | ||
Soini E, Kojola H. Time-resolved fluorometer for lanthanide chelates-a new generation of nonisotopic immunoassays. Clin Chem. 1983;29(1):65. | ||
Lopez E, Chypre C, Alpha B, Mathis G. Europium(III) trisbipyridine cryptate label for time-resolved fluorescence detection of polymerase chain reaction products fixed on a solid support. Clin Chem. 1993;39(2):196–201. | ||
Liu TC, Huang H, Dong ZN, et al. Development of an amplified luminescent proximity homogeneous assay for quantitative determination of hepatitis B surface antigen in human serum. Clin Chim Acta. 2013;426:139–144. | ||
He A, Liu TC, Dong ZN, et al. A novel immunoassay for the quantization of CYFRA 21-1 in human serum. J Clin Lab Anal. 2013;27(4):277–283. | ||
Xiong Y, Wu Y, Luo S, et al. Development of a novel immunoassay to detect interactions with the transactivation domain of p53: application to screening of new drugs. Sci Rep. 2017;7(1):9185. | ||
Bielefeld-Sevigny M. AlphaLISA immunoassay platform- the “no-wash” high-throughput alternative to ELISA. Assay Drug Dev Technol. 2009;7(1):90–92. |
Supplementary material
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






